Abstract
Background
Adams-Oliver Syndrome is characterized by the combination of aplasia cutis congenita and limb anomalies. It was initially described in 1945 by Adams and Oliver.
Main observations
We report a case of a 10-year-old girl with Adams-Oliver Syndrome with aplasia cutis congenita and limb defects only with no internal organ anomalies.
Conclusions
Adams-Oliver Syndrome is a rare multisystem disorder of unknown etiology. It may be presented by isolated aplasia cutis congenita and limb anomalies.
Keywords: Adams-Oliver syndrome, aplasia cutis, cicatricial alopecia, limb defects, scarring alopecia
Introduction
Adams-Oliver syndrome (AOS) is characterized by the combination of congenital scalp defects (aplasia cutis congenita) and terminal transverse limb defects of variable severity. Since its original description, many reports have highlighted the variable expression of this condition.[1,2,3] Multiple systems may be involved including central nervous, cardiovascular, gastrointestinal and genitourinary systems. Internal anomalies may be severe and lethal.[4]
We present a case of AOS with isolated terminal limb defects and aplasia cutis congenita.
Case Report
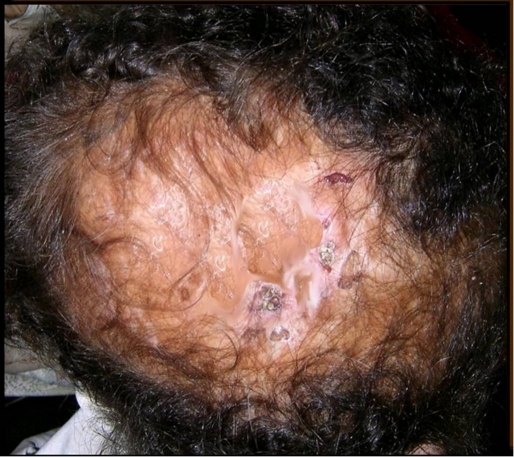
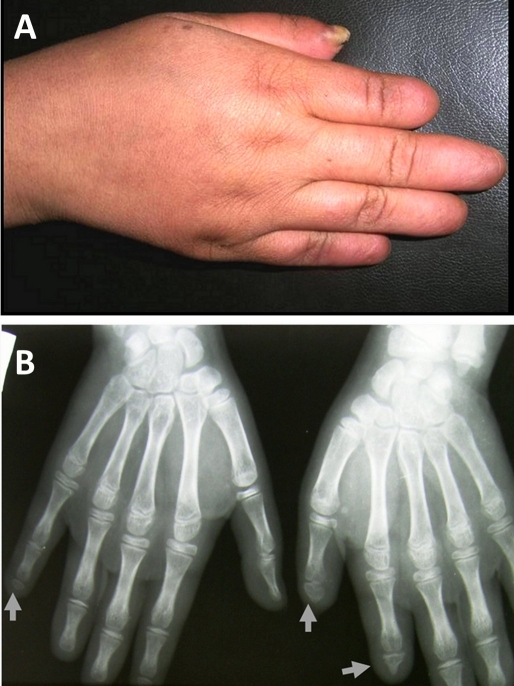
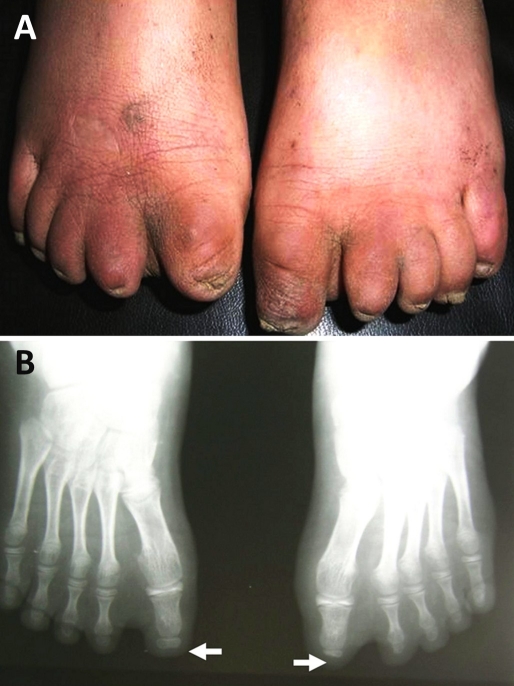
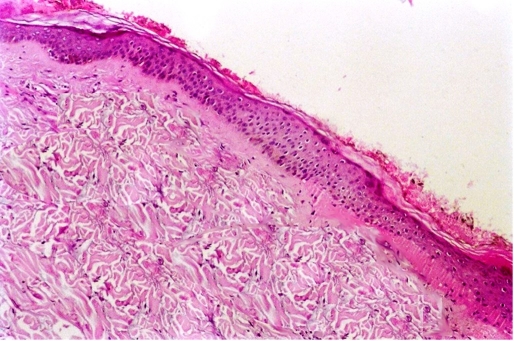
A 10-year-old female presented with atrophic bald plaque on the scalp [Fig. 1] with shortening of fingers and toes [Fig. 2A, 3A]. These anomalies were present since birth. The patient's mother reported that at birth, there was complete absence of scalp skin at the vertex that healed later on by scar. The mother denied any history of maternal drug intake, infection or radiation exposure during pregnancy and no family history of AOS, mental retardation, or central nervous system abnormalities. On scalp examination, we found a welldemarcated (15 × 12 cm) hairless atrophic plaque at its vertex. Hands and feet X-ray revealed hypoplasia of terminal phalanges of some fingers and total loss of terminal phalanges of other fingers [Fig. 2B]. Feet X-ray showed similar findings [Fig. 3B]. Skull X-ray, chest X-ray, echocardiography, abdominopelvic ultrasonography, intravenous pyelography, brain computed tomography were all normal. Trichoscopy was not performed. Biopsy was taken after taking mother's consent and histopathological examination of hematoxylin and eosin-stained section revealed flattening of rete ridge, thickening of collagen bundles in the dermis and loss of appendages [Fig. 4].
Figure 1.
Atrophic scaring of the vetex, representing aplasia cutis congenita.
Figure 2.
Shortening of terminal phalanges of fingers (A). Hand X-ray shows hypoplasia and/or complete absence of terminal phalanges (B).
Figure 3.
Bilateral shortening of terminal phalanges of toes (A). X-ray of feet shows hypoplasia and/or complete absence of terminal phalanges (B).
Figure 4.
Histopathology of the atrophic bald plaque showing flattening of rete ridges, excessive collagen deposition and loss of appendages (Hematoxylin and Eosin X 100).
The combination of aplasia cutis congenita and skeletal defects of the extremities suggests the diagnosis of AOS.
Discussion
Adams-Oliver syndrome is defined by the combination of limb abnormalities and scalp defects, often accompanied by skull ossification defects.[2] It was initially described in 1945 by Adams and Oliver.[5]
It is mostly inherited as an autosomal dominant (AD) trait but also a suggestive autosomal recessive (AR) mode of inheritance and sporadic cases have been described.[4,6]
Our case has no family history of AOS, so it is most likely a sporadic one. There are no differences in clinical manifestations between AD, AR or sporadic cases.[2]
The exact pathogenesis of AOS is unknown. AOS is the (syndrome of hypotheses). In their original description of the syndrome, Adams and Oliver[5] suggested that the underlying pathology could be an arrested development or agenesis of certain parts of the skeleton and soft tissue. Later on, intrauterine compression and amniotic band sequelae was suggested as a pathogeneic factor.[7] Pulmonary hypertension,[8] periventricular leukomalacia[9] and retinal folds[10] were also proposed as causative mechanisms.
Vascular impairment during embryogenesis has been proposed as a possible mechanism by several authors.
Hoyme et al.[11] found that the placentas from patients with AOS contained multiple organized thrombi throughout large fetal mainstem villous vessels. They hypothesized that an in-utero vascular thrombotic accident led to interruption of blood supply to developing structures. Others suggested that AOS is the result of the thrombotic interruption of embryonic blood supply in the subclavian or vertebral arteries[12] or other interruption of the early embyonic blood supply.[13] Swartz et al.[8] suggested that the abnormalities in AOS develop because of a generalized abnormality in small vessels causing disruption of blood flow. These small vessel irregularities would account for the aplasia cutis congenita, terminal transverse limb defects, as well as the cardiac, hepatic, and pulmonary vascular lesions. The vascular anomaly in AOS could be the result of a gene defect causing decreased stability of embryonic blood vessels toward tensile forces during the period of 6 to 8th week of embryonic life.[14]
More recently abnormal pericyte recruitment to blood vessels was postulated as a possible etiology.[15]
In order to identify the genetic cause of AOS, Verdyck et al.[16] evaluated a family with 10 affected individuals over 4 generations. However, they failed to find any disease-causing mutations in the studied genes (MSX 2, ALX4, MSX1, CART1, RUNX2, HOXD13, and P63).
Baskar et al.[17] suggested that the undelying genetic defect in AOS leads to aberrant morphogenesis rather than vascular disruption. They stated that bone morphogenetic proteins (BMPs) pathway is abnormal in these patients. BMPs are members of the transforming growth factor-b (TGF-b) superfamily that regulate growth, differentiation, chemotaxis and apoptosis and play a pivotal role in the morphogenesis of a variety of tissues and organs.[18] So the key to the underlying genetic basis of AOS, may lie in genes controlling proteins in this pathway. These if targeted for future candidate gene studies, could finally unravel the true etiology of this "syndrome of hypothesis".
Clinically, the limb defects are the most common feature of this disease and are usually asymmetric. The lower limbs are more susceptible than the upper limbs.[19]
The most frequently observed limb malformations in this disorder include syndactyly, brachydactyly, polydactyly, oligodactyly and hypoplastic finger/toenails. There is, however, a great variability in severity ranging from the complete absence of the foot or hand to only mild manifestations or normal appearance, as seen in obligate AOS carriers.[20]
Aplasia cutis congenita are scalp lesions most frequently found on the vertex of the skull that are variable in depth as well as in size. Often, skull defects underlying the scalp lesions are found.[16] Our patient has aplasia cutis congenita as proved by histopathological examination with normal underlying skull.
Various intracranial abnormalities have been described in AOS patients. They include encephalocele, microcephaly, hypoplasia of the left arteria cerebri, medial and right spastic hemiplegia, cortical dysplasia, pachygyria, hypoplastic corpus callosum, parenchymal calcifications, abnormal cerebral vasculature, ventriculomegaly and dysplasia of the cerebral cortex. As a consequence, secondary symptoms, such as epilepsy and mental retardation are frequently found in AOS patients.[4]
Cardiovascular malformations include obstructive defects in left heart, valvular anomalies, pulmonary vasular malformation and pulmonary hypertension.[8]
Other associated defects include cutis marmorata telangectasia congenita, gastrointestinal and hepatic malformations, accessory nipples, microphthalmia, hereditary hemorrhagic telangiectasia and cleft lip.[21] Our patient doesn't exhibit any of these anomalies as proved by examination and investigations.
The broad spectrum of symptoms and possible heterogeneity of AOS make genetic counseling difficult. It has been suggested that the severity of symptoms should be determined by fetoscopy if there is a positive family history. Prenatal diagnosis, as well as the assessment of severity is possible even in the first trimester.[22]
A question arises, whether the lifespan of an AOS patient without internal organs anomalies may be altered? Kuster et al.[19] reported a case of AOS without major organ abnormalities and stated that the lifespan of this category of patients is not affected. They indicated, however, that, if a newborn presents with aplasia cutis congenita and limb defects, dermatologists should consider AOS by conducting an evaluation of CNS and looking for cardiovascular, gastrointestinal, and genitourinary malformations.
Conclusion
AOS is a rare multisystem disorder that can be associated with extensive lethal anomalies. We report a case of AOS, most likely to be sporadic. The patient presented with isolated aplasia cutis congenita and terminal limb defects.
References
- Farrell SA, Warda LJ, LaFlair P, Szymonowicz W. Adams-Oliver syndrome: a case with juvenile chronic myelogenous leukemia and chylothorax. Am J Med Genet. 1993;47:1175–1179. doi: 10.1002/ajmg.1320470809. [DOI] [PubMed] [Google Scholar]
- Mempel M, Abeck D, Lange I, Strom K, Caliebe A, Beham A, Kautza M, Worret WI, Neubauer BA, Ring J, Schröder H, Fölster-Holst R. The wide spectrum of clinical expression in Adams-Oliver syndrome: a report of two cases. Br J Dermatol. 1999;140:1157–1160. doi: 10.1046/j.1365-2133.1999.02881.x. [DOI] [PubMed] [Google Scholar]
- Pereira-Da-Silva L, Leal F, Santos GC, Videira Amaral JM, Feijóo MJ. Clinical evidence of vascular abnormalities at birth in Adams-Oliver syndrome: Report of two further cases. Am J Med Genet. 2000;94:75–76. doi: 10.1002/1096-8628(20000904)94:1<75::aid-ajmg15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McGoey RR, Lacassie Y. Adams-Oliver syndrome in siblings with central nervous system findings, epilepsy, and developmental delay: refining the features of a severe autosomal recessive variant. Am J Med Genet. 2008;146:488–491. doi: 10.1002/ajmg.a.32163. [DOI] [PubMed] [Google Scholar]
- Adams FH, Oliver CP. Hereditary deformities in man: due to arrested development. J Hered. 1945;36:3–7. [Google Scholar]
- Koiffmann CP, Wajntal A, Huyke BJ, Castro RM. Congenital scalp skull defects with distal limb anomalies (Adams-Oliver syndrome--McKusick 10030): further suggestion of autosomal recessive inheritance. Am J Med Genet. 1988;29:263–268. doi: 10.1002/ajmg.1320290203. [DOI] [PubMed] [Google Scholar]
- Sybert VP. Aplasia cutis congenita: a report of 12 new families and review of the literature. Pediatr Dermatol. 1985;3:1–14. doi: 10.1111/j.1525-1470.1985.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Swartz EN, Sanatani S, Sandor GG, Schreiber RA. Vascular abnormalities in Adams-Oliver syndrome: cause or effect? Am J Med Gen. 1999;82:49–52. doi: 10.1002/(sici)1096-8628(19990101)82:1<49::aid-ajmg10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Sifakis S, Raissaki M, Germanakis I, Kalmanti M. Antenatal and postnatal evidence of periventricular leukomalacia as a further indication of vascular disruption in Adams-Oliver syndrome. Am J Med Genet A. 2008;146A:2545–2550. doi: 10.1002/ajmg.a.32410. [DOI] [PubMed] [Google Scholar]
- Prothero J, Nicholl R, Wilson J, Wakeling EL. Aplasia cutis congenita, terminal limb defects and falciform retinal folds: confirmation of a distinct syndrome of vascular disruption. Clin Dysmorphol. 2007;16:39–41. doi: 10.1097/MCD.0b013e328010b81c. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Jones KL, Van Allen MI, Saunders BS, Benirschke K. Vascular pathogenesis of transverse limb reduction defects. J Pediatr. 1982;101:839–843. doi: 10.1016/s0022-3476(82)80343-0. [DOI] [PubMed] [Google Scholar]
- Der Kaloustian VM, Hoyme HE, Hogg H, Entin MA, Guttmacher AE. Possible common pathogenetic mechanisms for Poland sequence and Adams-Oliver syndrome. Am J Med Genet. 1991;38:69–73. doi: 10.1002/ajmg.1320380116. [DOI] [PubMed] [Google Scholar]
- Fryns JP, Legius E, Demaerel P, van den Berghe H. Congenital scalp defect, distal limb reduction anomalies, right spastic hemiplegia and hypoplasia of the left arteri cerebri media: Further evidence that interruption of early embryonic blood supply may result in Adams-Oliver (plus) syndrome. Clin Genet. 1996;50:505–509. doi: 10.1111/j.1399-0004.1996.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Pousti TJ, Bartlett RA. Adams-Oliver syndrome: Genetics and associated anomalies of cutis aplasia. Plast Reconstr Surg. 1997;100:1491–1496. doi: 10.1097/00006534-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Patel MS, Taylor GP, Bharya S, Al-Sanna'a N, Adatia I, Chitayat D, Suzanne Lewis ME, Human DG. Abnormal pericyte recruitment as a cause for pulmonary hypertension in Adams-Oliver syndrome. Am J Med Genet A. 2004;129A:294–299. doi: 10.1002/ajmg.a.30221. [DOI] [PubMed] [Google Scholar]
- Verdyck P, Holder-Espinasse M, Hul WV, Wuyts W. Clinical and molecular analysis of nine families with Adams-Oliver syndrome. Eur J Hum Genet. 2003;11:457–463. doi: 10.1038/sj.ejhg.5200980. [DOI] [PubMed] [Google Scholar]
- Baskar S, Kulkarni ML, Kulkarni AM, Vittalrao S, Kulkarni PM. Adams-Oliver syndrome: Additions to the clinical features and possible role of BMP pathway. Am J Med Genet A. 2009;149A:1678–1684. doi: 10.1002/ajmg.a.32938. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Küster W, Lenz W, Kääriäinen H, Majewski F. Congenital scalp defects with distal limb anomalies (Adams-Oliver syndrome): report of ten cases and review of the literature. Am J Med Genet. 1988;31:99–115. doi: 10.1002/ajmg.1320310112. [DOI] [PubMed] [Google Scholar]
- Sankhyan N, Kaushal RK, Jaswal RS. Adams-Oliver syndrome: a case with complete expression. J Dermatol. 2006;33:435–436. doi: 10.1111/j.1346-8138.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Seo JK, Kang JH, Lee HJ, Lee D, Sung HS, Hwang SW. A case of Adams-Oliver syndrome. Ann Dermatol. 2010;22:96–98. doi: 10.5021/ad.2010.22.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Kunze J, Horn D, Gasiorek-Wiens A, Entezami M, Rossi R, Guschmann M, Sarioglu N. Autosomal recessive type of Adams-Oliver syndrome: prenatal diagnosis. Ultrasound Obstet Gynecol. 2002;20:506–510. doi: 10.1046/j.1469-0705.2002.00839.x. [DOI] [PubMed] [Google Scholar]