Abstract
Bloom's syndrome is a rare autosomal recessive disorder caused by germline mutation of the BLM gene. The objective of this study was to illustrate the clinical, biological and genetic characteristics of this syndrome through Tunisian series. We report in a retrospective study 8 case of bloom's syndrome observed during 20 years.
Results
Our patients were 4 males and 4 females issued from 5 families. For all patients, the parents were consanguineous. The age was 13 to 39 years. The telangiectatic erythema was developed in all the patients between 6 months and 2 years old on the cheeks, on the nose, on the lips and the lower eyebrows. The photosensitivity was constant and was complicated by vesicules and bullae for 5 patients who had extensive lesions, three patients noted accentuation of their telangiectasic erythema. An improvement with the age was noticed for the first four patients. The growth deficiency was observed for all patients. It was marked, between -2 and -4 DS (standard deviation). The number of sister chromatid exchange was increased to twelve fold comparatively to normal subjects. Two patients developed a breast cancer; the evolution was fatal in one. Another patient developed a leukaemia, the evolution was also fatal.
Conclusion
Bloom's syndrome is a rare genodermatitis. All the patients presented three symptoms: telangiectatic erythema, growth delay and photosensitivity associated with immunodeficiency. There is significant risk of cancer, so that follow up of patients is mandatory.
Keywords: erythema, growth retardation, malignancy, ocular abnormalities, photosensitivity, telangiectasia, teeth
Introduction
Bloom's syndrome (BS) is a rare autosomal recessive genetic disorder, characterized by chromosome instability.[1] Since the first case described by Bloom in 1954, and the creation of international registry, more then 180 cases have been reported.[1] BS is characterized clinically by major symptoms: telangiectatic erythema, photosensitivity, growth retardation, predisposition to develop early malignancies and inconstant other symptoms as cutaneous and bone dysplasia, teeth and ocular abnormalities.
The aim of the study was to specify the clinical, biological and genetic characteristics of the disease in a Tunisian series.
Patients and methods
Eight Bloom's syndrome patients' files were studied in a retrospective study. The patients have been followed between 1990 and 2010 in the Department of Dermatology of the university hospital of Sfax (TUNISIA). For each patient the age, gender, family and personal clinical history, clinical symptoms, biological and immunological data as well as the chromosomic changes were specified.
Cytogenetic analysis:
Blood samples were collected on heparine vacutainers for cell culture. Following Colcemid treatment metaphase chromosomes are prepared on slides:
G bands technique: After trypsinization, chromosome preparations are stained with Giemsa in order to produce G bands. The chromosomes were then examined for structural anomalies.
Sister chromatid exchange (SCE) method: Cell culture were incubated during two cell cycles with 5-bromo 2 deoxyuridine (BrdU). Incorporation of BrdU in the DNA discloses sister chromatid exchange during metaphase.
The SCE rate was evaluated for patients and sex and age matched controls. Three affected siblings from the same nuclear family underwent chromosomal study.
Results
The 8 patients with Bloom's syndrome were from 5 consanguineous families: 2 sisters, 3 brothers one male patient and 2 females from 3 separate families. The age at the first outpatient clinic visit varied from 6 to 39 years.
Table 1 summarizes physical and biological data collected during the follow up. All of patients had proportionate dwarfism and small stature between: -2 SD and -4 SD. A low birth weight and height were reported in all patients. The head circumference was reduced (-1 SD, -4 SD) in all patients.
Table 1.
| Patient | Age (years) | Height (cm) (SD) | Head circumference (cm) (SD) | Face | Sunlight sensitivity | Associated skin lesions | Associated clinical anomalies | Neoplasia | Associated biological anomalies | SCE |
|---|---|---|---|---|---|---|---|---|---|---|
| PA1, M | 6 | 110 (-1 SD) | 50 (-2 SD) | Narrow face | + in summer | Achromic patches | Ureter duplication | - | ↓ IgG ↓ IgM |
+ |
| PA2, M | 17 | 152 (-2 SD) | 53 (-2 SD) | Narrow face, malar hypoplasia, small mandibles | + vesicles, bullae | Café au lait spots Achromic patches |
Ureter duplication Brachyphalangea Skyphosis Testicular atrophy |
- | ↓ IgA ↓ IgM ↑ FSH |
+ |
| PA3, M | 14 | 152 (-1 SD) | 52 (-4 SD) | Narrow face | + vesicles, bullae | Café au lait spots Achromic patches Ectropion Loss of eyelashes |
Moderate mental retardation | - | ↓ IgA ↓ IgM |
+ |
| PB1, F | 36 | 132 (-4 SD) | 46 (-4 SD) | Narrow face, malar hypoplasia, low-set ears | + vesicles, bullae | Blepharitis Café au lait spots |
Cubitus valgus Toes deformity Delayed puberty Early menopause |
Breast carcinoma | ↓ IgG ↓ IgM ↑ FSH ↑ LH |
+ |
| PB2, F | 41 | 131 (-4 SD) | 46 (-4 SD) | Narrow face | + vesicles, bullae | Cafe au lait spots Lymphangioma of the toe |
Delayed puberty Early menopause Ectopic kidney |
- | ↑ FSH ↑ LH |
+ |
| PC, M | 6 | 94 (-3 SD) | 46 (4 SD) | Narrow face, small mandibles | + | Cafe au lait spots | Chronic myeloid leukaemia | ↓ IgA | + | |
| PD, F | 13 | 130 (-2 SD) | 48 (-3 SD) | Narrow face, small mandibles, low-set ears | + | Ectropion Hair loss Café au lait spots |
Mental retardation | - | ↓ IgA ↓ IgM |
+ |
| PE, F | 14 | 149 (-1 SD) | ND | Narrow face, small mandibles | + | Blepharitis Tinea capitis |
- | ↓ IgA ↓ IgM |
+ |
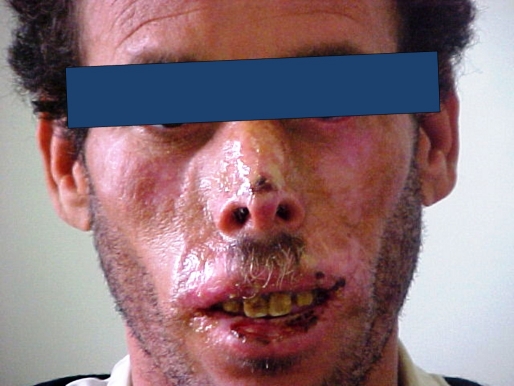
The telangiectatic erythema [Fig. 1] seen in all patients appeared at the age of 1 to 2 years. A butterfly like topography was constant with a variable extension.
Figure 1.
Erythematous, telangeictasic lesions of the face and cheilitis in a patient with Bloom's syndrome.
The erythema was extensive in the 3 brothers of the 1st family, involving the forehead, the chin and the perioral area.
Cheilitis, seen in all cases, varied from dry cheilitis to crusted and eroded lesions. Sun sensitivity was constant. Clinically it was characterized by vesicular and bullous extensive eruption in 5 patients. The 3 remaining patients complained from worsening of their telangiectatic erythema during summer season. The areas involved were the cheeks, forehead, nose, malar and perioral areas. The remaining photoexposed areas were spared. An improvement with age was seen in 4 patients.
Achromic patches were found in 3 cases and café-au-lait spots in 5 cases. Skeletal abnormalities were found in 3 patients: skyphosis of the backbone, cubitus valgus and brachyphalangae of the 5th finger. A moderate prognathism was seen in one patient.
A systematic ocular examination revealed a chronic blepharitis with loss of eyelash in 2 cases and an ectropion with loss of eyelash in one case.
Psychomotor development was normal in all cases excepted in one case with a moderate mental retardation.
A delayed puberty at the age of 19 with early menopause at the age of 38 and 29 years were reported in 2 female patients, whom presented elevated Follicular Stimulating Hormone (FSH) blood levels.
A testicular hypotrophy was detected in a 17-year-old boy, associated with a moderate increase of FSH.
Rhinopharyngitis and recurrent broncho-pulmonary infections were reported in all cases.
Three patients had recurrent urinary infections, related to an ectopic kidney (1 case) and duplication of ureter (2 cases).
Associated neoplasia was found in 3 patients. Two female patients issued from the same nuclear family developed a breast cancer. One of them deceased at the age of 39. A chronic myeloid leukemia occurred in the third patient, with fatal outcome.
Biological investigation showed a normal cellular blood count in all patients and a hypogammaglobulinemia in the protein electrophoresis. Endocrine investigations to rule out another cause of dwarfism were undertaken. Thyroid gland investigation (T3, T4, TSH) was normal except increase of TSH in one case. Testosteronemia was normal and FSH was increased in 2 adult post menopausal females. LH, cortisolemia, 17 ceto-steroid and 17 OH steroid were normal.
Immunologic investigation showed a decrease of 1 or 2 class immunoglobulins (IgA, IgM, IgE) in all patients. Intradermal PPD to explore immunity directed toward tuberculosis was normal.
Skin biopsies taken from the face in 2 patients, showed only epidermic atrophy while direct immunofluorescence showed granular IgG and complement deposition in the dermo epidermal junction.
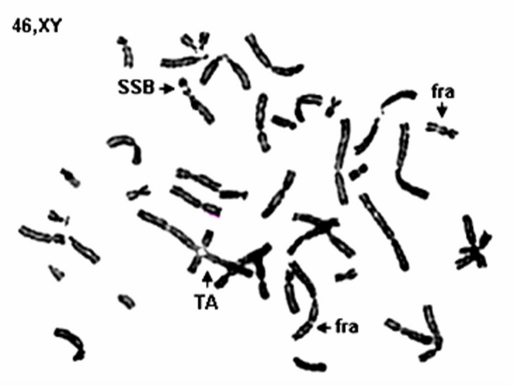
Cytogenetic study showed breakage of chromosome in blood samples taken from 7 patients and a bone marrow taken from another patient. Chromosomal aberrations observed include simple and double strand breaks, fragile sites and telomeric associations [Fig. 2].
Figure 2.
Chromosomal aberrations (SSB: simple strand break; TA: telomeric association; Fra: fragile site).
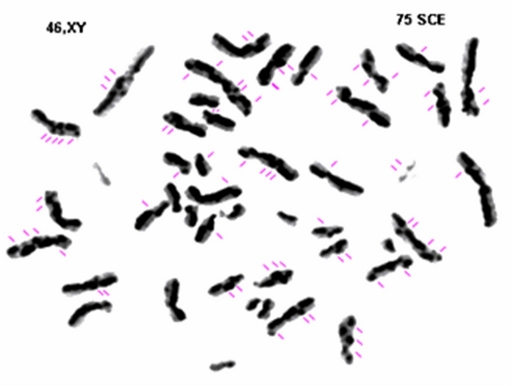
The number of SCE was increased to almost twelve fold when compared to normal subjects [Fig. 3].
Figure 3.
Sister chromatid exchange. Gray zone corresponds to partially incorporated bromodeoxyuridine. Black zone corresponds to completely incorporated bromodeoxyuridine. BS chromosomes with 75 SCE/ mitosis.
Discussion
Bloom's Syndrome is a rare autosomal recessive genodermatosis. The high rate of consanguineous marriages in our country would favour the occurrence of the disease.
In 1965, half of described cases were Ashkenazi Jews,[1] originated from Eastern Europe. A high frequency has been also reported in North America and Europe (Germany) in the International registry created by Bloom and German in the 60's.[3]
The male predominance was not found in our series, probably due to its small size.
The main clinical features are prenatal onset proportionate dwarfism, telangiectatic erythema and photosensitivity. All these cardinal clinical aspects were found in our patients. Some authors report a lupus-like facial lesions.[4]
Growth delay would not be related to organ failure or hormonal imbalance.[5,6] It starts as prenatal dwarfism responsible for the low weight at birth. Later on, the height and weight follow a slow progression (less than 1 cm per year until 21 years of age).[7] Investigation of the growth delay was made for all of our patients, and no endocrine disorders were found.
No major dysmorphic features were detected; however characteristic face was noted in all of our patients: narrow bird like faces with small mandible and low-set ears.
Photosensitivity with telangiectatic erythema was detected in all our patients with similar, clinical, evolutionary and topographic features reported in the literature.[5,6] The onset of telangiectatic erythema varied from 1 to 2 years of age. Its severity is variable, from moderate telangiectatic erythema to vesicular or bullous poïkiloderma. Involvement of the dorsal aspect of the hands and forearms has been reported,[6] however these features were lacking in our patients. The etiology of this photosensitivity remains unclear.[7]
Some other minor clinical signs as "café au lait" spots were found in more than half of cases and German series.[6] Achromic patches have been reported by several authors[2,6,8] and discovered in 3 of our patients, they differ from the ashleaf- shaped macules described in tuberous sclerosis.
Some other skin lesions may be seen in BS: twin spots which are pigmented or achromic macula,[9] hypertrichosis[2] and Norwegian scabies.[10]
Bone and articular abnormalities are frequently reported: malar and maxillar hypoplasia clindactyly, syndactyly and surnumerer finger.[15,6,11]
Neurological disorders are frequent, mainly major microcephaly described in most of the reported cases.[6,12] Usually the microcephaly is not associated with mental retardation, however memory deficiency and difficulties to make concentration efforts have been reported.
In our series neurologic development and motricity were normal in most of patients, except for one, who suffered from a mild mental retardation probably influenced by a poor social and economic environment.
Duplication of ureter and renal ectopy were frequent in our series. Gonadic abnormalities are sometimes reported mainly in male patients although, there is no puberty delay and the secondary sexual characters are present. Cryptorchidy and/or hypertrophic or atrophic testes are frequently reported.[6] In the female patients secondary sexual character are present, mainly the menses. However menopauses occurs early[6] as in the two female adult patients of our series.
BS is characterized by an immune deficiency with a decrease in Ig A, Ig G and Ig M with predisposition to recurrent diseases mainly of upper respiratory tract as well as urinary and digestive systems.[6] Recurrent bronchopulmonary infections as well as of the oropharynx, occurred in childhood in all of our patients. The consequences of these recurrent infections are severe in patients with delayed development.[6] However, the immunologic deficiency status is not correlated with predisposition to the infections. The cellular immunity is normal in these patients.[8,13]
BS is characterized by chromosome instability and sister chromatid exchanges with characteristic quadriradial formation figures. The gene responsible for BS has been mapped to chromosome 15 (15q26-1) which is closely linked to the proto-oncogene FES, and has been identified as a DNA helicase.[2,14,15]
Chromosomal instability is manifest by an increase in the frequency of chromosome breakage and somatic recombination, which associated with immunologic deficiency is a predisposing factor to develop cancer.[16]
The risk of occurrence of neoplasia is a major concern for BS patients. Solid tumors are the most common cancers involving the breast, uterus, larynx, colon, followed by leukemia and Hodgkin lymphoma.[16–19]
Colon adenomas, predisposing to cancer have been described in one patient.[17]
Conclusion
We reported 8 additional BS cases. We specified the clinical and biological features and compared our data with those reported in the literature.
References
- German J, Bloom D, Passarge E, Fried K, Goodman RM, Katzenellenbogen I, Laron Z, Legum C, Levin S, Wahrman. Bloom's syndrome. VI. The disorder in Israel and an estimation of the gene frequency in the Ashkenazim. Am J Hum Genet. 1977;29:553–562. [PMC free article] [PubMed] [Google Scholar]
- Reddy BS, Kochhar AM, Anitha M, Bamezai R. Bloom's syndrome - a first report from India. Int J Dermatol. 2000;39:760–763. doi: 10.1046/j.1365-4362.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- German J, Passarge E. Bloom's syndrome. XII. Report from the Registry for 1987. Clin Genet. 1989;35:57–69. doi: 10.1111/j.1399-0004.1989.tb02905.x. [DOI] [PubMed] [Google Scholar]
- McGowan J, Maize J, Cook J. Lupus-like histopathology in bloom syndrome: reexamining the clinical and histologic implications of photosensitivity. Am J Dermatopathol. 2009;31:786–791. doi: 10.1097/DAD.0b013e3181b3aa34. [DOI] [PubMed] [Google Scholar]
- German J, Takebe H. Bloom's syndrome. XIV. The disorder in Japan. Clin Genet. 1989;35:93–110. doi: 10.1111/j.1399-0004.1989.tb02913.x. [DOI] [PubMed] [Google Scholar]
- Gretzula JC, Hevia O, Weber PJ. Bloom's syndrome. J Am Acad Dermatol. 1987;17:479–488. doi: 10.1016/s0190-9622(87)70233-3. [DOI] [PubMed] [Google Scholar]
- Keller C, Keller KR, Shew SB, Plon SE. Growth deficiency and malnutrition in Bloom syndrome. J Pediatr. 1999;134:472–479. doi: 10.1016/s0022-3476(99)70206-4. [DOI] [PubMed] [Google Scholar]
- Rivera H, Garcia-Cruz D, Vaca G, Möller M, Ramos-Zepeda R, Cantú JM. Bloom syndrome in a Mexican mestizo girl. Ann Genet. 1986;29:39–41. [PubMed] [Google Scholar]
- Passarge E. Bloom's syndrome: the German experience. Ann Genet. 1991;34:179–197. [PubMed] [Google Scholar]
- Dick GF, Burgdorf WH, Gentry WC Jr. Norwegian scabies in Bloom's syndrome. Arch Dermatol. 1979;115:212–213. doi: 10.1001/archderm.1979.04010020058018. [DOI] [PubMed] [Google Scholar]
- Ishikiriyama S. Another case of Bloom's syndrome in Japan. Clin Genet. 1989;36:77–78. doi: 10.1111/j.1399-0004.1989.tb03372.x. [DOI] [PubMed] [Google Scholar]
- Legum C, Furman N, Diamant S. Bloom's syndrome in an Iranian Jewish male. Ann Genet. 1991;34:198–200. [PubMed] [Google Scholar]
- Weemaes CM, Bakkeren JA, Haraldsson A, Smeets DF. Immunological studies in Bloom's syndrome. A follow-up report. Ann Genet. 1991;34:201–205. [PubMed] [Google Scholar]
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- Blagoev KB, Goodwin EH, Bailey SM. Telomere sister chromatid exchange and the process of aging. Aging (Albany NY) 2010;2:727–730. doi: 10.18632/aging.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicken CH, Dewald G, Gordon H. Sister chromatid exchanges in Bloom's syndrome. Arch Dermatol. 1978;114:755–760. [PubMed] [Google Scholar]
- Lowy AM, Kordich JJ, Gismondi V, Varesco L, Blough RI, Groden J. Numerous colonic adenomas in an individual with Bloom's syndrome. Gastroenterology. 2001;121:435–439. doi: 10.1053/gast.2001.26259. [DOI] [PubMed] [Google Scholar]
- Karalis A, Tischkowitz M, Millington GW. Dermatological manifestations of inherited cancer syndromes in children. Br J Dermatol. 2011;164:245–256. doi: 10.1111/j.1365-2133.2010.10100.x. [DOI] [PubMed] [Google Scholar]
- Thomas ER, Shanley S, Walker L, Eeles R. Surveillance and treatment of malignancy in Bloom syndrome. Clin Oncol (R Coll Radiol) 2008;20:375–379. doi: 10.1016/j.clon.2008.01.007. [DOI] [PubMed] [Google Scholar]