Abstract
Purpose of review
Chagas disease is a complex ailment caused by infection with Trypanosoma cruzi. It afflicts millions in Latin America. Years of studies have focused on the development of pathology in Chagas disease and recent studies have helped us understand the cellular mechanisms behind differential clinical evolution of Chagas disease.
Recent findings
We discuss recent findings concerning the cellular immune response in human Chagas disease focusing on immunoregulation and the development of pathology. We seek to put several findings into the context of a disease that initially controls an extreme and patent infection, and later progresses to a chronic phase marked by the presence (cardiac and digestive forms), or not (indeterminate form), of associated pathology.
Summary
Several theories exist to explain differential clinical evolution of Chagas disease. A coherent understanding of these theories will certainly aid in determining what combination of them approximates the true development of chagasic pathology. For achieving the goal of developing a successful therapy or intervention, it is critical that no theory be excluded at this point, but. Rather, rather that a thoughtful analysis and assimilation of the best components of each system into a central theory that best fits the reality of human Chagas disease is desirable.
Keywords: Chagas disease, human, immunoregulation, pathology, T cells, Trypanosoma cruzi
Introduction
The interactions between Trypanosoma cruzi, a well adapted parasite, and its host lead to Chagas disease, a life-long illness with distinct clinical outcomes. While most chronic patients remain in an asymptomatic state (indeterminate form), a significant percentage develops deadly clinical forms (cardiac and/or digestive). Why differential clinical evolution occurs is a puzzling and, as of yet, unresolved question. Despite many uncertainties, it is acknowledged that the host immune response is critical in determining disease outcome. Here, we will discuss recent findings, focusing on human studies, which have added to our understanding of the processes associated with pathology development in Chagas disease.
Human Chagas disease: an old disease, many new concerns
Although Chagas disease was described in 1909 [1], evidence of T. cruzi infection in humans dates back 9000 years [2]. Approximately 18 million people are currently infected and 100 million at risk of infection with T. cruzi in Latin America. Despite the significant control of the insect vector, Triatoma infestans, in Uruguay, Chile and Brazil, the fact that neighboring countries still have high incidence of natural vector transmitted T. cruzi infection presents a constant threat to Chagas disease epidemiological control [3•]. Recent studies have shown that infected domestic animals are an important reservoir for sustaining natural transmission in endemic areas [4•,5]. Moreover, microepidemics due to ingestion of contaminated juices have been recently reported in Brazil, clearly showing the presence of contaminated insects even in areas where epidemiological control was thought to have been achieved [6]. For years, Chagas disease was considered one of the consequences of poor socioeconomic conditions in Latin America. While this statement is still true, Chagas disease transmission through blood transfusion and organ transplantation in nonendemic areas is currently recognized as a serious problem [7]. These data demonstrate that Chagas disease, although an old disease, is a contemporary concern and point to the importance of active epidemiological surveillance with efficient parasite-targeted veterinary care, dependable screening of human-derived blood and organs and reliable prophylaxis or therapeutic interventions. Multidisciplinary approaches and consistent funding are essential for achieving disease control, at all levels.
Progression of human Trypanosoma cruzi infection: a wide spectrum of diseases
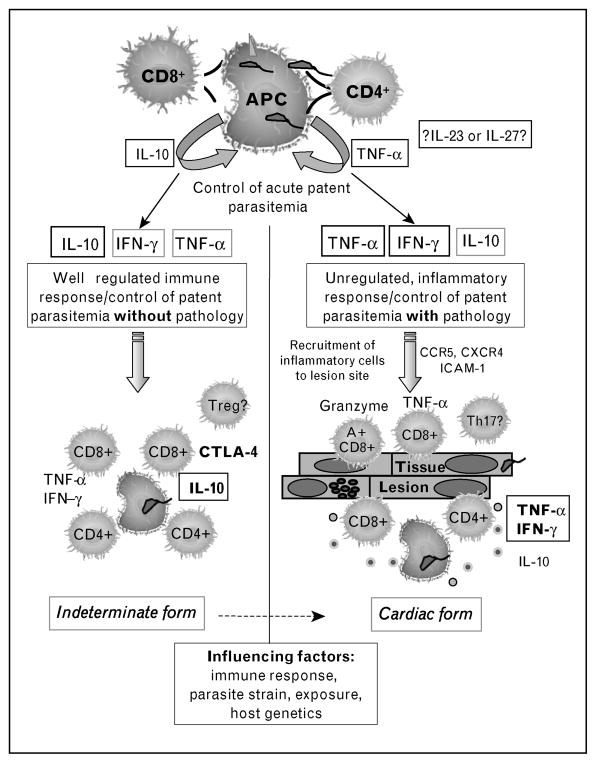
Upon infection, individuals undergo an acute phase that lasts approximately 2 months, characterized by high numbers of parasites in the bloodstream and tissues. Most acutely infected individuals in endemic areas are asymptomatic, which presents a serious challenge for diagnosis and treatment of disease. Treatment is successful in at least 70% of the individuals diagnosed shortly after infection [8,9]. If infection is not treated, roughly 5% of the individuals may die of acute myocarditis, but most individuals will enter into the chronic phase of Chagas disease. Progression to the chronic phase is concurrent with the establishment of a relatively efficient immune response, which brings parasitemia to subpatent levels in all immunocompetent individuals. Although parasite levels are drastically reduced, it is during the chronic phase that the most serious symptoms of Chagas disease may occur. While most chronic patients are asymptomatic, classified as ‘indeterminate’, approximately 30% develop serious clinical symptoms (Fig. 1). Some chagasic patients develop a destruction of the myoenteric nervous plexus leading to the digestive form, which presents as a variety of clinical syndromes, ranging from mild swallowing difficulty to severe dilations of intestinal structures known as the chagasic ‘mega’ syndrome. The cardiac clinical form is caused by an inflammatory reaction in the heart tissue, leading to a spectrum of debilitating and morbid cardiac diseases [10]. An important study recently demonstrated that it is possible to predict death by Chagas heart disease through analysis of clinical characteristics in cardiac patients [11]. Why T. cruzi-infected patients progress into distinct clinical forms is not known. Factors such as parasite strain and tissue tropism, parasite load, time of infection, nature of the immune response mounted and the host’s genetic background all play important roles.
Figure 1.
Immunoregulatory model in chronic indeterminate and cardiac Chagas disease
Theories for the generation and maintenance of (immuno) pathology in human Chagas disease
As mentioned earlier, several aspects are likely to influence the clinical evolution of human Chagas disease. Parasite load, time of infection and exposure to reinfection are extremely hard to ascertain while studying human populations. Using animal models, it has been possible to demonstrate that these factors affect protection and pathology following T. cruzi infection [12–14]. It has been shown, in experimental as well as human T. cruzi infection, that parasites isolated from different organs are genetically distinct, suggesting that the genetic variability of the parasite strains may influence disease outcome [15,16]. However, a recent study has shown a high variability of T. cruzi kDNA signatures amongst isolates from patients with the same clinical form [17].
Two major theories emerged in the literature to explain the generation of the immunopathology in human Chagas disease. One postulates that an antiparasite response is the major cause of pathology while the other postulates that autoimmune processes are the major cause of tissue destruction. These two theories have led to controversies for many years – and still do [18,19]. Nevertheless, it is fair to say that these theories are not necessarily mutually exclusive, and that the difference between tissue damage induced by an antiparasite response vs. that induced through indirect effects or directly against a specific self-antigen could very well be overlapping. A key point is to determine how protective immune responses are generated and maintained with limited pathology. Detailed immunological analysis of acutely infected individuals, as well as longitudinal studies of chronically infected patients will continue to be extremely valuable to help solve this puzzle.
How the early encounter of Trypanosoma cruzi with host cells can determine the fate of infection: more questions than answers …
The first step to ensure T. cruzi survival and successful infection is to enter host cells. Several molecules present on host cells and on the parasite surface are essential for the active process of cell invasion [20] and are capable of stimulating an innate immune response upon the first encounter [21••]. These early interactions will be critical for both the immediate control of parasitemia and for establishing a cytokine-rich microenvironment which will direct subsequent development of regulatory and effector T-cell populations, important for maintaining control of the parasite with or without pathology during the chronic phase.
A wealth of information concerning the immune response during early interactions in animals acutely infected with T. cruzi has identified important aspects to be studied in human disease [22••,23]. However, studies of early activation by T. cruzi using cells from acute Chagas patients are scarce. Although classic studies have suggested a suppressive response in the early phases of human T. cruzi infection [24,25], it is clear that the immune response mounted is efficient for controlling patent parasitemia. It has been suggested that B-cell activation leading to lytic antibody production is important in parasite elimination during the acute phase [26–28]. Although studies of endomyocardial biopsies from acute patients have shown the presence of CD4+ and CD8+ T cells in the inflammatory myocarditis [29], the function of these cells during the acute phase has not been determined. Interestingly, a decreased frequency of a particular CD4+ T-cell subpopulation expressing the Vbeta 5 region of the T-cell receptor (TCR) was seen in acute Chagas patients [30]. We speculate that this decrease was due to an activation-induced cell death, since the frequency of this population is increased upon lowering of antigenic concentration in chronic patients. Taken together, these data suggest that T cells play an important role during acute Chagas disease. Yet, a more extensive study is required to ascertain the exact role that T cells may play in early parasite control and how these cells may influence the subsequent development of distinct clinical forms of Chagas disease.
Immunoregulation in the chronic phase: balancing parasite control and pathology
Perhaps one of the most important points while studying the clinical evolution of human Chagas disease is to find out what determines whether an individual will develop severe disease or not. A plausible theory is that individuals who remain asymptomatic are capable of reducing parasite numbers in the early phase of infection, and go on to downmodulate the response in a way that it limits the development of pathology. On the other hand, individuals who will develop cardiac disease, although capable of parasite control, may not be capable of mounting efficient immunoregulatory mechanisms, thus leading to establishment of persistent inflammation. Alternatively, it is also plausible to hypothesize that the differential clinical evolution is due to qualitative differences in the response from the beginning, which could be due to several factors such as parasite strains and exposure, or host genetic background (Fig. 1). The high individual immunological variations inherent to humans, the impossibility of controlling for factors such as parasite exposure and the need for refined clinical characterization are some of the complicated issues when studying human Chagas disease; however, these difficulties can be dealt with by performing well designed studies [31].
Cellular responses are critical during the evolution of Chagas disease. Among the findings that support this concept is the inflammatory nature of the associated lesions and the fact that a robust proliferative response and cytokine expression is observed by cells from chronic patients. We demonstrated that indeterminate and cardiac chagasic individuals display high frequencies of activated T cells in their bloodstream [32]. Moreover, activated T cells are a major component of the inflammatory infiltrate observed in cardiac, as well as digestive patients [33•,34,35]. There is strong evidence for the involvement of these activated T cells in the development of pathology. It has been demonstrated that circulating activated T cells from asymptomatic and symptomatic patients express both inflammatory and anti-inflammatory cytokines, consistent with an active immunoregulation during the chronic phase [36,37]. However, a preferential expression of inflammatory cytokines, especially TNF-alpha and IFN-gamma, was observed in cardiac lesions [35,38]. A recent paper by Fonseca et al. [39•] suggested that IL-15 and IL-7 are important to sustain CD8+ T-cell survival in the cardiac inflammatory infiltrate. It has also been shown that CD8+ T cells from cardiac and digestive lesions express cytolytic molecules such as Granzyme or TIA-1 antigen [33•,35]. Adding to the importance of CD8+ T cells in chronic Chagas disease is the finding that a high percentage of these cells from chagasic patients lack CD28 expression, indicating chronic activation [40]. Albareda et al. [41] also observed a high frequency of CD8+CD28− cells in chronic patients and suggested that these cells may be exhausted due to chronic antigenic restimulation. These cells may be important for active control of the parasite during chronic phase and also in mediating tissue pathology. These possibilities are currently under investigation by several groups and may not be mutually exclusive.
Immunological analyses using well defined patients have recently been employed and have uncovered some important differences between indeterminate and cardiac patients, as well as among patients with different degrees of cardiac pathology, adding to our understanding of the role of the immune response in the differential clinical evolution of disease. Studies regarding T-cell repertoire showed that CD4+ T cells from indeterminate and cardiac patients displayed preferential expression of the Vbeta 5 TCR region [30]. However, recent studies showed that while CD8+CD28+ T-cells from indeterminate patients preferentially express Vbeta 3.1 and 5, the same cell population isolated from severe cardiac patients displayed a preferential expression of Vbeta 3.1, but not Vbeta 5 [42]. These data suggest that a dominant antigenic target may be important in eliciting T-cell responses in human Chagas disease and that there are differences in antigenic recognition between patients with distinct clinical forms. The high expression of inflammatory cytokines, especially IFN-gamma and TNF-alpha, has been associated with progressively severe cardiac disease [43,44]. However, other studies have described an opposite correlation between the expression of IFN-gamma and cardiac disease [45]. Considering the long lasting nature of chagasic cardiomyopathy, as well as the fact that indeterminate patients are capable of producing inflammatory cytokines, despite a lack of detectable pathology [37,43,46], it is clear that immunoregulatory mechanisms are present during the disease and might strongly influence its clinical evolution. Recent studies by our group [47] have shown that monocytes from indeterminate patients display higher expression of IL-10 upon exposure to the parasite, while monocytes from cardiac patients submitted to the same treatment preferentially express TNF-alpha. The higher expression of IL-10 by cells from indeterminate as compared with cardiac patients was also observed by other groups [43]. Moreover, a high frequency of CD4+CD25 cells has been observed in indeterminate patients [28]. An intriguing finding was that although CD8+ T cells from indeterminate patients display a high frequency of surface CTLA-4 expression, the same cells from cardiac patients have lower surface expression of CTLA-4, while intracellular expression was high [48••]. It is reasonable to hypothesize that decreased surface CTLA-4 expression by CD8+ T cells from cardiac patients may contribute to exacerbated cytolytic activity and tissue destruction. Together, these data suggest that cardiac patients may have a deficiency in downmodulating the inflammatory response, as compared with asymptomatic patients. Thus, it is possible that, although both groups of patients can maintain a low parasite burden, the ability to control inflammation (directly or indirectly) is ‘lost’ in cardiac patients (Fig. 1). Whether the ‘loss’ of this ability is genetically determined, a result of constant antigenic stimulation, or due to differences in the initial microenvironment are important questions to be answered. The search for parasite antigens that can potentially induce protective responses is a key aspect and will certainly be advanced by the availability of the parasite genome sequence and proteomic analysis underway in several laboratories. Moreover, recent studies have evaluated the occurrence of polymorphisms in genes that encode for molecules related to the control of the immune response in chagasic patients [49,50•,51]. This approach may help identify predisposing factors, and thus present an exciting field of research.
Conclusion
The data presented in this review clearly emphasize the complexity of the immune response generated during T. cruzi infection and strengthen the concept that the host immune response is critical for disease control and evolution. The antiparasitic response, crucial for chronification of infection, may work as a double-edged sword if not properly modulated. Luckily, as a result of thousands of years of coevolution, most individuals are able to coexist with the parasite for their lifetime. The lessons learned from studying this complex interaction will certainly be helpful when designing strategies for disease control and prevention. It is, however, unlikely that just one approach will lead to successful interventions. Given the severity of Chagas disease and the large contingent of infected and at risk individuals, the quest for efficient diagnosis, therapy and prevention is fully justified.
Acknowledgments
The authors would like to acknowledge WHO/TDR Programme, CNPq, FAPEMIG and NIH/NIAID for continued support of their work. We also present our apologies to the authors of many important papers not cited here due to format limitations and thank all the researchers whose scientific contributions have allowed for great progress towards the understanding of Chagas disease.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 331–332).
- 1.Chagas C. New human trypanosomiasis: studies on morphology and evolutionary cycle of Schizotrypanum cruzi n. gen., n. sp. ethiologic agent of a new human morbid entity [in Portuguese] Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- 2.Aufderheide AC, Salo W, Madden M, et al. A 9000-year record of Chagas’ disease. Proc Natl Acad Sci U S A. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Dias JC. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusion Chagas disease: historical aspects, present situation, and perspectives. Mem Inst Oswaldo Cruz. 2007;102(1):11–18. doi: 10.1590/s0074-02762007005000092. This paper brings into perspective the historical aspects and current reality of the largest Chagas disease control program in South America. [DOI] [PubMed] [Google Scholar]
- 4•.Gurtler RE, Cecere MC, Lauricella MA, et al. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134(Pt 1):69–82. doi: 10.1017/S0031182006001259. This paper emphazises the importance of peridomestic cycle in sustaining T. cruzi transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy MZ, Bowman NM, Kawai V, et al. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerg Infect Dis. 2006;12:1345–1352. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steindel M, Kramer Pacheco L, Scholl D, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60:25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Leiby DA, Herron RM, Jr, Read EJ, et al. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002;42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 8.Rassi A, Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115:1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 9.Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;19:583–587. doi: 10.1097/01.qco.0000247592.21295.a5. [DOI] [PubMed] [Google Scholar]
- 10.Rocha MO, Teixeira MM, Ribeiro AL. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther. 2007;5:727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- 11.Rassi A, Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante JM, Novarese M, Rivarola HW, et al. Reinfections and Trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. Parasitol Res. 2007;100:1407–1410. doi: 10.1007/s00436-006-0425-3. [DOI] [PubMed] [Google Scholar]
- 13.Hyland KV, Leon JS, Daniels MD, et al. Modulation of autoimmunity by treatment of an infectious disease. Infect Immun. 2007;75:3641–3650. doi: 10.1128/IAI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzelepis F, Persechini PM, Rodrigues MM. Modulation of CD4+ T cell-dependent specific cytotoxic CD8+ T cells differentiation and proliferation by the timing of increase in the pathogen load. PLoS ONE. 2007;2:e393. doi: 10.1371/journal.pone.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade LO, Machado CR, Chiari E, et al. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- 16.Vago AR, Andrade LO, Leite AA, et al. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lages-Silva E, Ramirez LE, Pedrosa AL, et al. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas’ disease in Brazil. J Clin Microbiol. 2006;44:2167–2171. doi: 10.1128/JCM.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyland KV, Engman DM. Further thoughts on where we stand on the auto-immunity hypothesis of Chagas disease. Trends Parasitol [Reply] 2006;22:101–102. doi: 10.1016/j.pt.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Kierszenbaum F. Where do we stand on the autoimmunity hypothesis of Chagas disease? Trends Parasitol. 2005;21:513–516. doi: 10.1016/j.pt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Andrade LO, Andrews NW. The Trypanosoma cruzi–host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 21••.Tarleton RL. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. Comprehensive review about parasite components recognized by host receptors, covering innate and adaptive responses mostly in murine models. [DOI] [PubMed] [Google Scholar]
- 22••.DosReis GA, Ribeiro-Gomes FL, Guillermo LV, Lopes MF. Cross-talk between apoptosis and cytokines in the regulation of parasitic infection. Cytokine Growth Factor Rev. 2007;18:97–105. doi: 10.1016/j.cytogfr.2007.01.009. Excellent review that presents an intriguing network between cytokines and apoptotic pathways as important for parasite persistence. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 24.Kierszenbaum F, Lopez HM, Sztein MB. Inhibition of Trypanosoma cruzi-specific immune responses by a protein produced by T. cruzi in the course of Chagas’ disease. Immunology. 1994;81:462–467. [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira AR, Teixeira G, Macedo V, Prata A. Acquired cell-mediated immunodepression in acute Chagas’ disease. J Clin Invest. 1978;62:1132–1141. doi: 10.1172/JCI109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antas PR, Medrano-Mercado N, Torrico F, et al. Early, intermediate, and late acute stages in Chagas’ disease: a study combining antigalactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am J Trop Med Hyg. 1999;61:308–314. doi: 10.4269/ajtmh.1999.61.308. [DOI] [PubMed] [Google Scholar]
- 27.Gazzinelli RT, Pereira ME, Romanha A, et al. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human antigal antibodies. Parasite Immunol. 1991;13:345–356. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 28.Vitelli-Avelar DM, Sathler-Avelar R, Massara RL, et al. Are increased frequency of macrophage-like and natural killer (NK) cells, together with high levels of NKT and CD4+CD25high T cells balancing activated CD8+ T cells, the key to control Chagas’ disease morbidity? Clin Exp Immunol. 2006;145:81–92. doi: 10.1111/j.1365-2249.2006.03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuenmayor C, Higuchi ML, Carrasco H, et al. Acute Chagas’ disease: immunohistochemical characteristics of T cell infiltrate and its relationship with T. cruzi parasitic antigens. Acta Cardiol. 2005;60:33–37. doi: 10.2143/AC.60.1.2005046. [DOI] [PubMed] [Google Scholar]
- 30.Costa RP, Gollob KJ, Fonseca LL, et al. T-cell repertoire analysis in acute and chronic human Chagas’ disease: differential frequencies of Vbeta5 expressing T cells. Scand J Immunol. 2000;51:511–519. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 31.Dutra WO, Rocha MO, Teixeira MM. The clinical immunology of human Chagas disease. Trends Parasitol. 2005;21:581–587. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Dutra WO, Martins-Filho OA, Cancado JR, et al. Activated T and B lymphocytes in peripheral blood of patients with Chagas’ disease. Int Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- 33•.da Silveira AB, Lemos EM, Adad SJ, et al. Megacolon in Chagas disease: a study of inflammatory cells, enteric nerves, and glial cells. Hum Pathol. 2007;38:1256–1264. doi: 10.1016/j.humpath.2007.01.020. Careful analysis of inflammatory infiltrate associated to digestive disease with or without occurrence of megacolon. [DOI] [PubMed] [Google Scholar]
- 34.Higuchi MD, Ries MM, Aiello VD, et al. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg. 1997;56:485–489. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 35.Reis DD, Jones EM, Tostes S, Jr, et al. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 36.Cunha-Neto E, Dzau VJ, Allen PD, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol. 2005;167:305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutra WO, Gollob KJ, Pinto-Dias JC, et al. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi Mde L, Gutierrez PS, Aiello VD, et al. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch A Pathol Anat Histopathol. 1993;423:157–160. doi: 10.1007/BF01614765. [DOI] [PubMed] [Google Scholar]
- 39•.Fonseca SG, Reis MM, Coelho V, et al. Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic Chagas disease cardiomyopathy. Scand J Immunol. 2007;66:362–371. doi: 10.1111/j.1365-3083.2007.01987.x. This paper suggests mechanisms for survival of CD8+ T cells in the heart of patients with Chagas heart disease. [DOI] [PubMed] [Google Scholar]
- 40.Dutra WO, Martins-Filho OA, Cancado JR, et al. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 41.Albareda MC, Laucella SA, Alvarez MG, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas’ disease patients. Int Immunol. 2006;18:465–471. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 42.Menezes CA, Rocha MO, Souza PE, et al. Phenotypic and functional characteristics of CD28+ and CD28− cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes JA, Bahia-Oliveira LM, Rocha MO, et al. Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect Immun. 2003;71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talvani A, Rocha MO, Barcelos LS, et al. Elevated concentrations of CCL2 and tumor necrosis factor-alpha in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943–950. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 45.Laucella SA, Postan M, Martin D, et al. Frequency of interferon-gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis. 2004;189:909–918. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 46.de Barros-Mazon S, Guariento ME, da Silva CA, et al. Differential regulation of lymphoproliferative responses to Trypanosoma cruzi antigen in patients with the cardiac or indeterminate form of Chagas disease. Clin Immunol. 2004;111:137–145. doi: 10.1016/j.clim.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Souza PE, Rocha MO, Rocha-Vieira E, et al. Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Souza PE, Rocha MO, Menezes CA, et al. Trypanosoma cruziinfection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas’ disease. Infect Immun. 2007;75:1886–1894. doi: 10.1128/IAI.01931-06. This work suggests mechanisms of immunoregulation in indeterminate patients on the basis of the expression of modulatory molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Florez O, Zafra G, Morillo C, et al. Interleukin-1 gene cluster polymorphism in chagas disease in a Colombian case-control study. Hum Immunol. 2006;67:741–748. doi: 10.1016/j.humimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 50•.Ramasawmy R, Fae KC, Cunha-Neto E, et al. Variants in the promoter region of IKBL/NFKBIL1 gene may mark susceptibility to the development of chronic Chagas’ cardiomyopathy among Trypanosoma cruzi-infected individuals. Mol Immunol. 2008;45:283–288. doi: 10.1016/j.molimm.2007.04.015. This paper presents possible markers of cardiac Chagas disease susceptibility in Brazilians. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Perez JM, Cruz-Robles D, Hernandez-Pacheco G, et al. Tumor necrosis factor-alpha promoter polymorphism in Mexican patients with Chagas’ disease. Immunology Lett. 2005;98:97–102. doi: 10.1016/j.imlet.2004.10.017. [DOI] [PubMed] [Google Scholar]