Abstract
Allelopathy, one type of direct plant competition, can be a potent mechanism through which plant communities are structured. The aim of this study was to determine whether allelopathic interactions occur between the opportunistic green tide-forming species Ulva prolifera and the native macroalga Gracilaria lichvoides, both of which were collected from the coastline of East China sea. In laboratory experiments, the presence of G. lichvoides at 1.25 g wet weight L−1 significantly inhibited growth and photosynthesis of U. prolifera at concentrations of 1.25, 2.50, and 3.75 g wet weight L−1 (p<0.05) in both semi-continuous co-culture assays and in co-culture assays without nutrient supplementation. In contrast, although U. prolifera had a density effect on G. lichvoides, the differences among treatments were not significant (p>0.05). Culture medium experiments further confirmed that some allelochemicals may be released by both of the tested macroalgae, and these could account for the observed physiological inhibition of growth and photosynthesis. Moreover, the native macroalgae G. lichvoides was a stronger competitor than the opportunistic species U. prolifera. Collectively, the results of the present study represent a significant advance in exploring ecological questions about the effects of green tide blooms on the macroalgal community.
Introduction
The introduction and spread of exotic species into the marine environment is considered to be a major threat to marine ecosystems, with potentially dramatic effects on biological diversity, productivity, habitat structure, and fisheries [1]. Beginning in the 1990 s, a vast increase in the worldwide spread of nonindigenous organisms has occurred, due mainly to dispersal via human-mediated transport [2], [3], [4]. One of the most representative examples linked with anthropogenic activities is an expansive “green tide” caused by the proliferation of green macroalgae belonging to the genus Ulva. Green tides have occurred almost in many enclosed marine water bodies, including in Europe, Australia, New Zealand, Hong Kong, the Philippines, Indonesia, India, Egypt, China, South Africa and Central America [5]. In China between May and July 2008, prior to the Olympic sailing competition, the large scale blooms of Ulva prolifera caused the world's largest green tide [6]; it consisted of more than 1 million tons of drifting biomass and covered an area of 13,000–30,000 km2 [7], [8].
While the occurrence and spread of U. prolifera have been well documented, the mechanism by which it invades a community and its impact on native communities has received little attention. The few studies conducted to date have shown that in addition to having negative effects on tourism, large algal mats can have deleterious ecological effects. These effects include uncoupling of the biogeochemical cycles in sediments from those in the water column [9], a negative impact on seagrass beds due to shading, disruption of feeding by wading birds [10], development of a lethal environment due to oxygen deficiency [11], and a shift from a high-diversity mixture to low-diversity assemblages of fast-growing annual algae [12].
Invasive macroalgae can impact native species through competition for different resources such as light, space, or nutrients [13], [14]; via modification of abiotic stress [15]; and by chemical means, such as allelopathy [16], [17]. Marine seaweeds produce a wide variety of secondary metabolites such as terpenes, sterols, polyphenols, and acetogenins [18]. This phenomenon of interactions among algal species has been called allelopathy. Several recent studies revealed that some of these compounds function as chemical defenses that are able to deter a broad range of natural enemies, including competitors, epiphytes, pathogenic bacteria, and herbivores [19], [20].
The bulk of research on allelopathy has focused on macroalga-microalga interactions, especially in the red tide-inhibition realm. There is now a considerable amount known about allelopathic effects of green macroalgal such as Ulva fasciata, Ulva lactuca, Ulva pertusa, and Ulva linza on the harmful microalgae Prorocentrum micans, Prorocentrum donghaiense, Heterosigma akashiwo, Alexandrium tamarense, and Chaetoceros gracile [17], [21], [22]. Additionally, microalga-microalga allelopathic interactions were also found between P. micans and Skeletonema costatum or Karenia mikimotoi [23]. However, much less is known about macroalga-macroalga allelopathic interactions. Our previous study showed that the green tide-forming macroalga U. linza could release allelochemicals that could inhibit the growth of the red macroalga Gracilaria lemaneiformis (unpublished data).
Like U. linza, U. prolifera is a dominant species responsible for forming green tides. However, Liu et al. (2010) [24] and Zhang et al. (2011) [25] reported that the dominant Ulva strain of the 2008 green algal bloom in the Yellow Sea was not detected in the coastal waters of Qingdao in the following winter. Compared to U. linza, much less is known about the allelopathic ecology of U. prolifera, and to date no studies have assessed what happens to the structure and biodiversity of a community when this opportunistic species invades it. In fact, few studies have addressed allelopathic interactions in the marine environment and the function of secondary metabolites as defenses against pathogens or other competing plants. Thus, in this study we examined the allelopathic interactions between the opportunistic species U. prolifera and the native macroalga Gracilaria lichvoides, both of which were collected from the coastline of East China Sea. We performed a series of laboratory experiments under controlled conditions in which we co-cultured the two species and also cultured them separately (i.e., mono-cultures). Physiological parameters such as algal growth, algal photosynthesis, nutrient assimilation, and changes of pH in the culture medium were examined to assess potential allelopathic effects between the tested macroalgal species.
Materials and Methods
Sampling and Culture Conditions
Floating specimens of U. prolifera and G. lichvoides were collected from the coastline of East China sea, in May 2011. In the laboratory, the intact samples were washed several times with sterile seawater, sterilized with 1% sodium hypochlorite for 2 min, and then rinsed with autoclaved seawater. Both U. prolifera and G. lichvoides were pre-cultured aseptically in f/2 medium in an incubator without N or P supplement for 48 h before running experiments. The temperature was maintained at 15°C. Illumination was provided by cool-white flouorescent lamps at 100 µmol photons m−2 s−1 and on a 12∶12 h light: dark cycle. All cultures were shaken manually twice at the same time every day. The pH and salinity of the seawater used for experiment were 8.0 and 30 ppt, respectively.
Effects of Fresh Thalli of U. prolifera/G. lichvoides on G. lichvoides/U. prolifera
To determine the allelopathic interactions between the fresh thalli of G. lichvoides and U. prolifera, in the batch culture experiment, G. lichvoides (1.25 g wet weight L−1) was co-cultured with three different density of U. prolifera (1.25, 2.50, and 3.75 g wet weight L−1). The experiments were conducted in 500 ml flasks containing 400 ml of culture medium with 882 µmol L−1 NaNO3 and 32 µmol L−1 KH2PO4 at 15°C and 100 µmol photons m−2 s−1. During the period of experiments, nutrients were not added into any flask to supply the decreasing of the nutrients. In addition, a serial of semi-continuous experiments were also conducted by regularly adding nutrients to 882 µmol L−1 NaNO3 and 32 µmol L−1 KH2PO4 every 24 h, while the culture conditions were the same as described above. G. lichvoides and U. prolifera were individually cultured (monocultured) as controls. All experiments in this study were conducted at in triplicate, and aseptic techniques were used in all experimental steps. Flasks were also monitored for pH levels during the experiments. Measurements were taken using a pH probe equipped with an electrode (Thermo Scientific Orion Star SeriesTM Benchtop pH meter; ±0.01 unit; calibrated prior each use with NIST traceable standards). These experiments lasted for 96 h. The growth of macroalgae U. prolifera and G. lichvoides was estimated by monitoring changes in algal wet weight at 0 h, 48 h, and 96 h.
Effects of Culture Filtrate of U. prolifera/G. lichvoides on G. lichvoides/U. prolifera
Macroalgal culture medium was prepared by separately culturing G. lichvoides and U. prolifera in sterilized seawater at a concentration of 10 g wet weight L−1 for 48 h without nutrient enrichment. Thereafter, the macroalgal thalli were removed and the macroalga-free culture medium was filtered through 0.45 µm acetate cellulose filters and diluted 2 and 4 times with sterilized seawater. The three gradient concentrations of culture filtrate of G. lichvoides or U. prolifera were used for experiments to study the effects of culture filtrates on fresh algal thalli of U. prolifera or G. lichvoides at a concentration of 1.25 g wet weight L−1. The media containing culture filtrates were resupplied with nutrients every 24 h, and the pH was adjusted to 8.0 by 2 mol L−1 HCl every day. The culture system was kept at 15°C with a light intensity of 100 µmol photons m−2 s−1 and a 12∶12 h light: dark cycle. The experiments lasted for 96 h, and the growth of macroalgae U. prolifera and G. lichvoides was estimated by monitoring changes in algal wet weight at 0 h, 48 h, and 96 h.
Nutrient Analysis
During the experiments, water samples (5 ml) in batch culture were collected daily, filtered immediately through acetate cellulose filters, and frozen in polyethylene flasks for storage until analysis. Concentrations of nitrate (NO3–N) and phosphas (PO4–P) were analyzed photometrically using an AutoAnalyzer (BRAN and LUEBBE AA3, Germany). Nutrient uptake rates were calculated as: NUR = (C0−Ct) V/DW/t, where NUR is the nutrient uptake rate (µmol of nutrient g FW wt−1 h−1); C0 and Ct are the nutrient concentrations (µmol L−1) at the beginning and the end of the experiment, respectively; V is the volume of water (L); FW is the algal fresh weight (g), and t is the time interval (h).
Photosynthesis Measurement
Simultaneously, the effective PSII quantum yield [Y(II)] of fresh algal thalli was measured using the pulse–amplitude modulated method on a Dual-PAM-100 (Walz, Effeltrich, Germ any) connected to a PC running WinControl software and calculated as follows: Y(II) = (F m′−F t)/F m′. The real-time fluorescence yield F t is obtained by averaging the fluorescence readings within 0.2 s and the maximum fluorescence yield (F m′) was detected when the samples were illuminated by actinic light of 100 µmol photons m−2 s−1.
Statistical Analysis
The significance of variance between treatments and the control or among treatments was tested using a one-way ANOVA or a multiple comparison test. All tests were run using the software SPSS 17.0. The significance level was set at 0.05 for all tests unless otherwise stated.
Results
Effects of fresh thalli of U. prolifera/G. lichvoides on G. lichvoides/U. prolifera
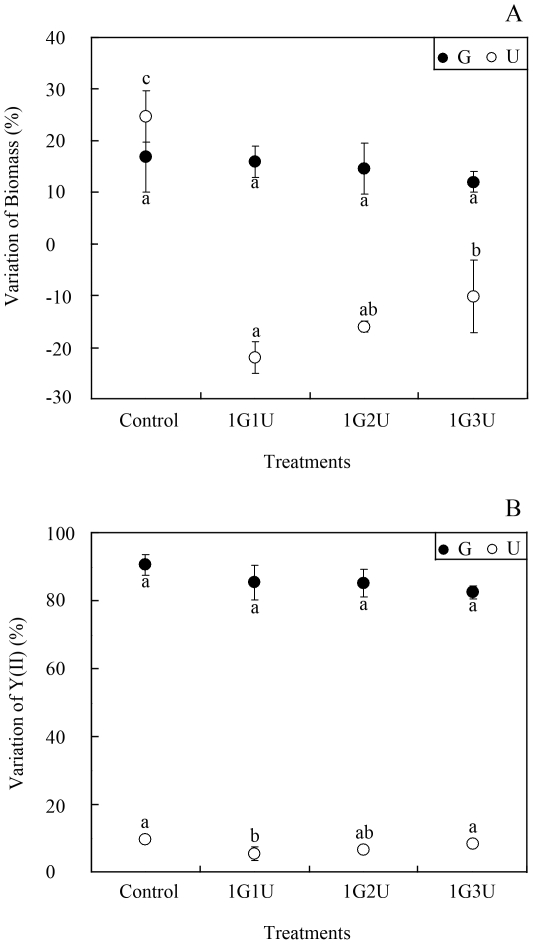
In the batch co-culture experiments without nutrient supplement, different quantities of U. prolifera had no apparent effects on the growth (df = 3, F = 0.467, p = 0.718) or photosynthesis (df = 3, F = 2.191, p = 0.232) of G. lichvoides compared to the control (Fig. 1A and B). After 96 h of incubation, the biomass and Y(II) of the mono-cultured G. lichvoides increased by 17.0±7.1% and 90.6±2.5%, respectively. In the three co-culture systems containing 1.25, 2.50, and 3.75 g wet weight L−1 of U. prolifera, G. lichvoides biomass increased by 16.0±2.8%, 14.7±4.6%, and 12.0±2.1%, respectively, and Y(II) increased by 85.4±4.5%, 84.4±4.2%, and 82.5±2.0%, respectively. In contrast, the presence of G. lichvoides at a concentration of 1.25 g wet weight L−1 had a dramatic negative effect on growth of U. prolifera at concentrations of 1.25, 2.50, and 3.75 g wet weight L−1 (growth declined by 22.0±2.8%, 16.0±1.0%, and 10.2±7.0%, respectively) (df = 3, F = 4.968, p = 0.046). Moreover, although the Y(II) of U. prolifera increased by 5.4±1.6%, 6.7±0.6%, and 8.4±0.7%, the effects of G. lichvoides on photosynthesis of U. prolifera were not significant compared to the control treatment (df = 3, F = 4.363, p = 0.073).
Figure 1. Interactions between U. prolifera and G. lichvoides in fresh thalli batch co-culture experiment.
A) Growth-inhibition effects, B) Photosynthetic effects. Values (means ± SD) in bars that have the same letter are not significantly different (p>0.05).
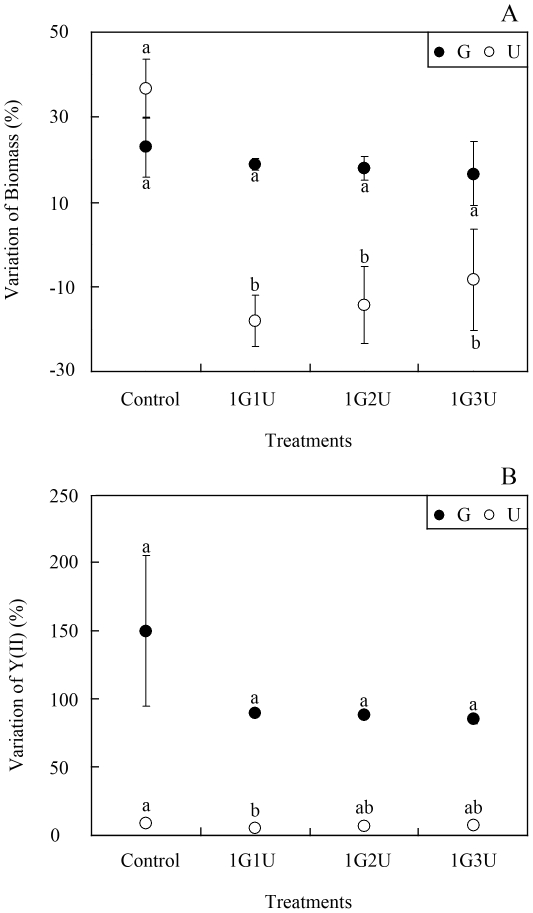
G. lichvoides also grew well both in monoculture and in co-cultures with U. prolifera (Fig. 2A and B) in semi-continuous cultivation conditions. By the end of the experiment, the biomass of mono-cultured G. lichvoides increased by 23.0±7.1% and that in the three co-culture treatments increased by 19.0±1.4%, 18.0±2.8%, and 16.7±7.6%, respectively. Similarly, Y(II) values in the co-culture treatments increased by 90.1±1.6%, 88.0±2.4%, and 85.4±3.4%, respectively, although all of these values were lower than that of the control, which increased by 150.2±55.2%. However, one-way ANOVA indicted that these differences were not significant (df = 3, F = 2.550, p = 0.194). In contrast, G. lichvoides had density-dependent effects on growth and photosynthesis of U. prolifera. After incubation for 96 h, the biomass of U. prolifera declined significantly by 18.0±5.7%, 14.3±9.5%, and 8.3±11.8%, respectively, compared to the control, which increased by 36.7±6.7% (df = 3, F = 17.157, p = 0.005). However, Y(II) of U. prolifera did not change significantly (df = 3, F = 1.619, p = 0.281).
Figure 2. Interactions between the fresh thalli of U. prolifera and G. lichvoides in semi-continuous cultivation.
A) Growth-inhibition effects, B) Photosynthetic effects. Values (means ± SD) in bars that have the same letter are not significantly different (p>0.05).
Nutrient changes in fresh thalli co-culture
Fig. 3 shows changes in nutrient concentrations with culture time in the fresh thalli batch culture systems. The NO3–N concentration in the monoculture of U. prolifera decreased more quickly (from 882 to 325.0±53.1 µm L−1) than that in the monoculture of G. lichvoides (882 to 359.4±47.1 µm L−1), except for the first 12 h. The NO3–N concentration in the monoculture of U. prolifera and in the monoculture of G. lichvoides was significantly correlated with the concentration in the co-culture systems; this relationship illustrates that NO3–N in the co-culture assays was absorbed jointly by G. lichvoides and U. prolifera (Fig. 3A). During the period the 96 h, the average N uptake rate of G. lichvoides (4.5±0.4 µmol N g−1 FW h−1) in monoculture experiment was lower than that of U. prolifera (4.8±0.4 µmol N g−1 FW h−1), but the difference was not significant (df = 1, F = 0.704, p = 0.449).
Figure 3. Variations of nutrient concentration with culture time in fresh thalli batch co-culture experiment.
A) Changes in nitrate concentrations, B) Changes in phosphorus concentrations.
The concentration of PO4–P in the monoculture of U. prolifera was significantly correlated with that in the co-culture of G. lichvoides with U. prolifera. Moreover, the PO4–P concentration in the co-culture system declined to much lower levels (from 32 to 2.6±1.8, 0.5±0.3, and 0.7±0.5 µm L−1, respectively) compared with that in the G. lichvoides monoculture (24 µm L−1) after 96 h. These results indicate that the PO4–P was mainly absorbed by U. prolifera in all co-culture assays (Fig. 3B). Moreover, the average P uptake rate of G. lichvoides (0.08±0.01 µmol P g−1 FW h−1) in monoculture experiment was dramatically higher than that of U. prolifera (0.25±0.01 µmol P g−1 FW h−1) (df = 1, F = 340.099, p = 0.000).
pH changes in fresh thalli co-culture
The pH of the culture medium used in both the monoculture and co-culture systems initially was 8.0. Over time, the pH values in all treatments increased slightly (no more than 1.12) in both the semi-continuous assays and in the co-culture assays without nutrient supplementation (Table 1). A concentration-dependent relationship was observed between the initial concentration of fresh thalli and pH values measured after 96 h of incubation.
Table 1. Changes of pH values with culture time in the fresh thalli co-culture.
| Treatment | In no nutrients added assays | In semi-continuous assays | ||
| Time(h) | 48 h | 96 h | 48 h | 96 h |
| 1G | 8.01 | 8.14 | 8.02 | 8.11 |
| 1U | 8.54 | 8.69 | 8.51 | 8.63 |
| 1G1U | 8.56 | 8.74 | 8.53 | 8.69 |
| 1G2U | 8.85 | 9.02 | 8.60 | 8.78 |
| 1G3U | 8.93 | 9.12 | 8.80 | 8.91 |
Effects of culture filtrates of U. prolifera/G. lichvoides on G. lichvoides/U. prolifera
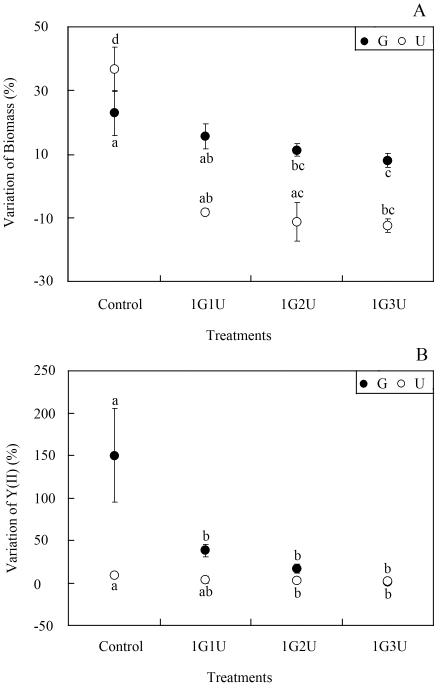
Fig. 4 shows results of the experiments in which U. prolifera or G. lichvoides was cultured with macroalgal culture filtrates of G. lichvoides or U. prolifera, respectively. The G. lichvoides culture filtrate dramatically inhibited growth (df = 3, F = 55.759, p = 0.001) and photosynthesis (df = 3, F = 2.923, p = 0.139) of U. prolifera in comparison to the control (Fig. 4). After 96 h of incubation, the biomass of U. prolifera decreased by 8.1±1.2%, 11.3±5.7%, and 12.4±1.5%, respectively, when treated with 4, 2, and 1 times diluted culture filtrate of G. lichvoides, whereas the biomass in the control increased by 36.7±6.7%. Additionally, although the Y(II) values of U. prolifera increased by 3.8±4.6%, 3.1±2.7%, and 1.9±1.7%, the 2 and 1 times diluted culture filtrates of G. lichvoides had significant effects on photosynthesis of U. prolifera. Moreover, the U. prolifera culture filtrate also caused significant inhibition of growth (df = 3, F = 7.239, p = 0.015) and photosynthesis (df = 3, F = 11.627, p = 0.019) of G. lichvoides compared with those of the control. In the monoculture, the biomass and Y(II) value of G. lichvoides increased by 23.0±7.1% and 150.2±55.2%, respectively. When treated with 4, 2, and 1 times diluted culture filtrate of U. prolifera, the biomass and Y(II) of G. lichvoides increased by only 15.7±3.6%, 11.3±2.1%, and 8.1±2.3% and 38.5±7.4%, 16.9±5.1%, and 1.3±0.1%, respectively.
Figure 4. Effects of macroalgal culture filtrates in semi-continuous cultivation.
A) Growth-inhibition effects, B) Photosynthetic effects. Values (means ± SD) in bars that have the same letter are not significantly different (p>0.05).
Discussion
The direct competitive effects of exotic plants on natives are among the leading causes of plant extinctions worldwide [26]. It's known that there are multiple mechanisms such as resource competition [13], environmental factors [16] and/or negative allelopathy [18], that may account for the negative interactions. Among direct competitive interactions, resource competition is frequently credited as being the principal competitive mechanism that affects plant success. Huo et al. (2011) [13] indicated that the growth of K. mikimotoi was suppressed by Gracilaria verrucosa mainly through competition for nutrients, especially nitrogen. Previous studies have reported that the green macroalgae Ulva spp., which have a high surface area/volume ratio, exhibit high rates of nutrient uptake. But many of these algae are often limited in their ability to concentrate and store nitrogen internally, and are, therefore dependent on a constant high level of nitrogen in the medium [27]. The red macroalge Gracilaria spp., on the other hand, have opposite qualities in that they show a high capability of storing nitrogen (partly as phycoerythrin), and require only pulse fertilization [28]. In the present study, high-level nutrient assimilation clearly occurred in the fresh thalli co-culture experiments with three U. prolifera concentrations (Fig. 3) without nutrient supplementation. And the U. prolifera appeared higher nutrient uptake than G. lichvoides. The NO3–N in the co-culture assays was absorbed jointly by G. lichvoides and U. prolifera, but PO4–P was mainly absorbed by U. prolifera. Correspondingly, U. prolifera biomass dramatically declined by 22.0±2.8%, 16.0±1.0%, and 10.2±6.7%, respectively, after incubation for 96 h (Fig. 1). Thus, it seemed that resource competition likely accounted for the observed growth suppression. However, in the semi-continuous assays (Fig. 2), in which nutrients were added every 24 h, G. lichvoides also had density-dependent effects on the growth of U. prolifera. After incubation for 96 h, the biomass of U. prolifera declined by 18.0±5.7%, 14.3±9.5%, and 8.3±1.7%, whereas that in the control increased by 37%. Therefore, nutrient limitation could be excluded as the cause of the observed negative effects.
Light competition was another mechanism that account for the species interactions. Tait and Schiel (2011) [29] indicated that the light intensity played an important role in productivity of canopy-forming macroalgae and their sub-canopy assemblages. At high cover, Sargassum muticum excludes native species and reduces richness through light competition by shading smaller, understory macroalgae [30]. Inversely, Svirski et al. (1993) [31] found that the growth inhibition of Gracilaria spp., when cultured in the presence of Ulva cf. lactuca, was not due to shading or nutrient depletion, but seemed to be caused by competition for inorganic carbon or some type of allelopathy. In the present study, the fresh algae was incubated in 500 ml flasks containing 400 ml of culture medium, and the space was big enough for the sample to growth. Meanwhile, the experiment was conducted in an illuminated incubator at 100 µmol photons m−2 s−1 and the algae can get the light from all directions. Additionally, all cultures were shaken manually twice every day and the samples could change their positions in the culture medium. Based on mentioned above, it makes light limit unlikely for growth.
Allelopathy, which is one type of direct plant competition, can play an important role in ecosystem structure and plant diversity [18]. Although the importance of allelopathy as a mechanism of competition is gaining prominence in terrestrial ecological research, the importance of allelopathy in aquatic ecosystems has received less attention, especially among macroalgae [32]. A recognized effect of growing macroalgae in culture is that they may increase the pH of the culture medium, making it unsuitable for the growth of microalgae in co-culture [33], [34]. In our experiments, the pH value of the culture medium was measured at the beginning and the end of the experiment, which increased (no more than 1.12) both in batch co-culture assays and in the semi-continuous assays. The pH changes may result in the growth inhibition of G. lichvoides or U. prolifera. However, in the culture filtrate experiments (Fig. 4), in which pH was adjusted to 8.0, the algal growth was also dramatically inhibited. Consequently, the elevated pH values may not the reason cause growth inhibition.
Because exotic plants are the major cause of declining plant diversity and abundance, determining the mechanisms through which exotic plants are able to become invasive could assist in the control and management of these species. It also could provide insight into how plant species interact and how plant communities are organized [26]. Based on the analysis above, neither nutrient and light limitation nor elevated pH was responsible for the observed effects in co-culture systems. In the culture filtrate assays, in which nutrient and pH changes were excluded, significant inhibition of growth and Y(II) was found in both experiments (G. lichvoides filtrate added to U. prolifera culture or U. prolifera filtrate added to G. lichvoides culture) (Fig. 4). These results indicate that allelochemical compounds may have been released by both of the tested algae. Moreover, the culture filtrate of G. lichvoides had a stronger ability to inhibit U. prolifera compared to the effect of the culture filtrate of U. prolifera on G. lichvoides. Collectively, these results provide a new insight about this macroalga-macroalga relationship: Although U. prolifera is the causative species of the world's largest green tide and its blooms have major ecological and economic impacts, the presence of a stable native algal canopy of G. lichvoides may inhibit its expansion.
Allelopathic effects of green tide blooms on the native community
Although green tides are widespread and invade many macroalgal ecosystems, they have been largely neglected in studies of the maintenance of biodiversity. The effects of green tide blooms are varied and have been summarized by Fletcher (1996) [35] and Raffaelli et al. (1998) [10]. However, our results suggest a more complex picture that involves a chemical-mediated system. Our findings illustrate that the native macroalgae G. lichvoides had strong allelopathic effects on the opportunistic species U. prolifera when the co-culture concentration of G. lichvoides was one-third times higher than that of U. prolifera. This may explain why the dominant U. prolifera strain of the bloom was absent in all the water-derived cultures during the sampling period [24], [25].
The effects of the introduction and spread of exotic species on community richness can be positive [36], negative [37], or neutral [38]. In addition, the impacts of exotic species are often species specific and context dependent. For example, Valentine and Johnson (2003) [39] reported that disturbance that reduced cover of the native algal canopy was critical in the establishment of Undaria pinnatifida, whereas the presence of a stable native algal canopy inhibited invasion. On the west coast of Vancouver Island in Canada, White and Shurin (2011) [30] found non-linear, density-dependent effects of Sargassum muticum on native macroalgal richness. In the present study, the highest concentration of U. prolifera in the co-culture system was 3.75 g wet weight L−1, and U. prolifera at this concentration had no significant effects on G. lichvoides (1.25 g wet weight L−1). However, the effect of higher co-culture concentrations of U. prolifera on G. lichvoides should be investigated in the future, as higher concentrations could significantly impact the native species.
Previous studies have reported that the green tide-forming species in the Yellow Sea were Ulva (formerly Enteromorpha) linza–procera–prolifera complex [40], [41]. U. prolifera, the causative species of the world's largest green tide, is distributed widely in the intertidal zones of shores and estuaries around the world because of its tolerance of a wide range of salinity and water temperature, its high growth rate, and its extraordinary capabilities for propagation [8], [42]. In a previous study, we found that the presence of U. linza could restrict growth and photosynthesis of G lemaneiformis, even when the co-culture density of U. linza was equal to that of G. lemaneiformis (unpublished data). The present study represents a significant advance in exploring ecological questions about the effects of green tide blooms on the macroalgal community. If hybridization between U. linza and U. prolifera occurred, a more destructive species could have more serious ecological effects on the marine community.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Shandong Science and Technology plan project (2011GHY11528), the Hi-Tech Research and Development Program (863) of China (2012AA052103), the Specialized Fund for the Basic Research Operating expenses Program (20603022012004, 2010-ts-03), National Natural Science Foundation of China (41176153), Natural Science Foundation of Shandong Province (2009ZRA02075), Qingdao Municipal Science and Technology plan project (11-3-1-5-hy), National Marine Public Welfare Research Project (200805069), and the National Science & Technology Pillar Program, (2008BAD95B11, 2010BAC68B03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williams SL, Smith JE. A global review of the distribution, taxonomy and ecological impacts of introduced seaweeds. Annual Review of Ecology Evolution Systematics. 2007;38:327–359. [Google Scholar]
- 2.Lapointe BE, Bedford BJ. Ecology and nutrition of invasive Caulerpa brachypus f. parvifolia blooms on coral reefs off southeast Florida, U.S.A. Harmful Algae. 2010;9:1–12. [Google Scholar]
- 3.Walters LJ, Brown KR, Stam WT, Olsen JL. Ecommerce and Caulerpa: unregulated dispersal of invasive species. Frontiers in Ecology and the Environment. 2006;4:75–79. [Google Scholar]
- 4.Wonham MJ, Pachepsky E. A null model of temporal trends in biological invasion records. Ecology Letters. 2006;9:663–672. doi: 10.1111/j.1461-0248.2006.00913.x. [DOI] [PubMed] [Google Scholar]
- 5.Frankenstein G. Blooms of Ulvoids in Puget Sound. 2000. Puget Sound Water Quality Action Team, Olympia, Washington.
- 6.Liu DY, Keesing JK, Xing QG, Shi P. Worlds largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin. 2009;58:888–895. doi: 10.1016/j.marpolbul.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Leliaert F, Malta EJ, Engelen AH, Mineur F, De Clerck O. Qingdao algal bloom culprit identified. Marine Pollution Bulletin. 2008;56:15–16. [Google Scholar]
- 8.Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, et al. ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world's largest example. Ecological Research. 2011;29:541–546. [Google Scholar]
- 9.Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, et al. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography. 1997;42:1105–1118. [Google Scholar]
- 10.Raffaelli DG, Raven JA, Poole LJ. Ecological impact of green macroalgal blooms. Oceanography and Marine Biology. 1998;36:97–125. [Google Scholar]
- 11.Charlier RH, Morand P, Finkl CW, Thys A. Green tides on the Brittany Coasts. Environmental Research, Engineering and Management. 2007;41:52–59. [Google Scholar]
- 12.Worm B, Heike K, Sommer U. Algal propagules banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia. 2001;128:281–293. doi: 10.1007/s004420100648. [DOI] [PubMed] [Google Scholar]
- 13.Huo YZ, Zhang JH, Xu SN, Tian QT, Zhang YJ, et al. Effects of seaweed Gracilaria verrucosa on the growth of microalgae: A case study in the laboratory and in an enclosed sea of Hangzhou Bay, China. Harmful Algae. 2011;10:411–418. [Google Scholar]
- 14.Wang Y, Yu ZM, Song XX, Tang XX, Zhang SD. Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates. Aquatic Botany. 2007;86:139–147. [Google Scholar]
- 15.Valentine JP, Johnson CR. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. Journal of Experimental Marine Biology and Ecology. 2003;295:63–90. [Google Scholar]
- 16.Wang Y, Zhou B, Tang XX. Effects of two species of macroalgae—Ulva pertusa and Gracilaria lemaneiformis—on growth of Heterosigma akashiwo (Raphidophyceae). Journal of Applied Phycology. 2009;21:375–385. [Google Scholar]
- 17.Tang YZ, Gobler CJ. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via Allelopathy. Harmful Algae. 2011;10:480–488. [Google Scholar]
- 18.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Natural Product Reports. 2010;27:165–237. doi: 10.1039/b906091j. [DOI] [PubMed] [Google Scholar]
- 19.Amsler CD, Fairhead VA. Defensive and sensory chemical ecology of brown algae. Advances in Botanical Research Incorporating Advances in Plant Pathology. 2005;43:1–91. [Google Scholar]
- 20.Pereira RC, da Gama BAP. Macroalgal chemical defenses and their role in structuring tropical marine communities. In: Amsler CD, editor. Algal Chemical Ecology. New York: Springer; 2008. pp. 25–55. [Google Scholar]
- 21.Nan CR, Zhang HZ, Lin SZ, Zhao GQ, Liu XY. Allelopathic effects of Ulva lactuca on selected species of harmful bloom-forming microalgae in laboratory cultures. Aquatic Botany. 2008;89:9–15. [Google Scholar]
- 22.Wang RJ, Xiao H, Wang Y, Zhou WL, Tang XX. Effects of three macroalgae, Ulva linza (Chlorophyta), Corallina pilulifera (Rhodophyta) and Sargassum thunbergii (Phaeophyta) on the growth of the red tide microalga Prorocentrum donghaiense under laboratory conditions. Journal of Sea Research. 2007;58:189–197. [Google Scholar]
- 23.Ji XQ, Han XT, Zheng L, Yang BY, Yu ZM, et al. Allelopathic interactions between Prorocentrum micans and Skeletonema costatum or Karenia mikimotoi in laboratory cultures. Chinese Journal of Oceanology and Limnology. 2011;29:840–848. [Google Scholar]
- 24.Liu F, Pang SJ, Chopin T, Xu N, Shan TF, et al. The dominant Ulva strain of the 2008 green algal bloom in the Yellow Sea was not detected in the coastal waters of Qingdao in the following winter. Journal of Applied Phycology. 2010;22:531–540. [Google Scholar]
- 25.Zhang XW, Xu D, Mao YZ, Li YX, Xue SY, et al. Vegetative fragments of Ulva prolifera in settlement confirmed as an important seed source for succession of a large-scale green tide bloom. Limnology and Oceanography. 2011;56:233–242. [Google Scholar]
- 26.Jarchow ME, Cook BJ. Allelopathy as a mechanism for the invasion of Typha angustifolia. Plant Ecology. 2009;204:113–124. [Google Scholar]
- 27.Friedlander M, Krom MD, Ben-Amotz A. The effect of light and ammonium on growth, epiphytes and chemical constituents of Gracilaria conferta in outdoor cultures. Botanica Marina. 1991;34:161–166. [Google Scholar]
- 28.Friedlander M, Gonen Y, Kashman Y, Beer S. Gracilaria conferta and its epiphytes: 3. Allelopathic inhibition of the red seaweed by Ulva cf. lactuca. Journal of Applied Phycology. 1996;8:21–25. [Google Scholar]
- 29.Tait LW, Schiel DR. Dynamics of productivity in naturally structured macroalgal assemblages: importance of canopy structure on light-use efficiency. Marine Ecology-Progress Series. 2011;421:97–107. [Google Scholar]
- 30.White LF, Shurin JB. Density dependent effects of an exotic marine macroalga on native community diversity. Journal of Experimental Marine Biology and Ecology. 2011;405:111–119. [Google Scholar]
- 31.Svirski E, Beer S, Friedlander M. Gracilaria conferta and its epiphytes. 2. Interrelationship between the red seaweed and Ulva cf. lactuca. Hydrobiologia. 1993;260/261:391–396. [Google Scholar]
- 32.Macías FA, Galindo JLG, García-Díaz MD, Galindo JCG. Allelopathic agents from aquatic ecosystems: potential biopesticides models. Phytochemistry Reviews. 2008;7:155–178. [Google Scholar]
- 33.Lundholm N, Hansen PJ, Kotaki Y. Lack of allelopathic effects of the domoic acid-producing marine diatom Pseudo-nitzschia multiseries. Marine Ecology-Progress Series. 2005;288:21–33. [Google Scholar]
- 34.Schmidt LE, Hansen PJ. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: effect of cell concentration, growth phase and pH. Ecology-Progress Series. 2001;216:67–81. [Google Scholar]
- 35.Fletcher RL. The British Isles. In: Schramm W, Nienhuis PH, editors. Marine benthic vegetation: recent changes, the effects of eutrophication. Berlin: Springer; 1996. pp. 7–223. [Google Scholar]
- 36.Mineur K, Johnson MP, Maggs CA. Non-indigenous marine macroalgae in native communities: a case study in the British Isles. Journal of the Marine Biological Association of the United Kingdom. 2008;88:693–698. [Google Scholar]
- 37.Wasson K, Fenn K, Pearse JS. Habitat differences inmarine invasions of Central California. Biological Invasion. 2005;7:935–948. [Google Scholar]
- 38.Klein J, Ruitton S, Verlaque M, Boudouresque CF. Species introductions diversity and disturbances inmarine macrophyte assemblages of the northwestern Mediterranean Sea. Ecology-Progress Series. 2005;290:79–88. [Google Scholar]
- 39.Valentine JP, Johnson CR. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. Journal of Experimental Marine Biology and Ecology. 2003;295:63–90. [Google Scholar]
- 40.Leliaert F, Zhang XW, Ye NH, Malta EJ, Engelen AE, et al. Identity of the Qingdao algal bloom. Phycological Research. 2009;57:147–151. [Google Scholar]
- 41.Pang SJ, Liu F, Shan TF, Xu N, Zhang ZH, et al. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Marine Environmental Research. 2009;69:207–215. doi: 10.1016/j.marenvres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XW, Wang H, Mao YZ, Liang CW, Zhuang ZM, et al. Somatic cells serve as a potential propagule bank of Enteromorpha prolifera forming a green tide in the Yellow Sea, China. Journal of Applied Phycology. 2010;22:173–180. [Google Scholar]