Abstract
Background
The mechanisms by which smoking cessation reduces cardiovascular disease risk are unclear. We evaluated longitudinal changes in carotid intima-media thickness among current smokers enrolled in a prospective, randomized smoking cessation clinical trial.
Methodology/Principal Findings
Subjects were enrolled in a randomized, double-blind, placebo-controlled trial of 5 smoking cessation pharmacotherapies and underwent carotid ultrasonography with carotid intima-media thickness measurement. Subjects were classified as continuously abstinent (biochemically confirmed abstinence at 6 months, 1 year, and 3 years post-quit attempt), intermittently abstinent (reported smoking at one of the three time points), or smoked continuously (reported smoking at all three time points). The primary endpoint was the absolute change (mm) in carotid intima-media thickness (ΔCIMTmax) before randomization and 3 years after the target quit date. Pearson correlations were calculated and multivariable regression models (controlling for baseline CIMTmax and research site) were analyzed. Among 795 subjects (45.2±10.6 years old, 58.5% female), 189 (23.8%) were continuously abstinent, 373 (46.9%) smoked continuously, and 233 (29.3%) were abstinent intermittently. There was a greater increase in carotid intima-media thickness among subjects who were continuously abstinent than among those who smoked continuously (p = 0.020), but not intermittently (p = 0.310). Antihypertensive medication use (p = 0.001) and research site (p<0.001) independently predicted ΔCIMTmax – not smoking status. The greatest increase in carotid intima-media thickness among continuous abstainers was related to increases in body-mass index (p = 0.043).
Conclusions/Significance
Smoking status did not independently predict ΔCIMTmax; increasing body-mass index and antihypertensive medication use were the most important independent predictors. The rapid reduction in cardiovascular disease events observed with smoking cessation is unlikely to be mediated by changes in subclinical atherosclerosis burden.
Trial Registration
ClinicalTrials.gov NCT00332644
Introduction
It has been hypothesized that the strong relationship between cigarette smoking and the development of cardiovascular disease (CVD) is mediated by inflammatory pathways, lipid oxidation, and vascular dysfunction [1], [2]. Smoking cessation reduces cardiovascular disease risk; however, the mechanisms and time-course of the effects of cessation on CVD risk are unknown [3]–[5]. In some studies cardiovascular disease risk begins to decrease within 3 years of smoking cessation [3], [4]. Carotid intima-media thickness (CIMT) is a measure of subclinical arterial injury that predicts future cardiovascular disease events. In cross-sectional analyses, we and others previously demonstrated that smoking intensity (pack-years) was associated with increased CIMT [6], [7]. The purpose of this paper was to describe longitudinal 3-year changes in CIMT observed in a large, modern cohort of current smokers enrolled in a prospective, randomized clinical trial of smoking cessation pharmacotherapies and to determine if smoking cessation is associated with a reduction in progression in CIMT.
Methods
The protocol for this trial and support CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1. Also included as supporting information is a manuscript by Piper, et al.; see Piper S1.
Study Procedures
Ethics Statement
This study was approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. It was conducted according to the principles expressed in the Declaration of Helsinki. All participants provided written informed consent.
Subjects were enrolled in a randomized, double-blinded, placebo-controlled comparative effectiveness trial of smoking cessation pharmacotherapies that also intended to study the natural history of smoking and cessation on subclinical atherosclerosis [6], [8]. Each participant’s consent for the randomized clinical trial included permission to evaluate the physiologic effects of active cigarette use and smoking cessation on atherosclerosis by ultrasonographic evaluation of CIMT at baseline and after 3 years. At the baseline and year 3 visits, anthropometric data, fasting laboratory tests, validated questionnaires, and interviews were completed. Participants were randomized to one of six treatment conditions: nicotine lozenge, nicotine patch, sustained-release bupropion, nicotine patch plus nicotine lozenge, sustained-release bupropion plus nicotine lozenge, or placebo. Inclusion and exclusion criteria were based on participation in the clinical trial. Inclusion criteria were age ≥18 years, current smoking of ≥10 cigarettes?day for the previous 6 months, an expired carbon monoxide level of >9 ppm, and stated motivation to try to quit smoking. Major exclusion criteria were uncontrolled hypertension (blood pressure >160?100 mmHg) and myocardial infarction in the previous 4 weeks. Additional study details, including the flowchart of participants, have been reported previously [6], [8].
Carotid Ultrasonography
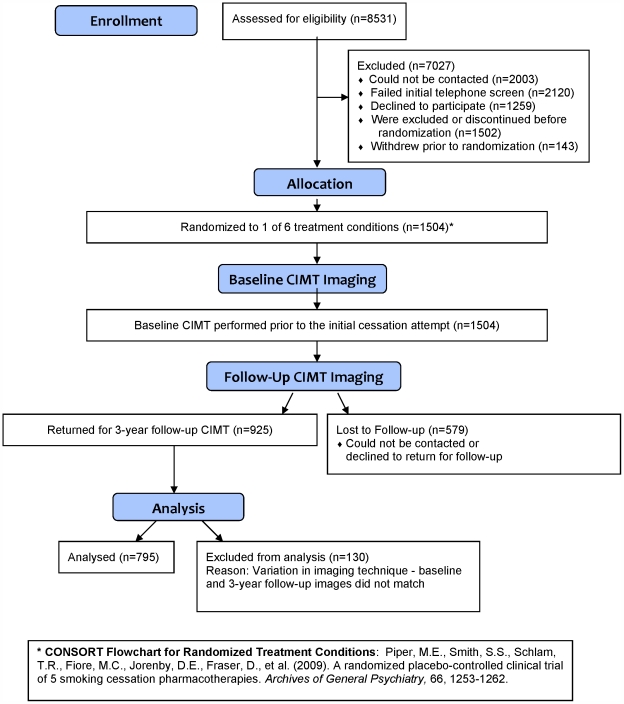
Subjects underwent carotid ultrasonography with CIMT measurement prior to randomization and 3 years after the target quit date at two research sites in Milwaukee and Madison, Wisconsin [6], [8]. Maximum right- and/or left-sided far wall common carotid artery CIMT measurements were averaged. The far wall CIMT of the distal 1 cm of each common carotid artery was measured in triplicate at the electrocardiographic R-wave using a high-resolution linear array transducer (L10–5) and cardiovascular ultrasound system (CV70; Siemens Medical Solutions, Mountain View, WA) [6]. The CIMT imaging protocol has been described previously [9]. Images were transferred via the Internet to a secure Web server at the University of Wisconsin Atherosclerosis Imaging Program, the core ultrasound laboratory. All scanners were trained and certified by the core lab. A single reader blindly measured all baseline and follow-up images using a semi-automated border detection tool [8]. At the time of CIMT image analysis, approximately 130 participants were excluded because the year 3 CIMT scan images did not match the baseline images closely enough for comparative analysis (Figure 1). This was a predefined exclusion criterion. The primary endpoint was the absolute change (mm) in CIMT (ΔCIMTmax) between baseline and year 3. The coefficient of variation for repeatability of CIMTmax measurements was 3.46%. Subjects were classified as continuously abstinent (biochemically confirmed abstinence at 6 months, 1 year, and 3 years post-quit attempt), intermittently abstinent (reported smoking at one of the three time points), or smoked continuously (reported smoking at all three time points).
Figure 1. CONSORT: Screening, Randomization, Baseline Imaging and Follow-up.
Statistical Analysis
Analyses were performed with SPSS software (SPSS, Inc., Chicago, IL). Our sample size estimate was based on the effect size of abstinence from smoking (compared to continued smoking) reported in statistical models from the Atherosclerosis Risk in Communities Study that adjusted for CVD risk factors and environmental tobacco smoke [7]. Our target sample size was 750 participants (300 abstainers and 450 smokers), based on a conservative 5–10% attrition rate from baseline to Year 3. We hypothesized that the ΔCIMTmax of continuing smokers would progress at a rate of approximately 0.043 mm/3 years and former smokers would progress at a rate of approximately 0.035 mm/3 years for an absolute difference of 0.007–0.008 mm/3 years, a relative decrease (treatment effect) of 17%. We used a two-tailed α = 0.05 to determine that we had 81% power to detect a 20% difference in progression between abstinent and smoking participants.
CVD risk factors and CIMT were described by means ± standard deviations, unless noted otherwise. Pearson correlations were estimated between ΔCIMTmax and current age, separately for the baseline values and the 3-year change values for the following continuous variables: body mass index (BMI), waist circumference, carbon monoxide levels, current pack-years smoking, heart rate, systolic blood pressure, diastolic blood pressure, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL), high-density lipoprotein (HDL) cholesterol, fasting glucose, hemoglobin A1C, high sensitivity C-reactive protein, average number of alcohol-containing beverages per month, and mean CIMT. Pearson correlations also were estimated between ΔCIMTmax and baseline Fägerstrom Test for Nicotine Dependence score (total). A one-way ANOVA was performed between ΔCIMTmax and smoking status groups and smoking cessation treatment arms (placebo, bupropion, lozenge, patch, bupropion + lozenge, patch + lozenge). T-tests were performed to compare the change in ΔCIMTmax between genders, races (white vs. non-white), research sites (Madison vs. Milwaukee, WI), and subjects who did and did not use lipid-lowering medications (yes/no) or anti-hypertensive medications (yes/no). The best-fitting multivariable linear regression models were developed using a backward model-building approach (Hosmer & Lemeshow) for ΔCIMTmax. All variables with p<0.20 in individual analyses were added to the model. Predictors were then systematically removed until only statistically significant (p<0.05) predictors remained, controlling for baseline CIMTmax and research site.
Results
The 795 subjects (45.2±10.6 years old, 58.5% female) who had CIMT scans at baseline and year 3 are described in Table 1. There were 189 (23.8%) subjects who were continuously abstinent, 373 (46.9%) smoked continuously, and 233 (29.3%) were abstinent intermittently. Abstinence rates did not differ by gender (p = 0.410).
Table 1. Subject Characteristics at Baseline and 3 Years after the Target Quit Date*.
| All subjects | Continuously Smoking | Intermittently Smoking | Continuously Abstinent | P values for one-way ANOVA | ||||||||||||
| Baseline | Baseline Mean (SD) | 3 Year Mean (SD) | Delta = Year 3-Baseline (SD) | Baseline Mean (SD) | 3 Year Mean (SD) | Delta (SD) | Baseline Mean (SD) | 3 Year Mean (SD) | Delta (SD) | Baseline | Delta (Year 3-Baseline) | |||||
| N | 795 | 373 | 233 | 189 | ||||||||||||
| Age | 45.3 (10.6) | 45.1 (9.8) | 44.5 (11.3) | 46.6 (11.3) | 0.101 | |||||||||||
| Body mass index (kg/m2) | 28.7 (6.1) | 29.0 (6.4) | 29.2 (6.6) | 0.3a,c (3.2) | 28.4 (5.8) | 29.5 (6.5) | 1.1 b,c (3.0) | 28.6 (5.8) | 30.7 (6.9) | 2.2 a,b (4.0) | 0.522 | <0.001 | ||||
| Waist circumference (cm) | 95.3 (15.67) | 95.3 (15.7) | 100.0 (34.2) | 4.3 (33.1) | 94.8 (16.0) | 98.2 (16.5) | 3.2 (12.2) | 100.0 (15.0) | 101.2 (16.1) | 5.0 (8.7) | 0.753 | 0.725 | ||||
| Carbon monoxide (ppm) | 25.6 (12.1) | 26.9 (12.1) | 19.8 (10.9) | -7.0 a,c (12.2) | 24.72 (12.9) | 8.8 (9.9) | -16.1 b,c (14.4) | 24.3 (11.0) | 1.6 (1.3) | -22.6a,b (11.1) | 0.026 | <0.001 | ||||
| Current Pack-years | 29.3 (20.0) | 30.8 (19.4) | 32.5 (19.1) | 1.9a,c (1.1) | 27.9 (21.6) | 28.8 (21.9) | 0.7 b,c (1.1) | 28.1 (19.2) | 28.1 (19.2) | 0.0a,b (0.0) | 0.139 | <0.001 | ||||
| Heart rate (beats/minute) | 73.5 (9.4) | 73.8 (9.6) | 74.3 (33.1) | 0.5 (34.0) | 73.4 (9.2) | 69.4 (9.1) | -3.6 (12.0) | 73.1 (9.5) | 71.5 (24.3) | -1.8 (25.3) | 0.721 | 0.180 | ||||
| Systolic blood pressure (mmHg) | 119.2 (14.6) | 119.2 (15.0) | 117.5 (17.5) | -1.8 (15.7) | 117.8 (13.4) | 114.3 (14.9) | -3.7 (15.3) | 120.9 (15.2) | 116.6 (15.6) | -4.2 (15.2) | 0.093 | 0.150 | ||||
| Diastolic blood pressure (mmHg) | 74.4 (10.1) | 74.1 (10.6) | 75.3 (33.0) | 1.2 (33.6) | 74.0 (9.7) | 75.1 (41.7) | 1.3 (42.6) | 75.7 (9.3) | 76.9 (43.8) | 1.4 (44.6) | 0.154 | 0.998 | ||||
| Total cholesterol (mg/dL) | 185.2 (35.2) | 186.1 (36.5) | 192.0 (38.5) | 5.6 (29.0) | 183.5 (35.2) | 189.2 (37.5) | 7.7 (29.4) | 185.7 (32.5) | 189.4 (37.3) | 4.7 (31.4) | 0.662 | 0.571 | ||||
| Low-density lipoprotein cholesterol (mg/dL) | 118.7 (90.0) | 118.9 (30.9) | 125.3 (33.5) | 6.0 c (26.1) | 116.8 (29.7) | 121.6 (31.9) | 6.3 (25.1) | 120.7 (28.4) | 120.2 (30.7) | 0.2 b (25.9) | 0.424 | 0.031 | ||||
| High-density lipoprotein cholesterol (mg/dL) | 42.7 (13.6) | 42.4 (13.6) | 44.6 (15.4) | 2.0 c (9.6) | 43.5 (13.8) | 46.4 (14.5) | 2.9 c (9.7) | 42.3 (13.4) | 48.3 (15.4) | 5.5 b (9.9) | 0.598 | 0.001 | ||||
| Triglycerides (mg/dL) | 147.9 (106.4) | 155.8 (123.7) | 145.0 (107.2) | -8.5 (99.5) | 143.1 (92.7) | 135.0 (86.2) | -5.2 (77.7) | 138.6 (81.9) | 135.5 (75.5) | -0.3 (76.4) | 0.148 | 0.608 | ||||
| Fasting glucose (mg/dL) | 94.2 (15.2) | 94.7 (17.3) | 95.1 (19.4) | 0.5 (17.5) | 94.0 (14.2) | 99.8 (62.2) | 6.6 (62.1) | 93.6 (11.5) | 100.0 (22.8) | 6.1 (18.1) | 0.702 | 0.116 | ||||
| Hemoglobin A1C (%) | 5.5 (0.5) | 5.5 (0.4) | 5.7 (0.6) | 0.1 (0.5) | 5.5 (0.5) | 5.6 (0.4) | 0.1 (0.3) | 5.5 (0.5) | 5.8 (0.6) | 0.2 (0.4) | 0.445 | 0.196 | ||||
| High-sensitivity C-reactive protein (mg/L) | 1.6 (2.7) | 1.7 (2.9) | 4.3 (9.5) | 2.7 (9.6) | 1.4 (2.5) | 3.7 (4.7) | 2.4 (4.4) | 1.6 (2.6) | 4.3 (5.7) | 2.7 (5.8) | 0.527 | 0.894 | ||||
| Alcohol use (drinks/month) | 18.3 (35.4) | 18.4 (44.1) | 18.2 (34.1) | 2.3 (29.5) | 15.9 (22.3) | 15.5 (26.2) | -1.2 (22.4) | 21.3 (29.5) | 20.9 (29.9) | -1.2 (22.8) | 0.329 | 0.226 | ||||
| Maximum CIMT | 0.88 (0.14) | 0.88 (0.13) | 0.88 (0.16) | -0.002 c (0.084) | 0.86 (0.14) | 0.87 (0.16) | 0.007 (0.082) | 0.89 (0.17) | 0.91 (0.20) | 0.019b (0.091) | 0.100 | 0.021 | ||||
all values are means (standard deviation); ANOVA = analysis of variance, CIMT = carotid intima-media thickness
= significantly different from intermittent smokers (p<0.05), based on a post-hoc Tukey test
= significantly different from continuous smokers (p<0.05), based on a post-hoc Tukey test
= significantly different from continuously abstinent (p<0.05), based on a post-hoc Tukey test
Age was significantly correlated with baseline cigarettes smoked/day (r = 0.17, p<0.001) but was not correlated with ΔCIMTmax (r = 0.04, p = 0.321). ΔCIMTmax was correlated weakly, but significantly, with 3-year changes in LDL cholesterol (r = -0.10, p = 0.007), total cholesterol (r = -0.09, p = 0.020), hemoglobin A1C (r = 0.08, p = 0.030), and use of antihypertensive medications at either visit (r = 0.11, p = 0.002). ΔCIMTmax did not differ between smoking cessation treatment arms (p = 0.993), by sex (p = 0.146), race (p = 0.338), or age (p = 0.321). There was a greater increase in CIMT among subjects at the Milwaukee site (p<0.001) and among subjects who were continuously abstinent compared to those who smoked continuously (p = 0.020) but not intermittently (p = 0.310).
Some CIMT predictors were significantly different between the Madison and Milwaukee research sites. Important demographic differences between sites were gender (p = 0.048), age (p = 0.003), race (p<0.001), marital status (p = 0.029), education (p<0.001), and Fägerstrom Test for Nicotine Dependence score (p = 0.021). Abstinence rate (p<0.001), antihypertensive medication use (p = 0.036), and 3-year changes in systolic blood pressure (p = 0.048), HgbA1C (p<0.001), and LDL cholesterol (p = 0.001) differed between sites.
ANOVA tests with post-hoc analysis were performed to compare 3-year changes in CIMT predictors to the three smoking classifications. Continuously abstinent participants smoked fewer cigarettes/day at baseline (p = 0.023) and by year 3 had a greater increase in HDL cholesterol (p = 0.001), less increase in LDL cholesterol (p = 0.031), and greater increase in glucose (p = 0.036) than both those who smoked continuously and those who were intermittently abstinent. Those who were intermittently abstinent had greater increases in body-mass index by year 3 than those who smoked continuously (p = 0.030), but not those who were continuously abstinent (p = 0.292).
Using all biological, demographic, site, and cessation parameters as candidate variables, and controlling for baseline CIMTmax and site, the only independent associations of ΔCIMTmax in the best-fitting linear regression model were anti-hypertensive medication use (standardized β = 0.15, p = 0.001) and site (β = 0.31, p<0.001). At the Milwaukee site, use of antihypertensive medications (p = 0.009) was the only variable independently associated with ΔCIMTmax; at the Madison site, male sex (p = 0.015) was the only variable independently associated with ΔCIMTmax. In the entire subject population, neither baseline smoking burden, baseline smoking intensity, nor abstinence status were independently associated with ΔCIMTmax. Change in BMI (p = 0.031), antihypertensive medication use (p = 0.019), and continuous abstinence (p = 0.040) were weakly but independently associated with being in the highest quartile of ΔCIMTmax. These effect sizes were modest (R2 adj = 0.032).
Based upon these findings we conducted exploratory mediation analysis using the conservative Baron and Kenny [10] approach and the joint significance test to examine how the direct path from smoking status to ΔCIMTmax was affected by the inclusion of the change in BMI over 3 years. Linear regression analyses were performed among continuously abstinent subjects whose ΔCIMTmax was in the top or bottom quartile. There were significant effects of smoking status on the change in BMI (β = 0.225, p <0.001) and significant effects of the change in BMI on ΔCIMTmax (β = 0.124, p = 0.015). Both of the indirect paths were sufficient to conclude mediation using the joint significance test [11]. Subsequently, we assessed the direct effect of smoking status on ΔCIMTmax when change in BMI was added to the model and the direct effect changed from β = 0.126 (p = 0.012) to beta = 0.102 (p = 0.048) suggestive of mediation.
Discussion
After smoking cessation, CVD risk decreases within 3 years [3], [4]. We hypothesized that CIMT progression would be reduced after 3 years of smoking cessation, but it was not. Male sex, increasing BMI, and antihypertensive medication use were the most important independent predictors of ΔCIMTmax. We also observed a powerful site effect that could not be explained by any patient or technical factors evaluated. Smoking status did not independently predict ΔCIMTmax; indeed, individuals who were continuously abstinent had a nominally greater increase in CIMT. In this same population we demonstrated previously that endothelial function improved [12] and HDL cholesterol, total HDL, and large HDL particles increased [13], within the first year after smoking cessation, despite weight gain. Therefore, the early decrease in CVD risk observed after smoking cessation may be due to improved endothelial function, CVD risk factors, and perhaps unmeasured factors related to hemostasis and thrombosis.
The longitudinal effects of smoking cessation on subclinical arterial disease are less clear. Such effects may require more than 3 years to emerge or might have been masked by BMI increases among abstainers. In this same cohort we previously described an increase in waist circumference and lack of a significant reduction in high-sensitivity C-reactive protein levels after 1 year of cessation [12], [14], both of which may influence short-term changes in CIMT. A previous study suggested that C-reactive protein levels require approximately 5 years to return to baseline after smoking cessation, even after controlling for other cardiovascular disease risk factors [15]. Both short- and long-term decreases in CVD risk after smoking cessation are well-established [3]–[5], and the beneficial effects of smoking cessation clearly outweigh any adverse metabolic effects. Thus, the decrease in CVD morbidity and mortality after cessation is likely due to multiple mechanistic pathways with differing temporal responses to smoking cessation. This study highlights the limitations of CIMT as a surrogate marker for short-term CVD risk reduction after smoking cessation due to the complex vascular pathophysiology associated with cessation.
Limitations of this study include between-site differences and a relatively small sample size of continuous abstainers. In addition, the lack of longitudinal carotid plaque assessment limits further evaluation of confounding factors associated with subclinical atherosclerosis and smoking cessation. It also is possible that changes in the internal carotid or bulb CIMT may have been different than we observed in the common carotid artery. We did not assess the incidence of CVD events during this short-term follow-up. Our 3-year drop-out rate (38.2%) is consistent with previous smoking cessation trials [12]. A larger prospective clinical trial with longer follow-up and combined CIMT and plaque assessment is needed to better understand the effects of smoking cessation on subclinical arterial disease.
Supporting Information
CONSORT Checklist.
(DOC)
A randomized placebo-controlled clinical trial of five smoking cessation pharmacotherapies.
(PDF)
Trial Protocol.
(DOC)
Acknowledgments
The authors express their appreciation to the subjects, whose participation in this study made these observations possible.
Footnotes
Competing Interests: HJ, MP, TB, and JS have no conflicts to disclose. Over the last three years, MF has served as an investigator in research studies at the University of Wisconsin that were funded wholly or in part by Pfizer, GlaxoSmithKline and Nabi. From 1997 to 2010, MF held a University of Wisconsin named Chair for the Study of Tobacco Dependence, made possible by a gift to University of Wisconsin from GlaxoWellcome. Medications for the study were provided under a research agreement with GlaxoSmithKline. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This research was supported by the National Institutes of Health (P50 DA019706, T32 HL07936, and K05 CA139871). Medications were provided under a research agreement with GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Kramer A, Jansen AC, Aalst-Cohen ES, Tanck MW, Kastelein JJ, et al. Relative risk for cardiovascular atherosclerotic events after smoking cessation: 6–9 years excess risk in individuals with familial hypercholesterolemia. BMC Public Health. 2006;6:262. doi: 10.1186/1471-2458-6-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121:750–758. doi: 10.1161/CIRCULATIONAHA.109.891523. [DOI] [PubMed] [Google Scholar]
- 6.Johnson HM, Piper ME, Jorenby DE, Fiore MC, Baker TB, et al. Risk factors for subclinical carotid atherosclerosis among current smokers. Prev Cardiol. 2010;13:166–171. doi: 10.1111/j.1751-7141.2010.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 11.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010;55:1988–1995. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, et al. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 2010;160:458–463. doi: 10.1016/j.ahj.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med. 2005;2:e160. doi: 10.1371/journal.pmed.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist.
(DOC)
A randomized placebo-controlled clinical trial of five smoking cessation pharmacotherapies.
(PDF)
Trial Protocol.
(DOC)