Abstract
Background
Pneumonectomy remains the ultimate curative treatment modality for destroyed lung caused by tuberculosis despite multiple risks involved in the procedure. We retrospectively evaluated patients who underwent pneumonectomy for treatment of sequelae of pulmonary tuberculosis to determine the risk factors of early and long-term outcomes.
Materials and Methods
Between January 1980 and December 2008, pneumonectomy or pleuropneumonectomy was performed in 73 consecutive patients with destroyed lung caused by tuberculosis. There were 48 patients with empyema (12 with bronchopleural fistula [BPF]), 11 with aspergilloma and 7 with multidrug resistant tuberculosis.
Results
There were 5 operative mortalities (6.8%). One patient had intraoperative uncontrolled arrhythmia, one had a postoperative cardiac arrest, and three had postoperative respiratory failure. A total of 29 patients (39.7%) suffered from postoperative complications. Twelve patients (16.7%) were found to have postpneumonectomy empyema (PPE), 4 patients had wound infections (5.6%), and 7 patients required re-exploration due to postoperative bleeding (9.7%). The prevalence of PPE increased in patients with preoperative empyema (p=0.019). There were five patients with postoperative BPF, four of which occurred in right-side operation. The only risk factor for BPF was the right-side operation (p=0.023). The 5- and 10-year survival rates were 88.9% and 76.2%, respectively. The risk factors for late deaths were old age (≥50 years, p=0.02) and low predicted postoperative forced expiratory volume in one second (FEV1) (<1.2 L, p=0.02).
Conclusion
Although PPE increases in patients with preoperative empyema and postoperative BPF increases in right-side operation, the mortality rates and long-term survival rates were found to be satisfactory. However, the follow-up care for patients with low predicted postoperative FEV1 should continue for prevention and early detection of pulmonary complication related to impaired pulmonary function.
Keywords: Tuberculosis, Pneumonectomy, Bronchopleural fistula, Empyema, Lung function
INTRODUCTION
The role of the thoracic surgeon in managing pulmonary tuberculosis has decreased remarkably since the advent of effective antimicrobial agents and better socioeconomic status. However, pneumonectomy or pleuropneumonectomy is still often required to treat complications of pulmonary tuberculosis such as destroyed lung, empyema, bronchopleural fistula (BPF), or multidrug resistant (MDR) tuberculosis [1].
Solid pleural symphysis and dense fibro-vascular adhesion around the hilum result in intraoperative difficulties in pneumonectomy for inflammatory lung disease. Also, the underlying disease of destroyed lung and a poor health status further worsen a patient's outcome in the postoperative period. For these reasons, some surgeons have advised to be cautious when deciding whether to perform pneumonectomy [2-4]. However, recent studies have reported acceptably low mortality rates after pneumonectomy for chronic infections [5-9].
We retrospectively evaluated the patients who underwent pneumonectomy or pleuropneumonectomy for treating sequelae of pulmonary tuberculosis to determine the risk factors for early and long-term unfaroble outcomes.
MATERIALS AND METHODS
Between January 1980 and December 2008, 73 consecutive patients who underwent pneumonectomy due to destroyed lung caused by tuberculosis were retrospectively reviewed. Preoperative data collection involved patient demographic data, medical and surgical histories, preoperative medications, presenting symptoms, chest roentgenogram, computed tomography, sputum smear and culture test for acid-fast bacilli, fiberoptic bronchoscopy, preoperative treatment modality, pulmonary function tests, and a quantitative pulmonary perfusion scan. Predicted postoperative (PPO) forced expiratory volume in one second (FEV1) was calculated as follows: PPO FEV1=preoperative FEV1×contribution of contralateral lung.
1) Operative technique
The patients were intubated with a double lumen endotracheal tube and placed in lateral decubitus position. In most cases, surgery was performed through a posterolateral thoracotomy incision. An extrapleural approach (pleuropneumonectomy) was used when the pleural space was completely obliterated due to previous inflammation or empyema. A bronchial stump was stapled and reinforced with pericardial fat or parietal pleura. In some cases, however, reinforcement was omitted based on the choice of the surgeons. In case of spillage to some extent taking place despite careful dissection, copious saline or antiseptic solution irrigation was performed during operation.
(1) Postoperative complications and mortality
Due to the chronic nature of the underlying diseases, in many instances postoperative complications occurred later than 30 days. Therefore, postoperative complications were included on either a 60-day or in-hospital basis. The occurrence of complications requiring treatment (postpneumonectomy empyema [PPE], BPF, bleeding, vocal cord palsy, wound infection or wound deheiscence) was investigated.
(2) Follow-up
The follow-up data were obtained from the outpatient clinic chart reviews or by telephone calls to the patients or their families.
(3) Statistics
Statistical analyses for any correlation between the risk factors and the occurrence of complications were performed with the Chi-square test or Fisher's exact test. For survival analysis, the Kaplan-Meier method was used. Parametric variables were expressed as mean¡¾standard deviation and non-parametric variables were expressed as frequency. A p-value less than 0.05 was considered to be statistically significant. All statistical analyses were performed with the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
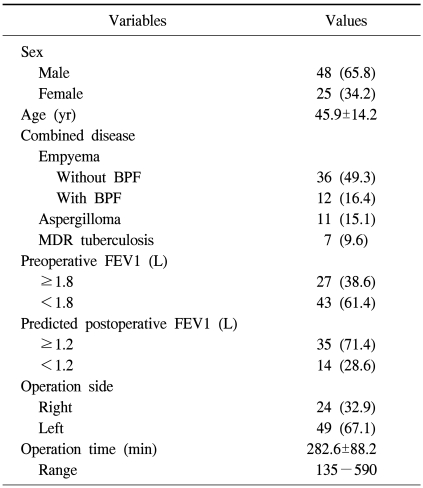
Out of 73 patients there were 25 women and 48 men with a mean age of 45.9 years (range, 19 to 70 years). The patients usually presented with the clinical signs of chronic infection and destroyed lung including cough with sputum, a mild to moderate degree of exertional dyspnea, chest discomfort, fever, or hemoptysis. There were 46 patients with empyema (12 with BPF), 11 with aspergilloma, and 7 with MDR tuberculosis. Drainage through the most dependent site was done initially in 34 patients. Sixty-one patients had a history of pulmonary tuberculosis, 28 patients were treated with anti-tuberculous drugs preoperatively, and 9 patients were treated with second line anti-tuberculous medication for pulmonary tuberculosis.
The mean operation time was 282.6±88.2 minutes (range, 135 to 590 minutes). Twenty-four patients underwent right-side operation and 49 underwent left-side resection. The preoperative mean FEV1 was 1.68±0.54 L/min (range, 0.66 to 3.50 L/min) and the mean PPO FEV1 was 1.43±0.39 L/min (range, 0.66 to 2.50 L/min) (Table 1). Intraoperative spillage took place in 17 patients during operation in 51 cases (33.3%).
Table 1.
Patient demographics
Values are presented as number (%) or mean±standard deviation. BPF=bronchopleural fistula; MDR=multidrug resistant; FEV1=forced expiratory volume in 1 second.
There were 5 operative mortalities (6.8%). One patient had excessive bleeding due to a subclavian vessel injury and developed ventricular fibrillation that could not be corrected. One patient had intraoperative ventricular fibrillation and expired due to arrhythmia during the postoperative course. Postoperative pneumonia and subsequent respiratory failure occurred in 3 patients.
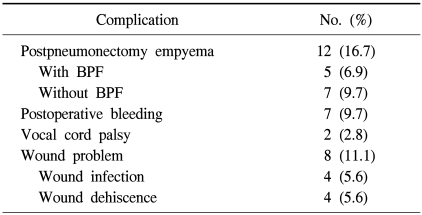
Among 72 patients, excluding one intraoperative death, a total of 29 patients (40.3%) suffered from postoperative complications: 12 (16.7%) patients had PPE, 4 (5.6%) wound infections, 4 (5.6%) wound deheiscence, 7 (9.7%) postoperative bleeding requiring re-exploration, and 2 (2.8%) vocal cord palsy requiring arytenoid reduction. BPF was documented in 5 patients (6.9%) (Table 2).
Table 2.
Postoperative complications
BPF=bronchopleural fistula.
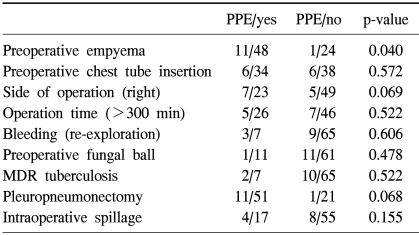
PPE was the most common postoperative complication. The prevalence of PPE increased in patients with preoperative empyema (p=0.04) (Table 3). Among 7 patients with PPE without BPF, 4 patients proceeded to undergo the Clagett procedure successfully and 3 patients were discharged with open-tube drainage. There were 5 patients with BPF and 4 occurred during right-side operation. The only risk factor for BPF was right-side operation (p=0.023).
Table 3.
Analysis of the risk factors for postpneumonectomy empyema
PPE=postpneumonectomy empyema; MDR=multi drug resistance.
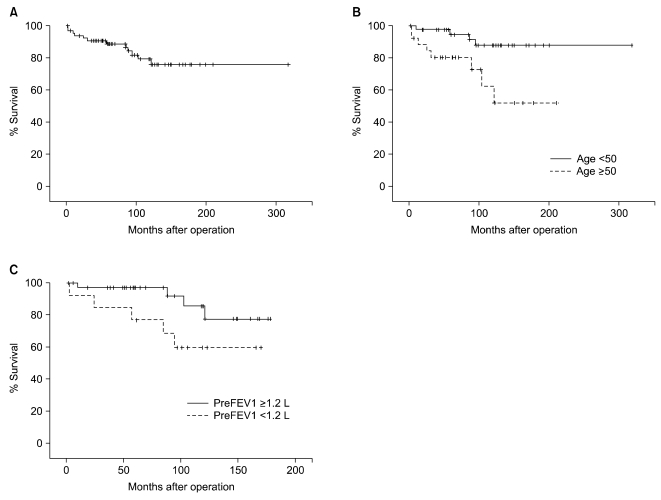
Sixty-eight patients were followed for a median of 96.7 months (range, 6 to 353 months). The 5- and 10-year survival rates were 88.9% and 76.2%, respectively. The causes of late death were pneumonia or respiratory failure in 8 and cor pulmonale in 2. The causes of the remaining 5 late deaths could not be determined.
The risk factors for late death were old age (≥50 years, p=0.02) and low predicted postoperative FEV1 (<1.2 L, p=0.02) (Fig. 1).
Fig. 1.
Long-term survival curve of patients after pneumonectomy for pulmonary tuberculosis. (A) overall survival (B) survival according to postoperative expected forced expiratory volume in one second (FEV1) and (C) survival according to age.
DISCUSSION
The present study shows that the mortality rate after pneumonectomy for pulmonary tuberculosis was acceptable considering the difficulties with the surgical technique and patients with poor health status. However, the postoperative complication rate (40.3%) was still high, the prevalence of PPE was higher in patients with preoperative empyema, and BPF was more common in right-side operation. Compared with the high complication rate, the long-term survival rate was found to be satisfactory.
Pneumonectomy for a benign inflammatory lung disease has been considered a high-risk procedure [2-4]. In particular, pneumonectomy for pulmonary tuberculosis is riskier than for other inflammatory disease [5,10-12]. There are three main reasons why the complication rate increases after pneumonectomy in patients with tuberculosis. First, tuberculosis commonly occurs in individuals with poor general health status and the progression to destroyed lung worsens the general status of tuberculosis patients. Second, preoperative empyema is much more commonly associated with tuberculosis than with other underlying diseases of destroyed lung. Third, most patients with tuberculosis have an infected cavity in the parenchyma that attaches strictly to the upper part of the thoracic wall, and it is sometimes impossible to separate it without perforation [5].
Although pneumonectomy for tuberculosis has been considered a high-risk procedure, there are situations when pneumonectomy or pleuropneumonectomy remains the only curative treatment modality. Those cases include destroyed lung, uncontrolled hemoptysis, main bronchial stenosis, MDR strain, and significant symptoms such as productive cough, or repetitive hospitalization. Several authors have reported a 1.1-8.5% operative mortality from pneumonectomy for inflammatory lung disease [2,6-9,13]. We experienced a 6.8% early mortality, which was not statistically significant compared with the mortality (4.5%) of pneumonectomy for lung cancer during the same period at our institution (p=0.841).
The major concerns among postoperative complications are PPE and BPF. The risk factors reported for these undesirable complications reported in the literature were the presence of preoperative empyema, aspergilloma, excessive blood loss, right-side operation, intraoperative spillage during operation, and re-exploration for hemorrhage [2,5,9,13]. The current study revealed that the presence of preoperative empyema was a risk factor for the development of PPE.
The presence of preoperative empyema has been considered an important risk factor for PPE [5]. Odell and Henderson [14] reported a PPE rate of 45.7% when pneumonectomy through an empyema was performed. On the other hand, Shiraishi et al. [13] recommended pleuropneumonectomy for the treatment of empyema with destroyed lung and reported operative mortality rates of 8.5% and PPE at 9.6% with this procedure. However, our data shows a high PPE rate of 21.6% in 51 pleuropneumonectomy patients. This result is thought to be due to an unrecognized intraoperative spillage due to the presence of preoperative empyema.
Due to the anatomical differences between the right and left main bronchi, destroyed lung occurs more frequently on the left side [15]. In this series, 49 out of 73 (67.1%) patients had left lung destruction. However, BPF followed by pneumonectomy occured less frequently in left-side operations due to anatomical peculiarities. The right bronchial stump is more exposed in the pleural space and less likely to be naturally buttressed by mediastinal tissues than the left bronchial stump [16]. This study also showed that there were 5 patients with BPF and 4 of these BPF occurred during right-side operation with statistical significance (p=0.023).
Although there are a few papers that have reported long term survival rates after pneumonectomy for chronic inflammatory lung disease, the long-term survival rates in our series would seem to be satisfactory. Shiraishi et al. [13] reported a 5-year survival rate of 83% and Kim et al. [9] reported 5- and 10-year survival rates of 94% and 88%, respectively. The risk factors for late deaths were old age and low predicted postoperative FEV1. Postoperative FEV1 is known as a risk factor for early and long-term survival after pneumonectomy and predicted postoperative FEV1 is correlated with actual postoperative FEV1 [17,18]. This study revealed that low predicted postoperative FEV1 is the risk factor for long-term survival after pneumonectomy for tuberculosis and most patients died of pulmonary complications although some patients were lost to follow-up eventually. These results suggest that pulmonary complications related to impaired pulmonary function may lead to late death. Therefore, continued surveillance should focus on prevention and early detection during the follow-up care of patients with low predicted postoperative FEV1, even if there are no early complications.
There are some limitations to this study. First, the study was retrospective and conducted at a single center. Second, we could not follow-up with some patients completely because we were short of regular surveillance 2 years after operation in the case of patients without postoperative complications. Third, preoperative FEV1 does not reflect the accurate postoperative FEV1, so a patient's prognosis can be predicted more reliably by measuring postoperative FEV1.
CONCLUSION
Although postoperative empyema increases in patients with preoperative empyema and postoperative BPF increases in right-side operation, the mortality rates and long-term survival rates seem to be satisfactory. However, the follow-up care of patients with low postoperative predicted FEV1 should continue for prevention and early detection of pulmonary complications related to impaired pulmonary function.
References
- 1.McLaughlin JS, Hankins JR. Current aspects of surgery for pulmonary tuberculosis. Ann Thorac Surg. 1974;17:513–525. doi: 10.1016/s0003-4975(10)65689-5. [DOI] [PubMed] [Google Scholar]
- 2.Massard G, Dabbagh A, Wihlm JM, et al. Pneumonectomy for chronic infection is a high-risk procedure. Ann Thorac Surg. 1996;62:1033–1037. doi: 10.1016/0003-4975(96)00596-6. [DOI] [PubMed] [Google Scholar]
- 3.Massard G, Roeslin N, Wihlm JM, Dumont P, Witz JP, Morand G. Pleuropulmonary aspergilloma: clinical spectrum and results of surgical treatment. Ann Thorac Surg. 1992;54:1159–1164. doi: 10.1016/0003-4975(92)90086-j. [DOI] [PubMed] [Google Scholar]
- 4.Shirakusa T, Ueda H, Saito T, Matsuba K, Kouno J, Hirota N. Surgical treatment of pulmonary aspergilloma and Aspergillus empyema. Ann Thorac Surg. 1989;48:779–782. doi: 10.1016/0003-4975(89)90670-x. [DOI] [PubMed] [Google Scholar]
- 5.Halezeroglu S, Keles M, Uysal A, et al. Factors affecting postoperative morbidity and mortality in destroyed lung. Ann Thorac Surg. 1997;64:1635–1638. doi: 10.1016/s0003-4975(97)00999-5. [DOI] [PubMed] [Google Scholar]
- 6.Ashour M. Pneumonectomy for tuberculosis. Eur J Cardiothorac Surg. 1997;12:209–213. doi: 10.1016/s1010-7940(97)00155-3. [DOI] [PubMed] [Google Scholar]
- 7.Blyth DF. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg. 2000;18:429–434. doi: 10.1016/s1010-7940(00)00526-1. [DOI] [PubMed] [Google Scholar]
- 8.Conlan AA, Lukanich JM, Shutz J, Hurwitz SS. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg. 1995;110(4 Pt 1):1118–1124. doi: 10.1016/s0022-5223(05)80181-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim YT, Kim HK, Sung SW, Kim JH. Long-term outcomes and risk factor analysis after pneumonectomy for active and sequela forms of pulmonary tuberculosis. Eur J Cardiothorac Surg. 2003;23:833–839. doi: 10.1016/s1010-7940(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.Stevens MS, de Villiers SJ, Stanton JJ, Steyn FJ. Pneumonectomy for severe inflammatory lung disease. Results in 64 consecutive cases. Eur J Cardiothorac Surg. 1988;2:82–86. doi: 10.1016/s1010-7940(88)80003-4. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz M, Madsen L, Goble M, Iseman M. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991;52:1108–1111. doi: 10.1016/0003-4975(91)91289-8. [DOI] [PubMed] [Google Scholar]
- 12.Brown J, Pomerantz M. Extrapleural pneumonectomy for tuberculosis. Chest Surg Clin N Am. 1995;5:289–296. [PubMed] [Google Scholar]
- 13.Shiraishi Y, Nakajima Y, Koyama A, Takasuna K, Katsuragi N, Yoshida S. Morbidity and mortality after 94 extrapleural pneumonectomies for empyema. Ann Thorac Surg. 2000;70:1202–1206. doi: 10.1016/s0003-4975(00)01612-x. [DOI] [PubMed] [Google Scholar]
- 14.Odell JA, Henderson BJ. Pneumonectomy through an empyema. J Thorac Cardiovasc Surg. 1985;89:423–427. [PubMed] [Google Scholar]
- 15.Ashour M. The anatomy of left bronchus syndrome. Clin Anat. 1995;8:256–261. doi: 10.1002/ca.980080404. [DOI] [PubMed] [Google Scholar]
- 16.Darling GE, Abdurahman A, Yi QL, et al. Risk of a right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg. 2005;79:433–437. doi: 10.1016/j.athoracsur.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Mineo TC, Schillaci O, Pompeo E, Mineo D, Simonetti G. Usefulness of lung perfusion scintigraphy before lung cancer resection in patients with ventilatory obstruction. Ann Thorac Surg. 2006;82:1828–1834. doi: 10.1016/j.athoracsur.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 18.Leo F, Scanagatta P, Baglio P, et al. The risk of pneumonectomy over the age of 70: a case-control study. Eur J Cardiothorac Surg. 2007;31:780–782. doi: 10.1016/j.ejcts.2007.01.036. [DOI] [PubMed] [Google Scholar]