Abstract
Background
The aim of this study is to evaluate our institutional results of the aortic valve replacement through minimally invasive approaches compared with conventional sternotomy.
Materials and Methods
From August 1997 to July 2010, 838 patients underwent primary isolated aortic valve replacement. Of them, 73 patients underwent surgery through minimally invasive approaches (MIAS group) whereas 765 patients underwent surgery through the conventional sternotomy (CONV group). Clinical outcomes were compared using a propensity score matching design.
Results
Propensity score matching yielded 73 pairs of patients in which there were no significant differences in baseline profiles between the two groups. Patients in the MIAS group had longer aortic cross clamp than those in the CONV group (74.9±27.9 vs.. 66.2±27.3, p=0.058). In the MIAS group, conversion to full sternotomy was needed in 2 patients (2.7%). There were no significant differences in the rates of low cardiac output syndrome (4 vs. 8, p=0.37), reoperation due to bleeding (7 vs. 6, p=0.77), wound infection (2 vs. 4, p=0.68), or requirements for dialysis (2 vs. 1, p=0.55) between the two groups. Postoperative pain was significantly less in the MIAS group than the conventional group (pain score, 3.79±1.67 vs. 4.32±1.56; p=0.04).
Conclusion
Both minimally invasive approaches and conventional sternotomy had comparable early clinical outcomes in patients undergoing primary isolated aortic valve replacement. Minimally invasive approaches significantly decrease postoperative pain.
Keywords: Aortic valve, surgery; Minimally invasive surgery
INTRODUCTION
The recent interest in minimal access surgery is based upon the theory that smaller surgical incisions lead to less postoperative pain, better cosmetic effects, less bleeding, shorter hospital stays, faster rehabilitation, and reduced cost. Early results of minimally invasive aortic valve (AV) surgery have been reported as promising by selected centers, with mortality rates of 0.8% to 4%, leading to increasing utilization of less invasive approaches for valve surgery throughout the world [1-8]. However, many surgeons remain skeptical about performing minimal access AV replacement (AVR), as this technique results in limited exposure and difficulties in handling, making surgery more difficult and dangerous [9,10]. Therefore, we performed a propensity-matched comparison of early outcomes in patients who underwent minimally invasive aortic valve surgery with those who underwent conventional full sternotomy.
MATERIALS AND METHODS
1) Patients
From August 1997 to July 2010, 838 patients underwent primary isolated aortic valve replacement by five surgeons. Of these patients, 73 underwent surgery using minimally invasive approaches (upper sternotomy in 51, transverse sternotomy in 20, right mini-thoracotomy in 1, and lower sternotomy in 1) whereas 765 underwent surgery through full sternotomy. The choice of procedure depended on the preference of the surgeons.
2) Surgical technique
(1) Upper sternotomy
For upper sternotomy, percutaneous internal jugular cannulation was done in 51 patients (69.8%) to facilitate venous drainage during cardiopulmonary bypass. A limited median skin incision (range, 6 to 8 cm) was made beginning at the sternal notch, and the upper partial sternotomy was made with the oscillating saw down to the fourth intercostal space, at which a transverse sternal osteotomy was made. The upper part of the sternum was then divided laterally. The upper portion of the pericardium was divided, exposing the upper part of the pericardial structures. The right common femoral vein was exposed through a 3 to 4 cm incision after heparinization and was cannulated. The arterial cannulation was performed at the ascending aorta (n=40), right femoral artery (n=9), or right axillary artery (n=2) depending on the presence of atheromatous lesions at the aorta or peripheral arteries. Mild-to-moderate hypothermic (range, 28℃ to 32℃) cardiopulmonary bypass was used and myocardial protection was achieved with cold or tepid blood cardioplegia. Initially, the cardioplegic solution was administered antegradely via aortic root cannulation (52%) or direct coronary cannulation (48%) according to the presence of aortic regurgitation. Maintenance of cardiac arrest thereafter was done by retrograde cardioplegic infusion through the coronary sinus or by direct infusion through a coronary ostium, intermittently. After opening the aorta, three traction sutures at the tip of each commissure were placed and suspended from the drapes under tension, elevating the valve for better exposure. After aortic declamping, one drainage tube was placed through a subxiphoid incision before weaning from cardiopulmonary bypass to avoid cardiac rupture during the tube insertion. Intraoperative transesophageal echocardiography was used routinely for assessment of the cardiac function, evaluation of surgical results, and confirmation of the de-airing process.
(2) Transverse sternotomy
An 8 to 10 cm transverse incision was made transversely at the level of the third or fourth intercostal space. The bilateral internal mammary arteries were sacrificed. Aortic cannulation was performed centrally (n=5) or peripherally (n=15). Venous cannulation was performed via the femoral vein. The rest of the operation was a fairly routine procedure as for any other minimal access aortic valve surgery.
(3) Right mini-thoracotomy
This procedure was performed with a 6 to 7 cm incision along the right anterior axillary line. The level of this incision was at the third intercostal space. Aortic and venous cannulation was performed through the right femoral vessels.
(4) Lower sternotomy
The sternum was incised from the tip of the xiphoid process up to the second intercostal space. Cardiopulmonary bypass was established via the right femoral access.
(5) Full median sternotomy
A full median sternotomy was performed from the suprasternal notch to the xiphoid process. Cannulation was performed according to the standard technique with the usual methods of myocardial protection.
3) Statistical analysis
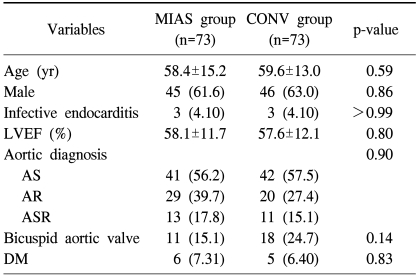
Categorical variables are presented as frequencies and percentages, and continuous variables are expressed as means with standard deviations or medians with ranges. To reduce the effect of treatment selection bias and potential confounding, we performed adjustments for the differences in the baseline characteristics by use of propensity score matching. The propensity scores were estimated with multiple logistic regression analysis. Prespecified covariates listed in Table 1 were included for the calculation of propensity scores. For the development of the propensity score-matched pairs (a 1:1 match), the greedy 5-to-1 digit matching algorithm was used. After propensity score matching, the data of the two groups were compared with the paired t-test or the Wilcoxon signed rank test for continuous variables (age, left ventricular ejection fraction, postoperative creatine kinase (CK)-MB and troponin-I levels, endotracheal intubation duration, intensive care unit stay; and the amount of bleeding or transfusion), and with the McNemar test or marginal homogeneity test for categorical variables (sex, infective endocarditis, aortic valve diagnosis, diabetes, low cardiac output syndrome, bleeding reoperation, cerebrovascular attack, pulmonary infection, requirement for dialysis, sternal infection, atrial fibrillation, and complete aortic valve [AV] block).
Table 1.
Preoperative baseline profiles of patients
Values are presented as mean±standard deviation or number (%).
MIAS=minimally invasive aortic surgery; CONV=conventional surgery; LVEF=left ventricular ejection fraction; AS=aortic stenosis; AR=aortic regurgitation; ASR=aortic steno-regurgitation; DM=diabetes mellitus.
All reported p-values were two-sided, and a value of p<0.05 was considered statistically significant. SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA), was used for statistical analyses.
RESULTS
Propensity score matching yielded 73 matched pairs of patients in which there were no significant differences in baseline characteristics between the minimally invasive approach group (MIAS group) and conventional sternotomy group (CONV group).
Aortic clamping time was slightly longer with the MIAS group (106.3±45.3 minutes vs. 101.9±39.6 minutes, p=0.06) whereas cardiopulmonary bypass time was equivalent between the two groups (106.3±45.3 minutes vs. 101.9±39.6 minutes, p=0.53). In the MIAS group, conversion to full sternotomy was needed in 2 patients (2.7%) due to operation site bleeding.
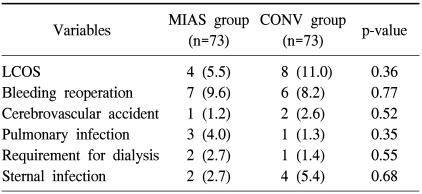
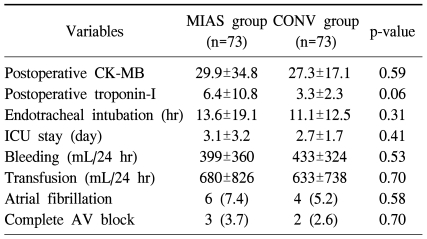
There were two early deaths only in the CONV group (p=0.36). Early postoperative complications are summarized in Table 2. There were no significant differences between the MIAS group and the CONV groups in the rate of postoperative complications including low cardiac output syndrome, reoperation due to bleeding, cerebrovascular accident (CVA), pulmonary infection, acute renal failure requiring dialysis, and wound infection. CK-MB and troponin T levels measured at 6 hours postoperatively were similar in both groups (Table 3). There were no significant differences in the duration of endotracheal intubation or intensive care unit stay, the amount of bleeding or amount of transfusion during the first 24 hours, or the rate of postoperative atrial fibrillation or complete AV block between the two groups (Table 3).
Table 2.
Early postoperative complications
Values are presented as number (%).
MIAS=minimally invasive aortic surgery; CONV=conventional surgery; LCOS=low cardiac output syndrome.
Table 3.
Postoperative outcomes
Values are presented as mean±standard deviation or number (%).
MIAS=minimally invasive aortic surgery; CONV=conventional surgery; CK-MB=creatine kinase-MB; ICU=intensive care unit; AV=aortic valve.
Postoperative echocardiography performed within 7 days of surgery revealed similar left ventricular ejection fraction in the two groups (59.6±9.3% in the MIAS group and 56.9±12.5% in the CONV group, p=0.15).
Patients in the MIAS group showed a higher PaO2/FiO2 ratio at the time of admission to the intensive care unit (333±145 in the MIAS group and 248±133 in the CONV group, p<0.001) and less pain during hospitalization (pain score, 3.79±1.67 in the MIAS group and 4.32±1.56 in the CONV group; p=0.04) than did those in the CONV group.
DISCUSSION
The present study demonstrated that minimally invasive approaches were associated with similar levels of hospital morbidity and mortality to conventional sternotomy. The points of controversy are whether minimally invasive AV surgery can provide good exposure for surgeons and beneficial results for patients. Those two approaches may have similar surgical exposures of the aortic root. This is partly related to the fact that since the total anterior mediastinum is not dissected in the upper sternotomy approach, the heart can be located in a relatively anterior position. The exposure of the aortic root can be enhanced by three traction sutures at the tip of each of the three commissures that will eventually lift up the valve [1].
At the beginning of minimally invasive AV surgery, several different methods of gaining access to the surgical field have been reported and different techniques regarding great vessel cannulation, aortic cross-clamp, and de-airing have been proposed [3-5,11-13]. Because aortic atherosclerosis is correlated with the increased incidence of aortic valve sclerosis [11], cannulation into the femoral artery using longer cannulae for minimally invasive approaches carries the potential risk of direct aortic injury, aortic dissection, atherothromboembolism, and acute limb ischemia [14,15]. We routinely evaluated the quality of the descending aorta and peripheral arteries by preoperative computed tomography scanning. The absence of peripheral vascular complications and the minimum risk of CVA in the current study may be attributed to this approach.
This study and other reports have shown no increase in the perioperative stroke rate associated with minimally invasive AV surgery [16,17]. Intraoperative transesophageal echocardiography was performed for the detection of residual air in the left ventricle before the release of the aortic cross-clamp and weaning from cardio-pulmonary bypass. Flooding the thoracic cavity with carbon dioxide gas decreases the danger of air embolism [17].
The application of all these modalities is thought to minimize the risk of neurologic complications [17]. Transverse sternotomy usually sacrifices the bilateral intrathoracic arteries [3,12,18,19]. The damage to the intrathoracic artery may have a great influence upon the recovery from a sternotomy wound [15,20] and can affect the prognosis of patients who are potential candidates for coronary artery bypass grafting in the future.
Since the extent of surgical trauma is considerably lower with minimally invasive incisions, the pulmonary function can be better preserved because only a small portion of the entire sternum was cut off. Obviously, smaller incisions also provide better cosmesis to patients.
The minimally invasive approach group tended to have a more favorable postoperative respiratory reserve, probably due to decreased chest wall instability and reduced postoperative pain. The increased sternal stability allowed patients to change position early and cough more efficiently.
Although conventional sternotomy, compared to other thoracic incisions, is a relatively less-painful incision, many patients still report considerable pain even long after surgery. In our survey of standard versus minimally invasive incisions, there appeared to be significantly less incisional pain with the minimally invasive incisions. We have routinely used intravenous fentanyl patient-controlled analgesia and oral non-steroid anti-inflammatory analgesics to control surgical site pain. The administration of analgesics was initiated immediately after surgery. Patients who complained of severe pain, despite the use of the above-mentioned medications, were given oral opioid (hydromorphone hcl) or transdermal opioid (fentanyl propanamide) for pain relief. These treatments were adopted for most patients regardless of the surgical approach (conventional or minimally invasive) and might have contributed to less perceived pain, postoperatively.
This study is subject to the limitations inherent in retrospective studies of observational data from a single center. The non-randomized design may have affected our results due to unmeasured confounders, procedure bias, or detection bias, even with the use of rigorous statistical adjustment.
CONCLUSION
Both minimally invasive approaches and conventional sternotomy had comparable early clinical outcomes in patients undergoing primary isolated AVR. Minimally invasive approaches significantly reduce postoperative pain and offer better postoperative respiratory function. Therefore, minimally invasive AVR is a more attractive approach.
Footnotes
This manuscript was presented at the 34th Autumn Academic Meeting of the Korean Society for Thoracic and Cardiovascular Surgery.
References
- 1.Konertz W, Waldenberger F, Schmutzler M, Ritter J, Liu J. Minimal access valve surgery through superior partial sternotomy: a preliminary study. J Heart Valve Dis. 1996;5:638–640. [PubMed] [Google Scholar]
- 2.Cosgrove DM, 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg. 1998;65:1535–1538. doi: 10.1016/s0003-4975(98)00300-2. [DOI] [PubMed] [Google Scholar]
- 3.Gundry SR, Shattuck OH, Razzouk AJ, del Rio MJ, Sardari FF, Bailey LL. Facile minimally invasive cardiac surgery via ministernotomy. Ann Thorac Surg. 1998;65:1100–1104. doi: 10.1016/s0003-4975(98)00064-2. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JG, Mitchell ME, Adams DH, Couper GS, Aranki SF, Cohn LH. Minimally invasive direct access mitral valve surgery. Semin Thorac Cardiovasc Surg. 1999;11:212–222. doi: 10.1016/s1043-0679(99)70062-6. [DOI] [PubMed] [Google Scholar]
- 5.Szwerc MF, Benckart DH, Wiechmann RJ, et al. Partial versus full sternotomy for aortic valve replacement. Ann Thorac Surg. 1999;68:2209–2213. doi: 10.1016/s0003-4975(99)00863-2. [DOI] [PubMed] [Google Scholar]
- 6.Colvin SB, Galloway AC, Ribakove G, et al. Port-Access mitral valve surgery: summary of results. J Card Surg. 1998;13:286–289. doi: 10.1111/j.1540-8191.1998.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 7.Svensson LG, Nadolny EM, Kimmel WA. Minimal access aortic surgery including re-operations. Eur J Cardiothorac Surg. 2001;19:30–33. doi: 10.1016/s1010-7940(00)00597-2. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JG, Hsin MK, Adams DH, et al. Minimally invasive direct access heart valve surgery. J Card Surg. 2000;15:21–34. doi: 10.1111/j.1540-8191.2000.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonchek LI, Ullyot DJ. Minimally invasive coronary bypass: a dissenting opinion. Circulation. 1998;98:495–497. doi: 10.1161/01.cir.98.6.495. [DOI] [PubMed] [Google Scholar]
- 10.Bonatti J, Schachner T, Bonaros N, et al. Technical challenges in totally endoscopic robotic coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131:146–153. doi: 10.1016/j.jtcvs.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 11.Agmon Y, Khandheria BK, Meissner I, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38:827–834. doi: 10.1016/s0735-1097(01)01422-x. [DOI] [PubMed] [Google Scholar]
- 12.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421–426. doi: 10.1097/00000658-199710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossi EA, Esposito R, Harris LJ, et al. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg. 1991;102:342–346. [PubMed] [Google Scholar]
- 14.Hendrickson SC, Glower DD. A method for perfusion of the leg during cardiopulmonary bypass via femoral cannulation. Ann Thorac Surg. 1998;65:1807–1808. doi: 10.1016/s0003-4975(98)00302-6. [DOI] [PubMed] [Google Scholar]
- 15.Arom KV, Emery RW. Minimally invasive mitral operations. Ann Thorac Surg. 1997;63:1219–1220. [PubMed] [Google Scholar]
- 16.Felger JE, Chitwood WR, Jr, Nifong LW, Holbert D. Evolution of mitral valve surgery: toward a totally endoscopic approach. Ann Thorac Surg. 2001;72:1203–1208. doi: 10.1016/s0003-4975(01)02978-2. [DOI] [PubMed] [Google Scholar]
- 17.Schroeyers P, Wellens F, De Geest R, et al. Minimally invasive video-assisted mitral valve repair: short and mid-term results. J Heart Valve Dis. 2001;10:579–583. [PubMed] [Google Scholar]
- 18.Lytle BW. Minimally invasive cardiac surgery. J Thorac Cardiovasc Surg. 1996;111:554–555. [PubMed] [Google Scholar]
- 19.Cosgrove DM, 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg. 1996;62:596–597. [PubMed] [Google Scholar]
- 20.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications: deep vs superficial infection. Chest. 1996;110:1173–1178. doi: 10.1378/chest.110.5.1173. [DOI] [PubMed] [Google Scholar]