Abstract
Background
Despite improved managements for acute respiratory distress syndrome (ARDS), its mortality remains high. Extracorporeal membrane oxygenation (ECMO) has emerged as the final option for the treatment of ARDS unresponsive to conventional measures. This study describes our experiences of venovenous ECMO support for the treatment of ARDS.
Materials and Methods
Between 2007 and 2010, 56 patients (aged 56.6±13.4 years, 43 males) received venovenous ECMO for the treatment of ARDS. The detailed clinical records were retrospectively reviewed.
Results
Before the institution of ECMO support, 35 patients (55.4%) required nitric oxide inhalation, 35 patients (55.4%) received continuous renal replacement therapy, and 20 patients (35.7%) were in shock status. The median duration of ECMO support was 164 hours (range, 5 to 1,413 hours). 27 (48%) patients could be successfully weaned from ECMO. Of them, 7 (13%) survived to discharge. On logistic regression analysis, a requirement for higher inspiratory pressure before ECMO support was the only significant factor that could predict ECMO weaning failure.
Conclusion
The outcome of venovenous ECMO support for the treatment of ARDS was suboptimal. Further improvements in outcomes should be made through the accumulation of experience and establishment of a standardized protocol for the management of ECMO.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a serious condition that is characterized by sudden-onset severe hypoxemia and diffuse bilateral pulmonary infiltrates [1]. Various causes or associated conditions may result in ARDS, including pneumonia, aspiration, sepsis, massive blood transfusion, and trauma. Mortality and morbidity rates associated with ARDS have improved on account of enormous advances in the understanding of the pathophysiology of ARDS and the development of more effective therapeutic strategies for ARDS. However, the ARDS mortality rate remains high [2-4].
Recently, interest has arisen regarding the role of extracorporeal membrane oxygenation (ECMO) as a final treatment option for ARDS unresponsive to conservative measures. Internationally, several previous studies have demonstrated the efficacy of ECMO support for this indication [5]. However, in the Republic of Korea, no clinical study has yet analyzed the efficacy of ECMO support for ARDS.
The purpose of this study was to describe our experience with venovenous ECMO support for ARDS at our institution and to discuss ways to improve the outcomes associated with this treatment option.
MATERIALS AND METHODS
Between January 2007 and November 2010, 153 patients received ECMO support for the treatment of respiratory failure. Excluding the patients who required venoarterial ECMO support due to concomitant heart dysfunction resulted in the final inclusion of 56 subjects who were treated with venovenous ECMO support. A retrospective chart review was conducted using data from these patients.
ECMO support was set up in the intensive care unit (ICU) in all cases. Heparin infusion was started intravenously before cannulation and was administered continuously to maintain an activated clotting time of 160 to 200 seconds. Antibiotics were given routinely and changed according to proven microbial infections. ECMO was established with venous drainage (17 to 28 Fr) from the femoral vein; arterialized blood was returned to the contralateral femoral vein or right internal jugular vein (17 to 22 Fr). System replacement was undertaken in the case of severe plasma leakage from the oxygenator, decreased ECMO blood flow, evidence of hemolysis, or deterioration of the oxygenator gas exchange function.
1) Data analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are expressed as mean±standard deviation or medians with ranges. All pre-ECMO demographic, clinical, and laboratory variables were assessed by a multiple logistic regression model to determine potential risk factors for ECMO weaning failure and in-hospital death. All data were analyzed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
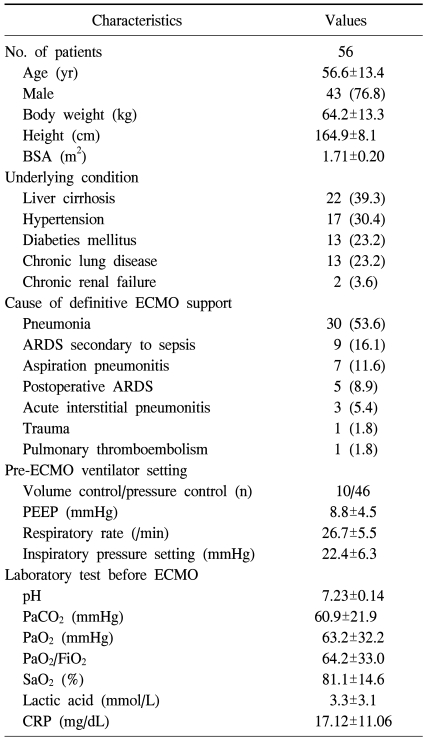
The baseline characteristics of the patients are summarized in Table 1. Among the 56 patients, 22 patients underwent surgical intervention before ECMO support; the surgical interventions included liver transplantation in 13 cases (23.2%), lung resection in 4 (7.1%), open heart surgery in 3 (5.4%), abdominal aorta replacement in 2 (3.6%), and colon resection in 1 (1.8%). The most common reason for ECMO support was pneumonia (53.6%), followed by ARDS secondary to sepsis (16.1%).
Table 1.
Baseline characteristics of all patients who received ECMO support
Values are presented as mean±standard deviation or number (%).
ECMO=extracorporeal membrane oxygenation; BSA=body surface area; ARDS=acute respiratory distress syndrome; PEEP=positive end expiratory pressure; CRP=C-reactive protein.
The median duration of ICU stay and ventilator care before ECMO support was 5 days (range, 0 to 64 days) and 4 days (range, 0 to 37 days), respectively. Before the initiation of ECMO support, 35 patients (55.4%) required nitric oxide inhalation, 35 patients (55.4%) received continuous renal replacement therapy, and 20 patients (35.7%) were in shock (systolic blood pressure <90 mmHg). The patients' respiratory parameters and ventilator settings before ECMO support are also shown in Table 1. The most common pre-ECMO ventilator setting was the pressure control mode (82.1%), with hypoxemia and hypercarbia being the predominant feature, even during ventilator support.
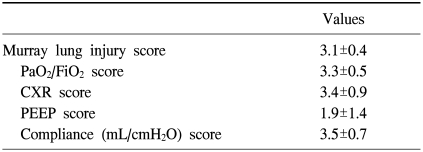
The Murray score for evaluating acute lung injury, which is calculated from the mean PaO2/FiO2 ratio, positive end-expiratory pressure, compliance, and chest radiography, for each patient is shown in Table 2. The mean Murray score was 3.1±0.4. The median size of the draining and inflow catheters was 20 Fr (range, 17 to 28 Fr) and 18 Fr (range, 17 to 22 Fr), respectively. The median duration of ECMO support was 164 hours (range, 5 to 1,413 hours), during which the oxygenators were changed 0.95±1.48 times for each patient.
Table 2.
Murray lung injury score of all patients
Values are presented as mean±standard deviation. The Murray score is a grading system for ARDS which uses 4 pieces of information graded 0-4 to give a severity index for ARDS. The data required are: 1) PaO2/FiO2: ≥300=0, 225-299=1, 175-224=2, 100-174=3, <100=4; 2) CXR: normal=0, 1 point per quadrant infiltrated; 3) PEEP: ≤5=0, 6-8=1, 9-11=2, 12-14=3, ≥15=4; 4) Compliance (mL/cmH2O): ≥80=0, 60-79=1, 40-59=2, 20-39=3, and ≤19=4.
CXR=chest X-ray; PEEP=positive end expiratory pressure; ARDS=acute respiratory distress syndrome.
Twenty-seven patients (48%) could be successfully weaned off ECMO. Of these patients, 7 (13%) survived and were eventually discharged. In mortality cases, causes of death were septic shock (35%), ARDS (31%), multiorgan failure (22%), heart failure (6%), and bleeding (4%). On univariate analysis, a requirement for higher inspiratory pressure before ECMO support was the only factor that significantly predicted ECMO weaning failure, at 20.7±6.9 mmHg in patients with weaning success compared with 24.0±5.4 mmHg in patients with weaning failure (p=0.012). No predictive factor for in-hospital death was identified in this study.
DISCUSSION
This article describes a retrospective analysis of our clinical experience with venovenous ECMO support in adults with ARDS unresponsive to conventional treatment. This report is the first clinical analysis of ECMO for ARDS in Korea. In the present study, the ECMO weaning success rate was 48% (27 of 56 patients) and the total survival to discharge rate was 13% (7 of 56 patients). Compared with recent reports, this study revealed disappointing results. Hemmila et al. [5] reported their experience with ECMO support for ARDS in 255 adults from 1989 to 2003. Their study revealed an ECMO weaning rate of 67% and a survival discharge rate of 52%. Their survival discharge rate for venovenous ECMO support specifically was 59.5% [5]. A recent multicenter randomized controlled trial, the conventional ventilation or ECMO for severe adult respiratory failure trial ('CESAR trial'), revealed an improvement in survival without severe disability at 6 months in patients who received ECMO treatment compared with those who received continued conventional ventilation [6]. Further, a previous study identified several factors predictive of ECMO mortality, including advanced patient age, a pre-ECMO arterial blood pH of less than 7.18, an increased duration of pre-ECMO ventilation, the underlying cause of respiratory failure, and complications associated with ECMO. Another report demonstrated that venovenous ECMO is as reliable as venoarterial ECMO in patients with no evidence of organ failure, apart from that in the lungs [7].
Our poor results could be explained, in part, by the relatively liberal patient selection. In our hospital, 21 patients underwent prolonged mechanical ventilation for more than 1 week before ECMO initiation, and all of these patients died. Additionally, compared with previous studies that have demonstrated good results based on a standardized protocol for disease management, our institution has insufficient experience with ECMO management and has no well-established standardized protocol. With additional accumulated experience and an established standardized protocol for ECMO support, better outcomes are expected.
In a clinical trial of ECMO management, Bonacchi et al. [8] introduced a new cannulation strategy for the venous ECMO "x-configuration." They suggested that their new method allows for higher separation between blood inflow and outflow, thus reducing recirculation and increasing systemic oxygen saturation and delivery [8]. A single institution's experience with a bicaval dual lumen catheter for adult venovenous ECMO has also been reported [9]. In order to improve outcomes associated with venovenous ECMO, more such novel innovations should be attempted and investigated.
This study has some important limitations. First, this was a retrospective analysis of nonrandomized patients. Second, our study included only a small number of patients, and risk factor analysis, therefore, could not be adequately performed.
CONCLUSION
The outcome of venovenous ECMO support for the treatment of ARDS was suboptimal in this single institution study. Futher improvements in outcomes should be undertaken through the accumulation of experience and establishment of a standardized institutional protocol for the management of ECMO.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome: the Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 4.Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes. Clin Chest Med. 2006;27:549–557. doi: 10.1016/j.ccm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–605. doi: 10.1097/01.sla.0000141159.90676.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 7.Oshima K, Kunimoto F, Hinohara H, et al. Extracorporeal membrane oxygenation for respiratory failure: comparison of venovenous versus venoarterial bypass. Surg Today. 2010;40:216–222. doi: 10.1007/s00595-008-4040-z. [DOI] [PubMed] [Google Scholar]
- 8.Bonacchi M, Harmelin G, Peris A, Sani G. A novel strategy to improve systemic oxygenation in venovenous extracorporeal membrane oxygenation: the "X-configuration". J Thorac Cardiovasc Surg. 2011;142:1197–1204. doi: 10.1016/j.jtcvs.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91:1763–1768. doi: 10.1016/j.athoracsur.2011.03.002. [DOI] [PubMed] [Google Scholar]