Abstract
Purpose
This study analyzed potentially functional polymorphisms in CASPASE (CASP) genes and their impact on the prognosis for Korean colorectal cancer patients.
Materials and Methods
A total of 397 consecutive patients with curatively resected colorectal adenocarcinoma were enrolled in this study. Genomic DNA from these patients was extracted from fresh colorectal tissue, and the 10 polymorphisms in the CASP3, CASP6, CASP7, CASP8, CASP9, and CASP10 genes were determined using a reverse transcription polymerase chain reaction genotyping assay.
Results
The median patient age was 63 years, and 218 (54.9%) patients had colon cancer, while 179 (45.1%) patients had rectal cancer. Univariate and multivariate survival analysis including pathologic stage, patient age, differentiation, and carcinoembryonic antigen level demonstrated that these polymorphisms were not associated with either disease-free or overall survival.
Conclusion
None of the 10 polymorphisms in the CASP genes investigated in this study was found to be an independent prognostic marker for Korean patients with curatively resected colorectal cancer.
Keywords: Colorectal neoplasms, Caspases, Genetic polymorphism, Prognosis
Introduction
Anatomic and pathologic staging remain the most accurate predictors of clinical outcomes in patients with colorectal cancer, enabling physicians to evaluate the benefit of adjuvant chemotherapy for individual patients. However, supplementing standard clinical and pathologic staging using molecular markers would allow more precise identification of those patients with the highest, or lowest, risk of relapse following colorectal cancer surgery. One of the most promising molecular markers that have been investigated in relation to colorectal cancer is the presence of tumor microsatellite instability. In a meta-analysis, colorectal cancers with microsatellite instability had a significantly better prognosis compared to those with intact mismatch repair, and these tumors may be resistant to treatment with 5-fluorouracil [1].
Apoptosis is an essential physiological process in maintaining normal tissue homeostasis, cellular differentiation and development [2,3]. It is well known that defects in apoptosis facilitate the accumulation of somatic mutations which thereby contribute to tumor initiation, progression, and metastasis [4-6]. Caspases (CASPs), a family of cystein-dependent aspartate-specific proteases, play a crucial role in the regulation and execution of apoptosis [7-9], and based on their proapoptotic function, they can be separated into initiator and effector CASPs. Initiator CASPs (CASP8, CASP9, and CASP10) transmit apoptotic signals, and activation of effector CASPs (CASP3, CASP6, and CASP7) function to execute the final cell death program [7-10].
Many polymorphisms identified in the CASP genes are putatively functional, and clinical studies have demonstrated that these polymorphisms are involved in the development of solid tumors such as colorectal [11], lung [12,13], breast [14], and malignant melanoma [15]. For example, CASP9 gene polymorphisms and their haplotypes, -1263A>G (rs4645978) and -712C>T (rs4645981), have been reported to both affect CASP9 expression and modulate lung cancer risk [12]. The V410I (rs13010627G>A) and I522L (rs13006529A>T) polymorphisms of the CASP10 gene have been reported to be associated with the risk of developing cutaneous melanoma and familial breast cancer [15,16]. Furthermore, the CASP7 rs2227310 and CASP9 rs4645981 polymorphisms have been identified as independent prognostic markers for patients with surgically resected, non-small cell lung cancer (NSCLC) [17]. Given these results, CASP gene polymorphisms appear to play a role in the carcinogenesis or prognosis of solid tumors.
Nonetheless, relatively few studies have investigated the single nucleotide polymorphisms (SNPs) in the CASP genes and their relationship to the clinical outcomes of colorectal cancer. Accordingly, this study analyzed 10 CASP gene polymorphisms and evaluated their impact on the prognosis of colorectal cancer patients.
Materials and Methods
1. Study population
All the tissues investigated in this study were obtained from 397 consecutive, ethnic Korean, colorectal cancer patients who had undergone a curative resection between January 2003 and August 2006, at Kyungpook National University Hospital (Daegu, Korea). Written informed consent for gene expression analyses was received from all participating patients prior to surgery, and the study was approved by the Kyungpook National University Hospital Institutional Research Board. The diagnosis and staging of the colorectal cancer data was performed according to World Health Organization (WHO) classifications [18] and the tumor, node, and metastasis (TNM) classifications set by the American Joint Committee on Cancer (AJCC) [19].
2. Selection of CASP gene polymorphisms
Due to the enormous number of SNPs in the human genome, an appropriate strategy for efficient selection of those SNPs most likely to contribute phenotypic effects was our first challenge. Thus, a prioritization scheme was created using public databases providing diverse information on potential phenotypic risks associated with specific SNPs. First, candidate SNPs from CASP genes were collected from web-based databases which included information on the biologic pathways and potential biologic effects of these polymorphisms. Next, based on the allele frequencies recorded for East Asian populations obtained from FASTSNP, those SNPs with frequencies less than 0.1 were excluded. The remaining CASP gene SNPs were then scored according to particular phenotypic risks, and then, based on the algorithm suggested in a previous report [20], they were ordered according to the sum of their risk scores. Among the 13 polymorphisms in the CASP3, CASP6, CASP7, CASP8, CASP9, and CASP10 genes that have been reported to be potentially functional or otherwise associated with cancer risk [11-16], ten polymorphisms (CASP3rs2705897; CASP6 rs1042891, rs2301717; CASP7 rs2227310, rs11593766; CASP8 rs3769818, rs3834129; CASP9 rs1052571, rs4645978; CASP10 rs13006529) were examined. CASP8 rs1045485 and CASP10 rs13010627 were excluded as they are rare or nonexistent in Asian populations [11,21].
3. Genotyping CASP gene polymorphisms
Genomic DNA was extracted from fresh colorectal mucosal tissue at the time of surgery using a Wizard genomic DNA purification kit (Promega, Madison, WI). The 10 selected CASP gene polymorphisms were then determined using a reverse transcription polymerase chain reaction (PCR) genotyping assay. For quality control, the genotyping analysis was performed blind as regards the subjects.
The selected, PCR-amplified DNA samples (n=2, for each genotype) were also examined by DNA sequencing to confirm the genotyping results.
4. Statistical analysis
The genotypes for each SNP were analyzed as a categorical variable for three-groups (reference model), and also grouped according to a dominant and recessive model. The survival estimates were calculated using the Kaplan-Meier method. As related to the appearance of SNPs of the CASP genes, the differences in patient overall survival (OS) or disease-free survival (DFS), were compared using log-rank tests. Cox's proportional hazard regression model was used for the multivariate survival analyses, whereby the analyses were adjusted for potential prognostic factors including age (median age, 63 years;≤63 years vs.>63 years), differentiation (well to poor differentiation), preoperative carcinoembryonic antigen (CEA) level (normal vs. elevated), and pathologic stage (0 to IV). The hazard ratio and 95% confidence interval were also estimated. A cutoff p-value of 0.05 was adopted for all statistical analyses. The statistical data were obtained using an SPSS ver. 11.5 (SPSS Inc., Chicago, IL) or SAS Genetic software (SAS Institute, Cary, NC).
Results
1. Patient characteristics and survival analysis
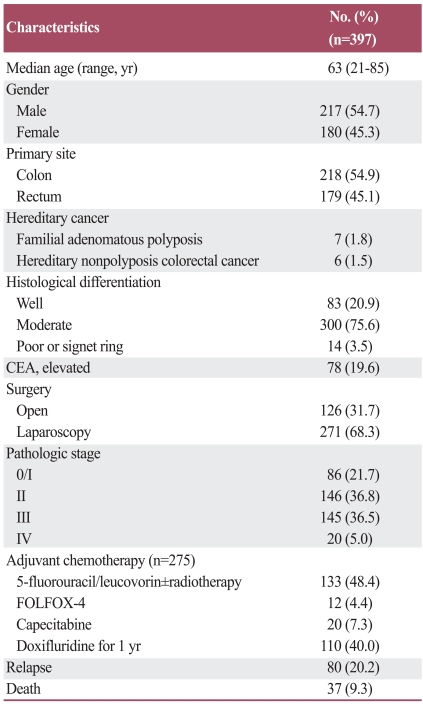
The median age of the patients was 63 years (range, 21 to 85 years), and 217 (54.7%) of the total patients were male. Of the total patients, 218 (54.9%) were diagnosed with colon cancer and the remaining with rectal cancer. Laparoscopic surgery was performed on 271 (68.3%) patients, while the others received an open colorectal resection. The pathologic stages after the surgical resection were as follows: stage 0/I (n=86, 21.7%), stage II (n=146, 36.8%), stage III (n=145, 36.5%), and stage IV (n=20, 5.0%). Among the 291 patients with stage II or III disease, 275 (94.5%) received adjuvant chemotherapy using either 6 cycles of 5-fluorouracil/leucovorin ± radiotherapy (n=133), 12 cycles of oxaloplatin, leucovorin, and fluorouracil (FOLFOX-4) (n=12), 8 cycles of capecitabine (n=20), or doxifluridine during a treatment period of one year (n=110) (Table 1).
Table 1.
Patient characteristics
CEA, carcinoembryonic antigen; FOLFOX-4, oxaloplatin, leucovorin, and fluorouracil.
At the time of the last analysis, 80 patients had experienced disease relapse and 37 had died as a result of colorectal cancer disease progression. However, the deaths of 2 patients were unrelated to colorectal cancer. At the median follow-up duration of 34.8 months (range, 1.1 to 65.7 months), the estimated 5-year OS and DFS for all the patients was 89.3±1.6% and 72.3±4.3%, respectively. Survival results differed according to the pathologic stage (p<0.001).
2. Association of genotype frequency with clinicopathologic features and survival
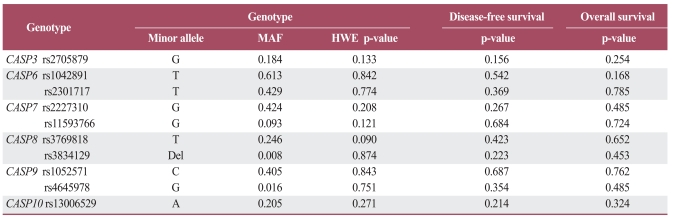
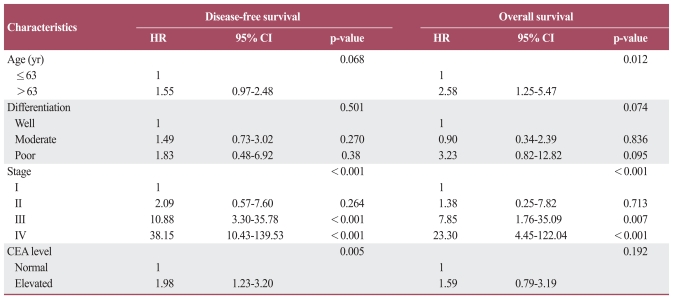
The 10 CASP gene SNPs were successfully amplified in more than 95% of patients. The frequencies of occurrence for each genotype also conformed to the Hardy-Weinberg equilibrium (p>0.05). There were no sexual differences observed in relation to any genotype and allele. No correlation was observed between any given frequency of the genotype or allele and clinicopathologic parameters, including for T, N, or M stage patients (data not shown). A multivariate survival analysis including pathologic stage, patient age, differentiation, and CEA level demonstrated there was no association between any genotypes with outcomes for DFS or OS (Table 2). In haplotype analysis of the CASP6, CASP8 and CASP9 genes, none were associated with colorectal cancer prognosis. In terms of clinicopathologic parameters in a Cox model, patient age, CEA level, and TNM stage were all significant prognostic factors for DFS and OS (Table 3).
Table 2.
Genotype frequencies and multivariate survival analysis
The genotypes for each single-nucleotide polymorphism were analyzed as categorical variables in three-groups (reference model). p-values correspond to multivariate analysis adjusted for age, differentiation, carcinoembryonic antigen level, and pathologic stage. MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium test; CASP, CASPASE.
Table 3.
Multivariate survival analysis
p-values correspond to multivariate Cox model adjusted for age, differentiation, CEA level, and pathologic stage. HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
Discussion
The prognostic impact of 10 SNPs of CASP genes was investigated in a large population of patients with curatively resected colorectal adenocarcinoma. However, no association was observed between the polymorphisms in the CASP genes and survival in these patients. Given the homogenous ethnic background of the patients (Korean), any potential confounding effect due to ethnicity is likely to be small in the present study.
Since the CASP pathway plays an important role in balancing cell growth and death, it is possible that germ line polymorphisms in these genes may affect an individual's cancer risk or prognosis. In a previous study by Yoo et al. [17], significant associations between the CASP7 rs2227310 and CASP9 rs4645981 polymorphisms and prognosis were found in 411 Korean patients with early stage NSCLC. However, these same polymorphisms were found to have no prognostic significance in the survival rate of colorectal cancer patients in the current study. In the Yoo et al.'s study [17], the CASP9 rs4645981 TT genotype was found to be significantly associated with poor survival outcomes, suggesting that a lower production genotype for CASP9 may have decreased the capacity for CASP9 to mediate the apoptotic activity required to eliminate mutated or transformed cells, which would thus increase the risk of lung cancer development and lead to poor survival outcomes in patients with early-stage NSCLC. Although it remains to be elucidated whether or not the CASP7 rs2227310C>G polymorphism itself affects protein characteristics, interaction sites, or protein solubility or stability, this polymorphism was associated with poor survival outcomes for patients suffering with NSCLC [17].
Relatively few studies have investigated these polymorphisms and their relationship to the risks or clinical outcomes of other solid tumors, including colorectal cancer, and the results are inconsistent [11,14,15,22-24]. For example Liu et al. [22], reported that 2 caspase-8 SNPs were not associated with colorectal cancer risk in a study conducted with 373 Chinese patients and 838 controls. In our previous study that analyzed 15 SNPs of apoptosis-related genes and their impact on the response to chemotherapy and survival of colorectal cancer patients, no significant association between patient response or survival and the polymorphisms of the CASP3 or CASP6 to CASP10 genes was observed [23].
Given that validation of genotype-phenotype association studies requires replication using an independent data set, additional studies are required to confirm the associations between CASP gene polymorphisms and survival outcomes in the cancer patients observed in our previous study.
Conclusion
None of the 10 CASP gene SNPs investigated in this study was found to be an independent prognostic marker for Korean patients with curatively resected colorectal cancer. However, since genetic polymorphisms often vary between ethnic groups, further studies clarifying the association between these polymorphisms and the prognosis of colorectal cancer in diverse ethnic populations are warranted.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 4.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 5.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 6.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: a review. J Cell Biochem. 2003;88:885–898. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 8.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 9.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 10.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384(Pt 2):201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J, et al. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat Genet. 2007;39:605–613. doi: 10.1038/ng2030. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Park JM, Jang JS, Choi JE, Kim KM, Cha SI, et al. Caspase 9 promoter polymorphisms and risk of primary lung cancer. Hum Mol Genet. 2006;15:1963–1971. doi: 10.1093/hmg/ddl119. [DOI] [PubMed] [Google Scholar]
- 13.Jang JS, Kim KM, Choi JE, Cha SI, Kim CH, Lee WK, et al. Identification of polymorphisms in the Caspase-3 gene and their association with lung cancer risk. Mol Carcinog. 2008;47:383–390. doi: 10.1002/mc.20397. [DOI] [PubMed] [Google Scholar]
- 14.MacPherson G, Healey CS, Teare MD, Balasubramanian SP, Reed MW, Pharoah PD, et al. Association of a common variant of the CASP8 gene with reduced risk of breast cancer. J Natl Cancer Inst. 2004;96:1866–1869. doi: 10.1093/jnci/dji001. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Zhao H, Hu Z, Liu Z, Wang LE, Gershenwald JE, et al. Genetic variants and haplotypes of the caspase-8 and caspase-10 genes contribute to susceptibility to cutaneous melanoma. Hum Mutat. 2008;29:1443–1451. doi: 10.1002/humu.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank B, Hemminki K, Wappenschmidt B, Meindl A, Klaes R, Schmutzler RK, et al. Association of the CASP10 V410I variant with reduced familial breast cancer risk and interaction with the CASP8 D302H variant. Carcinogenesis. 2006;27:606–609. doi: 10.1093/carcin/bgi248. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SS, Choi JE, Lee WK, Choi YY, Kam S, Kim MJ, et al. Polymorphisms in the CASPASE genes and survival in patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:5823–5829. doi: 10.1200/JCO.2009.23.1738. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SR, Aaltonen LA. Pathology and genetics: tumours of the digestive system. Lyon: IARC Press; 2000. World Health Oorganization classification of tumors. [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 20.Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, Lin YJ, et al. FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34(Web Server issue):W635–W641. doi: 10.1093/nar/gkl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son JW, Kang HK, Chae MH, Choi JE, Park JM, Lee WK, et al. Polymorphisms in the caspase-8 gene and the risk of lung cancer. Cancer Genet Cytogenet. 2006;169:121–127. doi: 10.1016/j.cancergencyto.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Zhang Y, Jin M, Ni Q, Liang X, Ma X, et al. Association of selected polymorphisms of CCND1, p21, and caspase8 with colorectal cancer risk. Mol Carcinog. 2010;49:75–84. doi: 10.1002/mc.20579. [DOI] [PubMed] [Google Scholar]
- 23.Kim JG, Chae YS, Sohn SK, Moon JH, Ryoo HM, Bae SH, et al. Prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) 8473T>C polymorphism associated with prognosis for patients with colorectal cancer treated with capecitabine and oxaliplatin. Cancer Chemother Pharmacol. 2009;64:953–960. doi: 10.1007/s00280-009-0947-3. [DOI] [PubMed] [Google Scholar]
- 24.Chae YS, Kim JG, Sohn SK, Lee SJ, Kang BW, Moon JH, et al. RIPK1 and CASP7 polymorphism as prognostic markers for survival in patients with colorectal cancer after complete resection. J Cancer Res Clin Oncol. 2011;137:705–713. doi: 10.1007/s00432-010-0929-1. [DOI] [PubMed] [Google Scholar]