Abstract
Purpose
The androgen receptor (AR) plays a central role in prostate cancer. Evidence from several groups indicates that epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) may enhance AR activity in prostate cancer cell lines. This study was designed to investigate the protein expression of AR, EGFR, and HER2 and to determine whether the EGFR and HER2 genes are amplified in prostate cancer tissues.
Materials and Methods
The protein expression levels of AR, EGFR, and HER2 in a tissue microarray block of 66 prostate cancer samples were investigated by immunohistochemical analysis and chromogenic in situ hybridization was used to determine whether the EGFR and HER2 genes were amplified in these tissues.
Results
The AR and EGFR proteins were expressed in 59.1% and 40.9% of prostate cancers, respectively, but their expression levels were not significantly associated with clinicopathologic factors. Of the cases in which tissues were negative for EGFR protein expression, 69.2% were positive for AR protein expression; however, AR protein expression was significantly reduced (44.4%) in tissues in which EGFR protein was expressed. HER2 expression was detected in only 1 case (1.5%). No amplification of the EGFR or HER2 genes was found in prostate cancer specimens.
Conclusion
This study was limited by small number of subjects, but it can still be inferred that the expression levels of the AR and EGFR proteins are inversely correlated in prostate cancer patients. The potential utility of EGFR and HER2 as prognostic factors or therapeutic targets warrants further study.
Keywords: Prostatic neoplasms, Androgen receptors, Epidermal growth factor receptor, HER2
Introduction
Androgen receptor (AR) plays a central role in prostate cancer, and androgen-deprivation therapy is therefore the standard initial systemic treatment; however, the tumors eventually recur despite androgen levels consistent with castration [1]. Such castration-resistant prostate cancers express high levels of AR mRNA, AR protein, and androgen-regulated genes, indicating that AR transcriptional activity has been reactivated. Prostate cancer therefore appears to androgen deprivation through multiple mechanisms that generate adequate AR activity despite castration-compatible levels of circulating androgens [2]. Previous studies have suggested that the progression to hormone-refractory disease may be associated with epidermal growth factor receptor (EGFR), epidermal growth factor (EGF), amphiregulin, and/or transforming growth factor-α (TGF-α). TGF-α and EGF bind to EGFR, and thus initiate tyrosine kinase activity, which can leads to the activation of gene expression, cell proliferation, and cell survival [3]. EGFR and human epidermal growth factor receptor 2 (HER2) also contribute to enhanced AR activity in castration-resistant prostate cancers. Studies on prostate cancer cell lines and xenograft models have found increased EGFR or HER2 expression levels in tumors that recur after castration, although this result is not consistently reproduced in patient samples [4,5]. EGF can increase AR transactivation when androgen levels are low, and activation of elements downstream of EGFR may also enhance AR activity [6].
HER2 has been shown to enhance AR activity and cell growth [7]. Other studies have shown that HER2 can enhance AR stability and that inhibition of HER2 decreases AR DNA-bindings activity in the presence of low androgen levels [4,8]. HER2 signaling has also been reported to negatively regulate AR expression and activity. In a previous study, EGF was shown to decrease the mRNA expression of AR and androgen-regulated genes in LNCap cells [9]. Other groups have shown that the binding of heparin to EGF decreases AR protein expression through activation of mammalian target of rapamycin and decreased translation AR mRNA [10,11].
Expression of HER2 and EGFR has been associated with advanced-stages disease, metastasis, shortened survival, poor response to chemotherapy, and even the failure of endocrine therapy [12]. Signoretti et al. [13] demonstrated that an initially minor population of HER2-positive tumor cells gradually increased, with progression toward androgen-independent prostate cancer, further justifying the targeting of HER2 in androgen-independent disease. However, Oxley et al. [14] detected increased HER2 oncogene copy number only rarely in prostate cancers. Therefore, the HER2 oncogene copy number would not likely useful biomarkers for identifying patients whose cancer was likely to recur after radical prostatectomy.
We undertook this study with the following aims: 1) to determine whether AR, EGFR, and HER2 proteins are expressed in human prostate cancer; 2) to assess whether the protein expression of AR, EGFR, and HER2 correlates with clinicopathologic factors in prostate cancer; and 3) to determine whether the EGFR and HER2 genes are amplified in human prostate cancer.
Materials and Methods
A total of 66 radical prostatectomy samples diagnosed as prostatic adenocarcinoma and 30 transurethral resection samples diagnosed as benign prostatic hyperplasia (BPH) collected between 2005 and 2009 were obtained from Chung-Ang University Hospital. The samples were fixed with formalin and embedded in paraffin.
Immunohistochemical analysis and chromogenic in situ hybridization were performed using the tissue microarray (TMA) technique; this method allows staining of a large number of specimens on 1 slide. TMAs were prepared manually using a punch biopsy needle (Beecher Instruments Inc., Sun Prairie, WI). To reduce the effects of tumor heterogeneity, cylindrical core biopsies (2.0 mm in diameter) were extracted from 3 different sites of each tumor, and these cores were arrayed in a recipient paraffin TMA block.
Tissue sections were mounted on poly-L-lysine coated slides for immunohistochemical analysis using the EnVision System Kit (Lab Vision, Fremont, CA). Anti-AR (1 : 50, Lab Vision), EGFR (1 : 50, Lab Vision), and HER2 (1 : 50, Lab Vision) primary antibodies were used in this study. Briefly, the TMA sections were deparaffinized, and hydrated and the endogenous peroxidase activity blocked using standard procedures, and then subjected to microwave antigen retrieval followed by incubation at 121℃ for 10 minutes. The sections were washed with normal goat serum for 10 minutes, and then incubated with pre-diluted primary antibodies at room temperature for 60 minutes. The sections were then allowed to react with peroxidase-conjugated streptoavidin for 45 minutes. The color developed using diaminobenzidine, and counterstaining performed with hematoxyline.
The samples were considered positive for AR expression if more than 10% of the neoplastic cells showed a nuclear staining. EGFR and HER2 expression levels were scored and categorized as 0, 1+, 2+, or 3+, as described for the Food and Drug Administration (FDA)-approved HercepTest; expression was considered to be positive at a level of 2+.
For in situ hybridization, the TMA sections were deparaffinized, dehydrated, air-dried, and incubated in boiling pre-treatment buffer for 15 minutes using a SPoT-Light FFPE reagent kit (Lab Vision). Enzymatic digestion was performed with SPoT-Light FFPE digestion enzyme (Lab Vision) at room temperature for 2-3 minutes. After dehydration, the histological slides were air-dried and the ready to use double-stranded DNA digoxigenin-labelled EGFR and HER2 probes (Lab Vision) applied. The samples were denature by incubating the slides, which were covered with a CISH cover-slip, on a 96℃ heat block for 5 minutes, and then hybridized in a humidity chamber at 37℃ overnight. The cover-slips were then removed and the samples stringently washed with 0.5× saline sodium citrate buffer at 80℃ for 5 minutes. Endogenous peroxidase activity and non-specific staining were blocked with 3% hydrogen peroxide and CAS-Block, respectively. A mouse antidigoxigenin antibody was added to the slides and allowed to hybridized with the EGFR and HER2 probes at room temperature for 45 minutes. The samples were then incubated with a peroxidase-conjugated goat anti-mouse antibody (Lab Vision) at room temperature for 45 minutes. A DAB chromogen substrate system was used to generate a sensitive signal that could be viewed after counterstaining with Mayer's hematoxylin.
The relationships between the different parameters were determined by Fisher's exact test and a Pearson chi-square test using SPSS ver. 12.0 (SPSS Inc., Chicago, IL) statistical software. A p-value less than 0.05 was considered statistically significant.
Results
The median age of the prostatic adenocarcinoma patient was 67.2 years (range, 49 to 80 years). The median pre-operative serum total prostate-specific antigen (PSA) was 15.26 ng/mL (range, 1.87 to 258.2 ng/mL). Twelve of 66 patients (18.2%) were classified as stage T2a/T2b, 32 (48.5%) as T2c, 14 (21.2%) as T3a, and 8 (12.1%) as T3b. At the time of radical prostatectomy, 3 patients (4.5%) had cancer in lymph nodes and 10 (15.2%) had positive surgical margins.
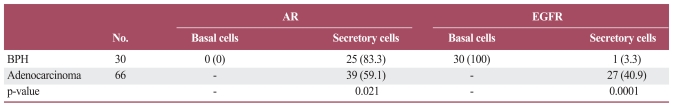
AR expression was observed in 25 of 30 (83.3%) cases of BPH (Table 1). The staining was observed predominantly in the nuclei of secretory cells. Benign prostate glands stained homogeneously, whereas both intensity and extent of staining were heterogeneous in prostate cancers (Fig. 1A and B). AR expression was observed in 39 of 66 (59.1%) cases prostatic adenocarcinoma. AR expression did not correlate significantly with age, pre-operative PSA level, Gleason score, nodal metastasis, or surgical margin status. The frequency of AR expression was highest in the group with the highest pre-operative PSA levels, the highest Gleason scores, and the presence of nodal metastasis, although these association were not statistically significant (Table 2).
Table 1.
Expressions of AR and EGFR proteins in BPH and adenocarcinoma
Values are presented as number (%). AR, androgen receptor; EGFR, epidermal growth factor receptor; BPH, benign prostatic hyperplasia.
Fig. 1.
Expression of androgen receptor. (A) Positive immunoreactivity in benign prostatic glands and stroma (AEC staining, ×40). (B) Strong immunoreactivity in prostatic adenocarcinoma (AEC staining, ×400).
Table 2.
Correlation of AR and EGFR expression with clinicopathologic factors in 66 prostate adenocarcinomas
Values are presented as number (%). AR, androgen receptor; EGFR, epidermal growth factor receptor; Pre-op PSA, pre-operative serum total prostate-specific antigen; NS, not significant.
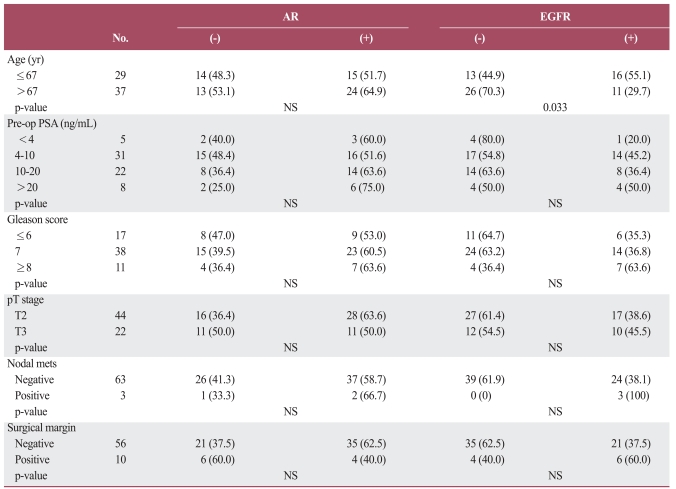
EGFR expression was observed in the basal cells of all cases and secretory cells of 1 case (3.3%) of BPH (Table 1). EGFR expression was observed in 27 of 66 (40.9%) cases of prostatic adenocarcinoma (Fig. 2A and B). The median age of the patients was 67.2 years, when the patient 67 years of age or younger were compared with those over 67 years of age, the percentage of cancers positive for EGFR expression was found to be significantly higher (p=0.033) in the younger (≤67 years old) group. EGFR expression did not correlate significantly with pre-operative PSA levels, Gleason scores, tumor stage, or surgical margin status. The frequency of EGFR expression was highest in the group with the highest pre-operative PSA levels, and the highest Gleason scores, the most advanced stages of cancer, the presence of nodal metastasis, and positive surgical margins, although these association were not statistically significant.
Fig. 2.
Expression of epidermal growth factor receptor. (A) Strongly positive immunoreactivity in benign prostatic glands and weakly positive immunoreactivity in prostatic adenocarcinoma (AEC staining, ×40). (B) Strong membrane staining in prostatic adenocarcinoma (AEC staining, ×400).
Of the cases in which tissues were negative for EGFR protein expression, 69.2% were positive for AR protein expression; however, AR protein expression was significantly reduced (44.4%, p=0.044) (Table 3) in tissues in which EGFR protein was expressed.
Table 3.
Correlation of EGFR and AR expression levels in 66 prostate adenocarcinomas
Values are presented as number (%). EGFR, epidermal growth factor receptor; AR, androgen receptor.
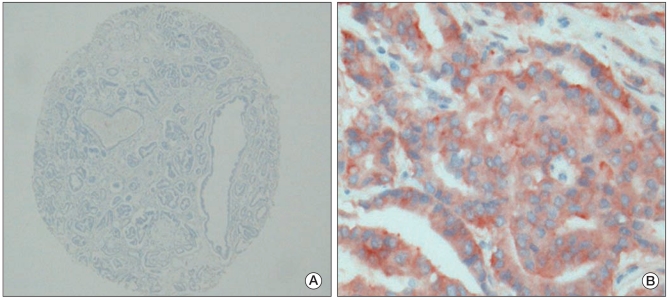
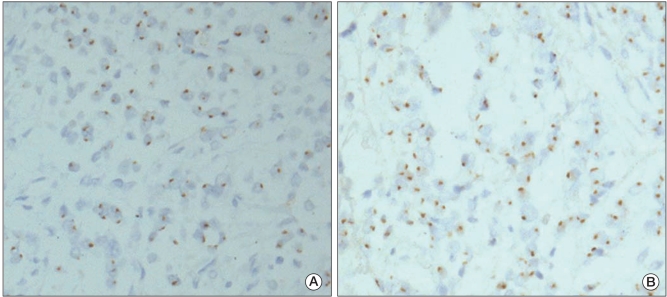
An HER2 protein expression levels of 2+ was observed in only 1 (Fig. 3A and B). The results of chromogenic in situ hybridization indicated no amplification of either the EGFR gene or HER2 gene in any of the 66 cases (Fig. 4A and B).
Fig. 3.
Expression of human epidermal growth factor receptor 2. (A) Negative immunoreactivity in normal prostatic glands and prostatic adenocarcinoma (AEC staining, ×40). (B) Weakly membrane staining in prostatic adenocarcinoma (AEC staining, ×400).
Fig. 4.
Epidermal growth factor recptor (EGFR) (A) and human epidermal growth factor recptor 2 (HER2) (B) gene amplification examined by chromogenic in situ hybridization. One or 2 signals are visible in nuclei of adenocarcinoma cells. There is no evidence of gene amplification (CISH staining, ×400).
Discussion
Prostate cancer is the most frequent cancers in Western countries and one of the most rapidly increasing malignant diseases in Korean men. This disease varies greatly over its clinical course, ranging from indolent to lethal. The investigation of the pathogenesis of prostate cancer is very challenging, and only a handful of the molecular and genetic factors involved have been identified thus far [15]. However, androgen-related factors, including androgen metabolism and receptor signaling, correlate strongly with tumor development and progression [1].
The prostate gland depends on androgen stimulation for its development and growth. However, testosterone is not the major androgen responsible prostate growth. Testosterone is converted into dihydrotestosterone (DHT) in prostatic stromal and basal cells by the enzyme delta 3-ketosteroid 5-alpha-reductase. DHT is primarily responsible for prostate development and the pathogenesis of BPH [16]. Androgens significantly alter prostate cancer growth rates, and the progression of prostate cancer from preclinical to clinically significant forms may result in part from altered androgen metabolism. Elevated concentrations of testosterone and its metabolite, DHT may increase prostate cancer risk over many decades, but results have been inconsistent in this area. Both endogenous and exogenous factors may affect hormone levels [17]. Studies of AR gene regulation have implicated the AR itself as a self-regulating transcription factor that binds the AR gene to increased AR mRNA levels. Other mechanisms such as increased kinase pathway signaling (HER2, Ras, or mitogen-activated protein kinase) and altered co-activator/co-repressor ratios, are postulated to increase AR activity and may also lead, albeit indirectely, to increased AR mRNA levels. Any primary molecular event that alters AR activity could thus increase AR mRNA levels, suggesting a common final pathway for escape from standard hormone therapy [18]. Despite treatment, prostate cancer eventually recurs as an androgen- or hormone-independent tumor. The molecular basis for progression to hormone independent-cancer is poorly understood. One hypothesis is that the function of AR is altered by gene amplification of AR or a mutation in the hormone-binding domain, the latter occurs in 20-30% of androgen-independent prostate tumors. A previous study showed that mutation can alter the specificity of the ligand-binding domain to allow the mutant AR to bind and respond to other steroid hormones, such as estrogen [2]. An alternative model is that AR activation despite clinical androgen deprivation is due to the recruitment of non-steroid receptor signal transduction pathways [4].
In this study, AR expression was observed in 59.1% and 83.3% of prostatic adenocarcinoma and BPH cases, respectively. The frequency of AR expression in adenocarcinoma cases was highest in the group with the highest pre-operative PSA levels, the highest Gleason scores, and the presence of nodal metastasis, although these association were not statistically significant. Together, these results support the hypothesis that increased levels of AR transcripts may be of particular importance in promoting tumor cell growth, as indicated in a previous study [2].
EGFR is overexpressed in many cancer, including prostate, kidney, and urinary bladder carcinomas. The overexpression of EGFR in these carcinomas correlates with poor prognosis and decreased survival. Many agents are used to inhibit EGFR function, including neutralizing monoclonal anti-EGFR antibodies, receptor tyrosine kinase inhibitors, and monoclonal antibody-conjugated toxins. Of these antibodies, cetuximab has received the most attention and has recently been approved by the US FDA for use in cases of refractory colorectal cancer [19]. Marks et al. [3] found that EGFR gene expression was detectable in 35% of a large series of hormone-treated prostate cancers, and that EGFR protein was frequently expressed in tissues from these patients. EGFR over-expression may be a reasonable target for therapeutic intervention in this otherwise difficult to treat subset of prostate cancers.
In this study, EGFR protein expression was observed in 40.9% and 3.3% of prostatic adenocarcinoma and BPH cases, respectively. The frequency of EGFR expression was highest in the group with the highest pre-operative PSA levels, the highest Gleason scores, most advanced stages of cancer, the presence of nodal metastasis, positive surgical margins, although these association were not statistically significant. Early studies using immunohistochemical analysis found EGFR expression in 35.2% of prostate cancers. Of the several variables examined by univariate analysis, high serum PSA values (≥20), extraprostatic extension, seminal vesicle invasion, and EGFR expression were significant predictors of biochemical recurrence [20]. These results are consistent with those of several previous studies describing a correlations between EGFR expression and prostate cancer progression.
Cai et al. [21] described negative regulation of AR mRNA expression by EGFR and HER2, which may provide an approach to the suppression of AR expression in castration-resistant prostate cancer. Previous studies have indicated that EGF can enhance AR activity and HER2 can enhance AR activity and responses to low levels of androgen [7]. However, other studies in LNCap cells found that EGF and heparin binding-EGF decrease AR expression [10]. In the present study, we observed that AR protein was expressed in 69.2% of cases in which EGFR protein was not expressed, but that AR protein expression was significantly reduced when EGFR protein was expressed. These data suggest that the expression levels of the AR and EGRF proteins are inversely correlated in prostate cancer patient.
Our results indicated no amplification of the EGFR gene in any of the 66 cases. Changes in EGFR gene copy number are infrequent in prostate cancers. Most altered tumors have only a low-level EGFR copy number increase (3-4 copies), such increase were found in 2.7% of prostate cancer. Schlomm et al. [22] reported that fluorescence in situ hybridization analysis with a dual-labeling probe for centromere 7 and EGFR showed increased EGFR copy numbers in 3.3% of cases. This amplification was heterogeneous, involving only approximately 30% of the cancer volume. No EGFR mutations were found in 35 of the cases analyzed. Taken together, these results showed no specific increase in gene copy number and no gene amplification.
Previous studies suggested that, statistically, there might be 2 or 3 cases of abnormal gene copy number in our study. However, no such abnormalities were observed in this study. This may be because the relatively small number of specimens in our study, did not allow detection of significant correlation between EGFR protein expression and gene amplification.
The HER2 oncogene encodes a transmembrane tyrosine kinase receptor with extensive homology to EGFR. HER2 testing is used to predict the response of breast cancer to trastuzumab [23]. The HER2 oncogene is involved in the biology of many different types of tumors and serves as a prognostic marker and a therapeutic target in breast cancer. In contrast to the consensus for breast cancer, studies on HER2 overexpression and gene amplification in prostate cancer have yielded variable results. In a previous study, a significant increase in copy number was present in 1.75% of prostate cancers, but chromosome 17 hybridization showed that the increase was the result of polysomy rather than true amplification [14]. In our study, a HER2 protein expression levels greater than 2+ was observed in only 1 case; unlike in previous studies, no gene amplification was observed. Minner et al. [24] reported that detectable HER2 immunostaining in 17.2% of prostate cancers, with the vast majority of cases showing 1+ or 2+ staining. Low-level HER2 overexpression occurs with relevant frequency in prostate cancer and in the absence of gene amplification [24]. These data argue for reconsidering anti-HER2 therapy.
Conclusion
No clinicopathologic factors was statistically significantly correlated with AR or EGFR protein expression. AR and EGFR protein expression levels are inversely correlated in prostate cancer patients. The frequency of detectable HER2 protein expression was very low, and no amplification of the EGFR or HER2 genes was observed. Further investigation of EGFR and HER2 as prognostic factors and therapeutic targets in prostate cancer is warranted.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks RA, Zhang S, Montironi R, McCarthy RP, MacLennan GT, Lopez-Beltran A, et al. Epidermal growth factor receptor (EGFR) expression in prostatic adenocarcinoma after hormonal therapy: a fluorescence in situ hybridization and immunohistochemical analysis. Prostate. 2008;68:919–923. doi: 10.1002/pros.20715. [DOI] [PubMed] [Google Scholar]
- 4.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 5.Calvo BF, Levine AM, Marcos M, Collins QF, Iacocca MV, Caskey LS, et al. Human epidermal receptor-2 expression in prostate cancer. Clin Cancer Res. 2003;9:1087–1097. [PubMed] [Google Scholar]
- 6.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Gregory CW, Whang YE, McCall W, Fei X, Liu Y, Ponguta LA, et al. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005;11:1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 8.Mellinghoff IK, Tran C, Sawyers CL. Growth inhibitory effects of the dual ErbB1/ErbB2 tyrosine kinase inhibitor PKI-166 on human prostate cancer xenografts. Cancer Res. 2002;62:5254–5259. [PubMed] [Google Scholar]
- 9.Henttu P, Vihko P. Growth factor regulation of gene expression in the human prostatic carcinoma cell line LNCaP. Cancer Res. 1993;53:1051–1058. [PubMed] [Google Scholar]
- 10.Adam RM, Kim J, Lin J, Orsola A, Zhuang L, Rice DC, et al. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143:4599–4608. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 11.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 12.Souder C, Leitzel K, Ali SM, Demers L, Evans DB, Chaudri-Ross HA, et al. Serum epidermal growth factor receptor/HER-2 predicts poor survival in patients with metastatic breast cancer. Cancer. 2006;107:2337–2345. doi: 10.1002/cncr.22255. [DOI] [PubMed] [Google Scholar]
- 13.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 14.Oxley JD, Winkler MH, Gillatt DA, Peat DS. Her-2/neu oncogene amplification in clinically localised prostate cancer. J Clin Pathol. 2002;55:118–120. doi: 10.1136/jcp.55.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon CY, Byun SS. Pathogenesis of prostate cancer. J Korean Med Assoc. 2010;53:98–106. [Google Scholar]
- 16.Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001;58(6 Suppl 1):17–24. doi: 10.1016/s0090-4295(01)01299-7. [DOI] [PubMed] [Google Scholar]
- 17.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 18.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 19.Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11:397–405. [PubMed] [Google Scholar]
- 20.Lee JW, Cho KS, Han KS, Kim EK, Joung JY, Seo HK, et al. Epidermal growth factor receptor as predicting factor on biochemical recurrence after radical prostatectomy: a prospective study. Korean J Urol. 2008;49:974–980. [Google Scholar]
- 21.Cai C, Portnoy DC, Wang H, Jiang X, Chen S, Balk SP. Androgen receptor expression in prostate cancer cells is suppressed by activation of epidermal growth factor receptor and ErbB2. Cancer Res. 2009;69:5202–5209. doi: 10.1158/0008-5472.CAN-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlomm T, Kirstein P, Iwers L, Daniel B, Steuber T, Walz J, et al. Clinical significance of epidermal growth factor receptor protein overexpression and gene copy number gains in prostate cancer. Clin Cancer Res. 2007;13(22 Pt 1):6579–6584. doi: 10.1158/1078-0432.CCR-07-1257. [DOI] [PubMed] [Google Scholar]
- 23.Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 24.Minner S, Jessen B, Stiedenroth L, Burandt E, Köllermann J, Mirlacher M, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]