Abstract
Purpose
Non-small cell lung carcinoma (NSCLC) comprises 75-85% of all lung cancers, and approximately 25% of all NSCLC patients develop brain metastasis. There are no reliable markers for predicting in which patients this metastasis will occur. DCUN1D1, also known as squamous cell carcinoma-related oncogene, is associated with tumor progression and poor outcomes in NSCLC. The objective of this study was to investigate the role of DCUN1D1 expression in cases of brain metastasis due to NSCLC.
Materials and Methods
Primary tumor samples from a total of 71 cases of NSCLC, either with (n=40) or without (n=31) brain metastasis, were evaluated for DCUN1D1 expression by immunohistochemistry analysis.
Results
DCUN1D1 expression was detected in 16 patients (23%) and tended to correlate with T classification (15% of T1-2 tumors vs. 30% of T3-4 tumors, p=0.083). DCUN1D1 expression was significantly associated with tumor stage. It was observed in none of the patients with stage I disease, 10% of those with stage II disease, and 29% with stage III disease (p=0.009). In addition, 14 of 16 DCUN1D1-positive patients resulted in brain metastasis (p=0.01). The odds ratio of brain metastasis for patients with DCUN1D1 expression was 3.112 (p=0.009).
Conclusion
DCUN1D1 expression may play a role in tumor progression and development of brain metastasis in patients with NSCLC. Evaluation of DCUN1D1 expression may provide assistance in identifying those patients who are at higher risk for brain metastasis.
Keywords: Non-small cell lung carcinoma, DCUN1D1 protein, Neoplasm metastasis
Introduction
Incidence rates of non-small cell lung carcinoma (NSCLC) are rising worldwide while survival rates remain stagnant. Despite advances in cancer treatment, overall survival at 5 years after diagnosis of NSCLC is only 15%. NSCLC is frequently associated with brain metastasis during the course of the illness [1]. Invasive progression and metastasis is a complex, multi-step process which involves various interactions between tumor cells and host tissues including alterations in tumor cell proliferation, adhesion, proteolysis and invasion of the extracellular matrix, angiogenesis, and colonization and growth at the target organ [2]. During progression, tumor cells may acquire the ability to preferentially colonize particular organs, such as the brain. Identification of the molecular factors involved in brain metastasis from primary NSCLC is imperative, because overall survival and quality of life will depend on the control of the metastasis as the intrathoracic management of the locally advanced NSCLC improves. Several studies have suggested that brain metastasis is related with p53, Ki-67, and proteins mediating cell adhesion [3-6]. However, it remains unclear which molecular and biological mechanisms are responsible for metastasis of primary tumors to the brain.
DCUN1D1, also known as squamous cell carcinoma-related oncogene, is a novel gene initially identified as a result of investigation of 3q amplification in head and neck squamous cell carcinoma [7]. DCUN1D1-transformed NIH-3T3 cells demonstrated a significantly higher invasive capacity, both in vitro and in vivo [8-10]. The mechanism by which DCUN1D1 expression induces extracellular matrix invasion may involve activation of matrix metalloproteinase 2 transcription in an activator protein-2 and p53-dependent manner. In addition, DCUN1D1 expression is correlated with vascular endothelial growth factor-A expression in lung squamous cell carcinomas, implicating a role in angiogenesis [11-13].
In the present study, lung tumor samples obtained from NSCLC patients, either with or without evidence of brain metastasis, were subjected to immunohistochemistry evaluation to examine DCUN1D1expression and to determine its significance in the development of brain metastasis from primary tumor(s).
Materials and Methods
1. Patient characteristics
A total of 71 NSCLC patients identified with primary tumors, either adenocarcinoma or squamous cell carcinoma, from January 1, 2005 to December 31, 2009, were included in this study. All patients were available for follow-up evaluation of clinical outcomes (40 with the brain as the first site of metastasis and 31 without brain metastasis). None of the patients had received therapy prior to the diagnosing biopsy performed at our institution. Tumors were rated according to World Health Organization (WHO) classifications, and staged according to the tumor-node-metastasis staging system recommended by the American Joint Committee on Cancer. The study protocol was approved by the Institutional Review Board of St. Vincent's Hospital at The Catholic University of Korea (IRB No. VC10SIS10030). Informed consent was waived by the Institutional Review Board. Clinical information was obtained through a computerized database of the tumor registry. Clinical follow-up for the patients in this study was conducted until December 2010, for a time period ranging from 1 to 65 months, with a mean of 29 months. Brain metastases were documented by computed tomography or magnetic resonance imaging records.
2. Immunohistochemical analysis
Tissue sections were obtained from 71 formalin-fixed, paraffin-embedded NSCLC specimens. Immunohistochemical studies were performed using a sensitive peroxidase-streptavidin method for the detection of DCUN1D1 (GenWay Biotech, Inc., San Diego, CA), as described previously [14].
Briefly, 4 µm thick histology sections were placed on positively charged slides, deparaffinized in xylene, and then rehydrated in graded alcohols and water. The endogenous peroxidase activity was blocked by soaking the slides in 3% H2O2 at 45℃ for 5 minutes. For antigen retrieval, the sections were immersed in a citrate buffer (2.1 g/L, pH 6.0) and then autoclaved for 15 minutes. The slides were treated with a protein blocking reagent before incubation overnight with primary antibody at a 1 : 100 dilution in a humidified chamber at 4℃, as recommended by the supplier. After extensive washing with tris buffer, the immunoreactions were assessed using the UltraVision LP detection system (Thermo Fisher Scientific, Fremont, CA). Diaminobenzidine was used as a chromogen and hematoxylin as a nuclear counterstain.
Tissue from the renal tubule was used as a positive control. The primary antibody was omitted during processing to create the negative control. All immunostained slides were examined independently by two experienced pathologists (S.H.L. and K.I.L.) in a blind-fashion. The results of immunostaining against DCUN1D1 cytoplasmic expression were graded by a scoring system where 'N0' indicated DCUN1D1 expression was absent, 'N1' indicated low expression, 'N2' indicated moderate expression, and 'N3' indicated high levels of expression. Scores of 2 and 3 were considered 'positive' results.
3. Statistical analysis
Statistical analyses were carried out using the SPSS ver. 13.0 (SPSS Inc, Chicago, IL). Correlations between clinicopathologic factors and DCUN1D1 expression were estimated using Spearman's chi-square and Fisher's exact tests. Associations between brain metastasis and various parameters were evaluated by the age-adjusted Mantel-Haenszel estimate of the odds ratio. Two-sided p-values were determined with a log-rank test. The level of significance was set at 0.05.
Results
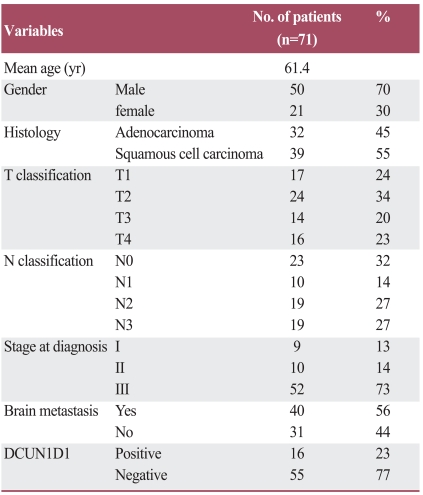
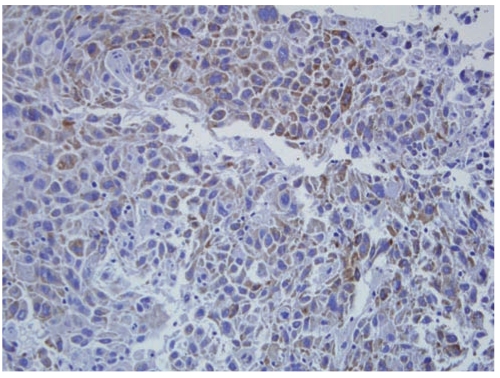
A total of 50 men and 21 women ranging in age from 19 to 79 years (mean age, 61.4 years) were included in this study (Table 1). Pathology review confirmed histologic subtypes of adenocarcinoma in 32 patients (45%) and squamous cell carcinoma in 39 patients (55%). The patients were staged at time of diagnosis and 9 had stage I disease, 10 had stage II disease, and 52 had stage III disease. Brain metastasis appeared in 40 patients (56%), 2-37 months after diagnosis. The negative and positive controls produced expected results. All samples were investigated for DCUN1D1expression, which was identified in 16 cases (23%) (Fig. 1).
Table 1.
Patient characteristics and DCUN1D1 expression
Fig. 1.
Immunohistochemistry results for DCUN1D1, showing cytoplasmic staining (ABC staining, ×100).
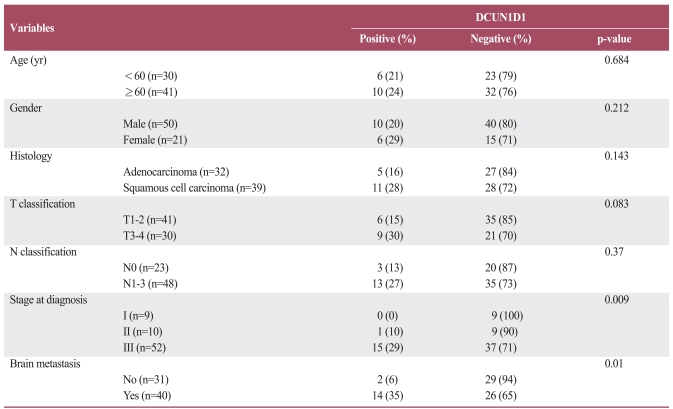
DCUN1D1 expression was observed in 15% of T1-2 tumors and in 30% of T3-4 tumors (Table 2). This difference approached statistical significance for a positive correlation (p=0.083). DCUN1D1 was more frequently expressed in patients with N1-3 status than in patients with N0 status (27% vs. 13%), but no statistical significance was found. DCUN1D1 expression was not detected in patients with stage I disease. However, DCUN1D1 was expressed in 10% of patients with stage II disease and in 29% of patients with stage III disease (p=0/009). Of the 40 patients who developed brain metastasis, 14 (35%) demonstrated DCUN1D1 expression, whereas 2 of 31 (6%) with no evidence of brain metastasis tested positive for DCUN1D1 immunoreactivity. This difference was statistically significant (p=0.01). Other clinicopathologic parameters including age, gender and histology, demonstrated no association with DCUN1D1expression.
Table 2.
Correlation between clinicopathologic factors and immunohistochemical profiles
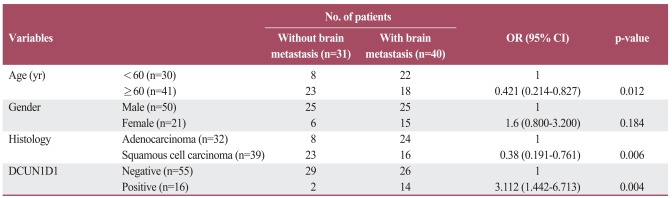
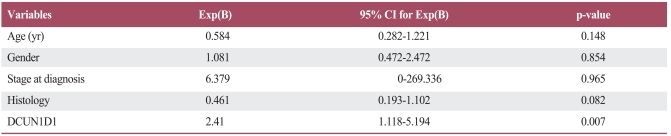
Table 3 presents the odds ratio for brain metastasis corresponding to each variable. The risk of developing brain metastasis was low in patients ≥60 years in age compared to patients <60 years in age (odds ratio, 0.421; 95% confidence interval [CI], 0.214 to 0.827; p=0.012), and was also low in patients with squamous cell carcinomas when compared to patients with adenocarcinomas (odds ratio, 0.38; 95% CI, 0.191 to 0.761; p=0.006). The hazard of brain metastasis for patients with positive DCUN1D1 expression was 3.112 times that of patients with negative DCUN1D1 expression (95% CI, 1.442 to 6.713; p=0.004). No significant risk of developing brain metastasis was associated with gender (p=0.184). Multivariate analysis revealed that DCUN1D1 expression was an independent, unfavorable factor (Table 4).
Table 3.
Risk of developing brain metastasis according to clinicopathologic factors and DCUN1D1 expression
OR, odds ratio; CI, confidence interval.
Table 4.
Cox regression multivariate analysis
CI, confidence interval.
Discussion
DCUN1D1 expression was assessed because of its well established role in the metastatic process to other organs. Although specific molecular abnormalities may be relevant to the occurrence of metastasis at other sites, our study focused specifically on brain metastasis due to the prospect of prophylactic cranial irradiation in NSCLC patients who receive adjuvant chemoradiation. Furthermore, with advances in intrathoracic local control of NSCLC, a favorable overall outcome increasingly depends on early detection of brain metastasis. We compared patients who developed brain metastasis with a control group of NSCLC patients who had no evidence of brain metastasis during the 29 month mean follow-up period. The two major findings of our study were as follows: 1) DCUN1D1 expression was associated with tumor progression as evidenced by its correlation with T classification and tumor stage and 2) the brain as the initial site of metastasis occurred in 88% (14/16) of patients with DCUN1D1-positive tumors and in 47% (26/55) of DCUN1D1-negative patients (p=0.01). The hazard ratio of brain metastasis increased approximately threefold in patients with positive results for DCUN1D1 expression.
DCUN1D1 expression, or amplification, is observed in many malignancies, with a predilection for squamous cell carcinomas of mucosal origin [7]. To date, only a few studies on the role of this gene in lung cancer have appeared in the literature. DCUN1D1 was detected in 23% (16/71) of our samples, which is lower than the prevalence of expression (50%) reported in a previous study [15]. There are a few ways to address the discrepancy in results between these studies. The difference in results may be due to differences in the analytical methodologies used, as their study was based on the analysis of DCUN1D1 mRNA levels while ours was based on protein expression. In a study by Sarkaria et al. [10] using an immunohistochemistry analysis method similar to the one used in our work, the overall prevalence of DCUN1D1 expression in lung adenocarcinomas was 16.3%, which was consistent with our findings (16%). Other factors, such as histologic subtype and progression (early vs. late) of the tumors that were included in the studies may have played a role in the difference in study results. The specificity of 3q amplification in squamous cell carcinomas has been indicated by comparative genomic hybridzation analysis, which results in a higher frequency in squamous cell carcinomas as compared to adenocarcinomas [7-10]. Results may vary according to the proportion of tumor types included in the analysis. Our specimens consisted of 39 squamous cell carcinomas and 32 adenocarcinomas, with a higher prevalence of positive DCUN1D1 phenotype resulting in the squamous cell carcinomas than in the adenocarcinomas (28% vs. 16%). The previous study reporting 50% positivity in cases of NSCLC did not divide the tumors into subtypes [15].
Our data demonstrated that the risk for brain metastasis for patients with DCUN1D1 overexpression was 3.112 times higher than that of DCUN1D1-negative patients. Furthermore, DCUN1D1 expression was an independent parameter as observed by multivariate analysis and may have value in identifying NSCLC patients who are at higher risk for brain metastasis. However, we observed that 47% of DCUN1D1-negative patients also had brain metastasis. No differences in histology results or stage were found in DCUN1D1-negative cases, with or without brain metastasis. It is of interest that metastasis occurred more often in patients less than 60 years of age (69% vs. 31%). Based on our findings, age may attribute to brain metastasis results. Metastasis to a distant organ location is a complex, multistep process, and our study did not offer a fully objective view because of the small sample sizes. Further studies with larger numbers of the age-adjusted patients are needed.
To the best of our knowledge, this is the first report demonstrating the association between DCUN1D1 expression and brain metastasis in lung cancer. The exact mechanisms underlying this association are unclear. However, DCUN1D1 has been shown to correlate with tumor progression as revealed in the present study. Amplification of 3q has been reported to be encountered in 3% of normal mucosa, 25% of carcinoma in situ, and 56% of invasive squamous cell carcinomas of the head and neck [13,15]. Similarly, studies using pure bronchioloalveolar carcinoma, bronchioloalveolar carcinoma with focal invasion, and adenocarcinoma with bronchioloalveolar carcinoma features, have found statistically significant prevalence of DCUN1D1 expression in tumors with increasing degrees of invasive components [10]. To metastasize to the brain, tumor cells must adhere to the brain microvasculature, penetrate the blood-brain-barrier (BBB), and grow within the brain parenchyma [4]. DCUN1D1-positive tumor cells may have the ability to disrupt the BBB and colonize the brain. Other studies have shown DCUN1D1-mediated glioma formation and progression is associated with malignant glioblastoma [9]. Those findings, as well as our data, suggest that DCUN1D1 may play a role in the brain parenchyma invasion found in both primary brain tumors and metastatic tumors. Investigations on several genes linked to these steps, along with the downstream targets of DCUN1D1, are warranted. Such studies are currently in progress at our laboratories.
Conclusion
DCUN1D1 was associated with tumor progression and represented a threefold increase in risk factor for brain metastasis, suggesting that DCUN1D1-positive primary NSCLCs carry a biological predisposition for metastasis to the brain. Measurement of DCUN1D1 protein may provide assistance in identifying those who are at higher risk for brain metastasis. There is a need to conduct further studies on a larger scale for validation of our results.
Acknowledgments
This study was supported by Grants from St. Vincent's Hospital Research Center in 2010.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45(Suppl 2):S253–S257. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13:1656–1662. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 3.Saad AG, Yeap BY, Thunnissen FB, Pinkus GS, Pinkus JL, Loda M, et al. Immunohistochemical markers associated with brain metastases in patients with nonsmall cell lung carcinoma. Cancer. 2008;113:2129–2138. doi: 10.1002/cncr.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold SM, Young AB, Munn RK, Patchell RA, Nanayakkara N, Markesbery WR. Expression of p53, bcl-2, E-cadherin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 in paired primary tumors and brain metastasis. Clin Cancer Res. 1999;5:4028–4033. [PubMed] [Google Scholar]
- 5.Shabani HK, Kitange G, Tsunoda K, Anda T, Tokunaga Y, Shibata S, et al. Immunohistochemical expression of E-cadherin in metastatic brain tumors. Brain Tumor Pathol. 2003;20:7–12. doi: 10.1007/BF02478941. [DOI] [PubMed] [Google Scholar]
- 6.Grinberg-Rashi H, Ofek E, Perelman M, Skarda J, Yaron P, Hajdúch M, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15:1755–1761. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Gogineni SK, Sacks PG, Shaha AR, Shah JP, Stoffel A, et al. Molecular cytogenetic characterization of head and neck squamous cell carcinoma and refinement of 3q amplification. Cancer Res. 2001;61:4506–4513. [PubMed] [Google Scholar]
- 8.Sarkaria I, O-charoenrat P, Talbot SG, Reddy PG, Ngai I, Maghami E, et al. Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res. 2006;66:9437–9444. doi: 10.1158/0008-5472.CAN-06-2074. [DOI] [PubMed] [Google Scholar]
- 9.Broderick SR, Golas BJ, Pham D, Towe CW, Talbot SG, Kaufman A, et al. SCCRO promotes glioma formation and malignant progression in mice. Neoplasia. 2010;12:476–484. doi: 10.1593/neo.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkaria IS, Pham D, Ghossein RA, Talbot SG, Hezel M, Dudas ME, et al. SCCRO expression correlates with invasive progression in bronchioloalveolar carcinoma. Ann Thorac Surg. 2004;78:1734–1741. doi: 10.1016/j.athoracsur.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 11.O-charoenrat P, Sarkaria I, Talbot SG, Reddy P, Dao S, Ngai I, et al. SCCRO (DCUN1D1) induces extracellular matrix invasion by activating matrix metalloproteinase 2. Clin Cancer Res. 2008;14:6780–6789. doi: 10.1158/1078-0432.CCR-08-0719. [DOI] [PubMed] [Google Scholar]
- 12.Kim AY, Bommeljé CC, Lee BE, Yonekawa Y, Choi L, Morris LG, et al. SCCRO (DCUN1D1) is an essential component of the E3 complex for neddylation. J Biol Chem. 2008;283:33211–33220. doi: 10.1074/jbc.M804440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talbot SG, O-charoenrat P, Sarkaria IS, Ghossein R, Reddy P, Ngai I, et al. Squamous cell carcinoma related oncogene regulates angiogenesis through vascular endothelial growth factor-A. Ann Surg Oncol. 2004;11:530–534. doi: 10.1245/ASO.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Yoo J, Jung JH, Lee MA, Seo KJ, Shim BY, Kim SH, et al. Immunohistochemical analysis of non-small cell lung cancer: correlation with clinical parameters and prognosis. J Korean Med Sci. 2007;22:318–325. doi: 10.3346/jkms.2007.22.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh B, Stoffel A, Gogineni S, Poluri A, Pfister DG, Shaha AR, et al. Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol. 2002;161:365–371. doi: 10.1016/S0002-9440(10)64191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]