Abstract
The reported incidence of synchronous multiple primary cancer (SMPC) is rare, and it is even less common to observe synchronous solid tumor with a hematological malignancy. We report five cases of solid tumor presented synchronously with hematological malignancy, all observed within a 2 year period at the oncology department of a university hospital in Shanghai, China. These individual cases included lung adenocarcinoma with chronic myelogenous leukemia, colon cancer with solitary plasmocytoma, gastric adenocarcinoma with diffuse large B cell non-Hodgkin's lymphoma, lung adenocarcinoma with multiple myeloma, and colon cancer with diffuse large B cell non-Hodgkin's lymphoma. It is challenging to therapeutically control the biological behavior of concurrent multiple primary tumors, and there is no standard treatment for such rare conditions. In this paper we discuss these five cases of SMPC and their treatments.
Keywords: Synchronous multiple primary neoplasms, Solid neoplasms, Hematologic neoplasms
Introduction
Multiple primary cancer (MPC) is a specific malignant tumor type, manifesting as more than one primary tumor diagnosed in the same patient, either simultaneously or sequentially. The diagnostic criteria [1] include: 1) the cancer must be clearly malignant as determined by histological evaluation; 2) each cancer must be geographically separate and distinct; 3) the possibility that the second tumor represents a metastasis should be excluded. Synchronous multiple primary cancer (SMPC) is defined as two or more tumors occurring within six months of each other, while heterochronic multiple primary cancer is a subset of SMPC where the second cancer occurs more than six months after the first. As previously reported [2], the major hematological conditions in patients with multiple malignancies include multiple myeloma, myelodysplastic syndromes, non-Hodgkin's lymphoma, and chronic myelogenous leukemia (CML). The major sites of MPC occurrence, sans hematological malignancies, include the stomach, colon, breast, and esophagus. The incidence of SMPC is very low, and it is even less common than synchronous presentation of a solid tumor with a hematological malignancy. The mechanism of pathophysiology remains poorly understood, and risk factors involved in MPCs may include tobacco and alcohol intake, infections and immunosuppression, genetic predisposition, and toxic effects related to treatment by chemotherapy or radiotherapy [2]. Herein, we report five cases of synchronous solid tumor and hematological malignancy, and discuss the treatment of such rare malignancies.
Case Report
From January 2008 to January 2010, five patients diagnosed with synchronous solid tumor and hematological malignancy were admitted to the medical oncology department at Zhongshan Hospital at Fudan University in Shanghai, China. The SMPC diagnosis for these five cases accounted for 0.5% of all newly diagnosed cancerous patients during this time period. The mean age of these patients was 53.8 years old and their median survival time was 14.0 months (95% confidence interval, 6.12 to 21.88 months). The detailed clinical data is listed in Table 1.
Table 1.
Clinical data for five synchronous multiple primary cancerous patients
The intervals between two primary tumors were all less than six months. OS, overall survival; F, female; Ope, operation; Chemo, chemotherapy; M, male; CML, chronic myelogenous leukemia.
1. Case 1
A 56-year-old female with a mass found at her right groin. The biopsy pathology result for the mass indicated plasmablast myeloma. Immunohistochemistry assay revealed leukocyte common antigen (LCA, +++), CD20(-), CD3(-), CD79α (weak positive), CD45RO(-), plasmacyte antibody(-), epithelial membrane antigen (weak positive), κ(+++), λ(-), CD5(-), and CD10(-). The patient's immunoglobins were observed to be normal, and serum electrophoresis and immunoelectrophoresis showed no M protein. Dysplastic plasma cells accounted for less than 5% of the bone marrow aspirate and biopsy results. Isolated extramedullary plasmacytoma (EMP) was diagnosed and she did not receive any therapeutic treatment. At her six month follow-up visit, spiral computed tomography (CT) scan revealed a lesion located in the right lung with hilar lymph node enlargement. The patient received the video-assisted thoracoscopic surgery (VATS) in radical operation for lung tumor.
The post-surgery pathology result revealed the lesion to be adenocarcinoma (pT2N0M0, stage IB) and the hilar lymph nodes to be negative. Concurrent laboratory tests revealed a serum beta-2 microglobulin result of 3.45 mg/L. The right groin mass remained palpable and had grown larger.
Elevated tumor markers were observed for cancer antigen 125 (CA125, 86.8 IU/mL) and neuron-specific enolase (51.2 ng/mL). Because the patient's beta-2 microglobulin had increased, we chose to use vinorelbine as it is effective for treating myeloma [3,4]. The patient received vinorelbine plus cisplatin (NP) regimen (cisplatin 40 mg d1-3 plus vinorelbine 40 mg d1, d8, q21d) for four cycles. The right groin mass reduced in size after chemotherapy and the tumor marker decreased to a normal level, at which point, 45 Gy radiotherapy was successfully delivered to the right groin. On the fifth month post-treatment follow-up, multiple masses were found in the bilateral lung. Percutaneous biopsy revealed recurrent adenocarcinoma of the lung. Single agent pemetrexed at a dose of 1,000 mg was administered to the patient for two cycles in keeping with disease progression. This patient's progression free survival duration was three months and she died of respiratory failure at 22 months from diagnosis.
2. Case 2
A 50-year-old male with cough and dyspnea which was aggravated by movement. A malignant cell was found in his pleural effusion. A concurrent, routine blood test revealed total white blood cell (WBC) count of 116.20×109/L, hemoglobin 132 g/L and granulocytes 45.3×109/L. A color Doppler abdominal ultrasound examination found splenomegaly. Various immature granulocytes were observed in the peripheral blood smear. The bone marrow aspirate assay uncovered active karyocyte hyperplasia, granulocyte/erythroid 61.7/1, active granulocyte hyperplasia, and granular left shift with relatively restrained erythrocyte series hyperplasia. Neutrophil alkaline phosphatase staining produced negative results and the bone marrow biopsy revealed granulocyte series neoplastic hyperplasia with myeloperoxidase granulocyte(++), lysozyme granulocyte(+), CD34(+), CD68 histiocyte(+), CD61 macronucleus(+), CD10(+), TdT(-), LCA partial(+), CD20(-), CD3(-), and CD56(-). The sum of these results confirmed a diagnosis of CML. The patient was orally administered hydroxyurea for one week and his WBC counts decreased progressively after treatment (WBC, 13.65×109/L; granulocytes, 9.3×109/L). Left pleura biopsy pathology under VATS revealed adenocarcinoma. The tumor markers for CA199, CA125 and CA153 in blood were 199.1 IU/mL, 114.9 IU/mL and 103.9 IU/mL, respectively. A bone scan found multiple bone metastases. He was diagnosed with non-small cell lung cancer (NSCLC) with bone metastasis (stage IV). NP regimen (cisplatin 40 mg d1-3 plus vinorelbine 40 mg d1,8, q21d) was administered for six cycles, and the response was partial remission. He was then shifted to a maintenance therapy of gefitinib at a dose of 250 mg/day and zoledronic acid at a dose of 15 mg once/month. Two months later, the tumor marker was observed to rise further and the bone metastasis progressed. Pemetrexed of 1,000 mg dose was administered for three cycles as a second-line salvage therapy. The disease continued to progress with metastasis to the brain and liver. The patient's post-diagnosis survival time was 14 months.
3. Case 3
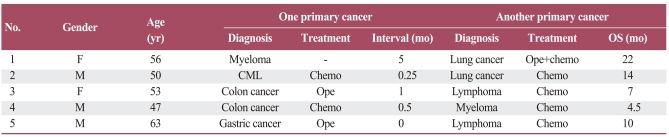
A 53-year-old female initially presented with several masses on the left neck. A biopsy was performed and the pathological diagnosis was reactive hyperplasia with mild dysplasia of the lymphoid tissue. It was a kind of inflammatory reaction. The patient reported severe constipation in the prior month, so a colonoscopy was performed which found a mass at the sigmoid colon. After radical resection, the pathological diagnosis was mucinous adenocarcinoma (pT3N0M0, stage II). Meanwhile, the left neck masses progressively enlarged, and a biopsy confirmed diffuse large B cell non-Hodgkin's lymphoma with LCA(+), CD20(++), CD79α(++), CD3(partial positive), CD10(-), BCL-6(-), BCL-2(50%+), Ki-67(20%+), MUM(-), CD68(+), CD4(partial positive), and CD8(+), as illustrated in Fig. 1. After two cycles of R-CHOP (rituximab 600 mg d0, cyclophosphamide 1.0 d1, epirubicin 80 mg d1, vindesine 4 mg d1 plus methylprednisolone 80 mg d1-5, q21d), the patient attained complete remission. Two additional cycles of R-CHOP were administered. The patient survived until the final, scheduled follow-up visit. Her survival duration was seven months from time of diagnosis.
Fig. 1.
Case 3 pathological figures. (A) Post surgery colon adenocarcinoma (H&E staining, ×40). (B) Biopsy result revealing lymphoma (H&E staining, ×40).
4. Case 4
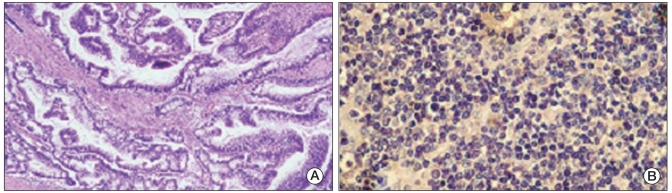
A 47-year-old male with a liver mass was found in conjunction with a report of weight loss during a routine health examination. A colonoscopy found adenocarcinoma at the cecum, and his blood test revealed a WBC count of 2.60×109/L, hemoglobin of 78 g/L, and platelet count of 121×109/L. A CT scan found metastasis of the lung and liver, and his carcinoembryonic antigen elevated to 601.0 ng/mL. He was diagnosed with stage IV colon cancer. Concurrent serum electrophoresis and immunoelectrophoresis revealed abnormal kappa light chain. The bone marrow aspirate and biopsy demonstrated more than 25% dysplastic plasma cells, as seen in Fig. 2. The patient's serum beta-2 microglobulin was 12.83 mg/L. A plain skeletal X-ray revealed multiple lytic bony lesions (MM, stage III). He was treated with dexamethasone 40 mg d1-4, d9-12, adriamycin 15 mg d1-4, plus capecitabine 1,500 mg bid d1-14. After two cycles, the serum beta-2 microglobulin and kappa light chain reduced. However, the patient succumbed to the progressing lung and liver metastasis from the colon cancer, with survival duration of 4.5 months from time of diagnosis.
Fig. 2.
Case 4 pathological figures. (A) Computed tomography scan result identifying liver metastasis from colon cancer. (B) Computed tomography scan identifying lung metastasis from colon cancer. (C) Colon adenocarcinoma revealed by colonoscopy (H&E staining, ×10). (D) Bone marrow aspiration smear showing dysplastic plasma cells (Giemsa staining, ×10).
5. Case 5
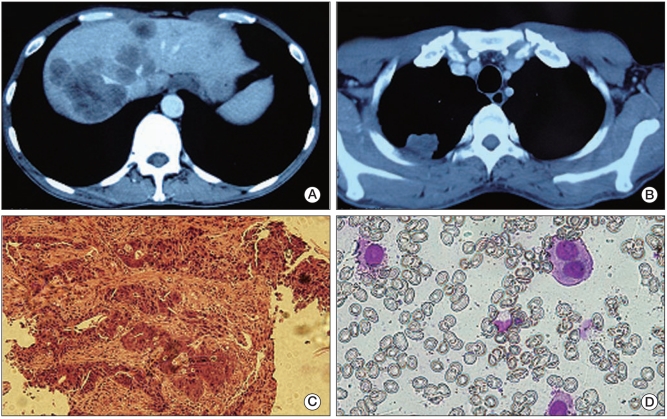
A 63-year-old male with an uneven mass found in his right upper abdomen by ultrasound during a routine medical examination. Gastroscopy revealed low-grade differential adenocarcinoma on the gastric angle. Percutaneous biopsy of the right upper abdomen mass verified diffuse large B cell non-Hodgkin's lymphoma, with cytokeratin (CK)(-), CK19(-), CD45 (LCA, +++), CD20(+++), CD3(±), CD30 (-), CD79α(++), and CD45RO(+), as seen in Fig. 3. A therapeutic regimen was administered including rituximab 600 mg d0, etoposide 100 mg d1-3, epirubicin 40 mg d1-2, cisplatin 30 mg d1-3, and methylprednisolone 40 mg d1-4 plus vincristine 1 mg d1. After two cycles, the abdominal mass disappeared and the mass in the stomach appeared stable as observed by ultrasonic gastroscopy. Because the patient complained of anorexia and weight loss, after discussion of alternatives and indications, he received radical subtotal gastrectomy (pT3N1M0). Administration of one cycle of rituximab 600 mg d0, epirubicin 40 mg d1-2, cisplatin 30 mg d1-3, and methylprednisolone 40 mg d1-4 plus vincristine 2 mg d1, was delivered after surgery. At his follow-up hospital visit, a mass was found in his right upper abdomen during CT scan. He then received a second-line R-ICE chemotherapy regimen (rituximab 600 mg d0, ifosfamide 2.0 d1-4, and carboplatin 150 mg d1-3 plus etoposide 150 mg d1-3) for three cycles. The result was complete remission. Two months later, the patient suffered vomiting from intestinal obstruction. Because his symptoms couldn't be relieved by conservative treatment, an exploratory laparotomy was performed which discovered peritoneal metastasis from gastric cancer. The pathology of the small peritoneal metastatic lesions revealed adenocarcinoma. The patient's performance status deteriorated after surgery and his survival duration from time of diagnosis was 10 months.
Fig. 3.
Case 5 pathological figures. (A) biopsy result revealing lymphoma in right upper abdominal mass (H&E staining, ×40). (B) Gastric adenocarcinoma identified after radical subtotal gastrectomy (H&E staining, ×40). F18-FDG high uptake lesion at gastric sinus (C) and right upper abdomen (D), as observed by positron emission tomography/computed tomography).
Discussion
The precise mechanism of SMPC has remained unknown and there are no uniform treatment guidelines. Recent studies have demonstrated evidence of a possible spread of cancerous cells from a single clone to multiple sites [5,6]. It was reported whether or not genetic defects involving the mismatch repair system constituted an important risk factor in patients with MPC [7]. An Argentine research group found 32% of MPC patients had a family history of oncologic diseases [8]. In this report, we found only one patient (case 5, accounting for 20%) who carried this genetic tendency. Both his father and uncle had died from cancer.
A literature review of 1,104,269 cancer patients concluded that the prevalence of MPC is between 0.73% and 11.7% [9]. The wide variation in reporting was concluded to be associated with the varying capabilities and experience of hospitals and their doctors. At our one hospital we received five SMPC patients in a relatively short time, and it reminded us to carefully consider the appearance of such diseases. In these cases, there is a change not only in disease spectrum, but also the cancer spectrum. With increased availability of methods with greater sensitivity for screenscreening organs susceptible to MPCs, there may be an increase of frequency in the identification of multiple primaries in the coming years.
Although it is both impractical and unnecessary to obtain biopsies for every metastatic lesion that may appear in a case of MPC, awareness of the different biological behaviors between solid tumors and hematologic malignancies should be considered. In this report, some patients came to the hospital presenting solid tumors symptoms, and others for hematologic malignancies. But some symptoms couldn't be controlled effectively while treating a single tumor, and other lesions appeared to defy the routine metastatic rule. These clinical observations should remind us to investigate the possibility of SMPC. A re-biopsy is the only way to verify suspicion of SMPC. Published literature has reported that such diseases are often located at the same organ, such as simultaneous Hodgkin's lymphoma and adenosquamous carcinoma in the uterine cervix, and adenocarcinoma and lymphoma in the stomach [10-12].
Lacking guidelines in the management of such diseases, the treatment modality should be determined using the doctor experience and close attention to the characteristics of the patient's illness and performance status. The EMP in case 1 was early stage with good prognosis, so therapy was not delivered as it was not expected to affect patient survival time. We chose the NP regimen instead of gemcitabine plus cisplatin or paclitaxel plus cisplatin, because vinorelbine is a kind of vinca alkaloid drug, and the regimen is appropriate to both lung cancer and plasmatic disease. Because epidermal growth factor receptor (EGFR) mutation status was not detected, pemetrexed instead of gefitinib was chosen as the second-line chemotherapy. In case 2, the patient's WBC count decreased rapidly after just one week of hydroxyurea treatment, but the patient did not recover from the dyspnea symptoms. This raised the suspicion that another primary cancer was involved, which was revealed by VATS to be lung cancer. Because CML and lung cancer were found at the same time, hydroxyuria was chosen for its effectiveness in reducing WBC counts rapidly. Because the CML was controlled well and had little effect on long-term survival, the patient received six cycles of NP regimen and gefitinib maintenance therapy. During treatment for NSCLC, the patient did not receive any therapy for CML because routine blood tests were normal. The initial diagnosis for case 3 was lymphoid tissue reactive hyperplasia of the neck, but then, sigmoid colon cancer was found and a radical resection was administered. After the operation, the left neck mass enlarged. This observation triggered the biopsy to be repeated and lead to a diagnosis of lymphoma. As the pair of tumors were both early stage, the patient had a good prognosis with a long survival time. The case 4 patient presented two severe tumors, MM (stage III) and colon cancer (stage IV). Choosing a proper regimen was difficult because both the WBC and hemoglobin counts were low. We treated the MM mainly with dexamethasone and adriamycin, and controlled the colon cancer using low dose intensity capecitabine in hopes that the blood test results would improve. Then, we administered the standard chemotherapy to treat the advanced colon cancer, but the disease had progressed too quickly. Simultaneous abdominal lymphoma and gastric cancer were found in case 5. Because the abdominal lymphoma was extensive, the treatment regimen followed the lymphoma guideline. Consideration was also made for the gastric cancer. Cyclophosphamide was displaced by cisplatin in the regimen.
After the lymphoma was satisfactorily controlled, only then was the gastric cancer operated on. After the operation, two lymph nodes of twenty remained. Unfortunately the tumors were not early stage and overall survival time was short.
SMPCs are difficult to deal with because clinicians might not be able to provide simultaneous treatment for both disease types, such as one treatment resulting in competing toxicities for treating the other. The treatment choice depends on the tumor location and may involve curative surgical resection of each cancer, radiotherapy, and chemotherapy [13,14]. Our main approach to treating SMPCs was to abide by the therapeutic principles associated with each tumor and to consider both when treating one of the malignant diseases. We recommend that doctors formulate individual therapeutic strategies according to the patient's illness. The therapeutic effects and patient endurance must be evaluated at the same time. Case discussion with a multiple-disciplinary team may be helpful.
According to the literature, the prognosis of MPC patients can be determined independently as a function of the stage of each cancer. We found that the SMPC prognosis would be good if the tumors were all at early stage, otherwise the prognosis was likely poor. When the tumors are advanced stage at diagnosis, it is always a question of whether this is due to a delay in diagnosis or dependent on the biology of the tumor, such as a high grade tumor, etc. As a summarizing thought to consider, when encountering SMPCs, the fewer reported cases available to reference makes treatment that much more of a challenge.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Johnson C. SEER program coding and staging manual 2004. NIH publication no. 04-5581. 4th ed. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 2.Fraumeni JF, Jr, Curtis RE, Edwards BK, Tucker MA. Introduction. In: Curtis RE, Freedman DM, Ron E, Ries LA, Hacker DG, Edwards BK, et al., editors. New malignancies among cancer survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 3.Annunziata M, Celentano M, Pocali B, D'Amico MR, Palmieri S, Viola A, et al. Vinorelbine plus intermediate dose cyclophosphamide is an effective and safe regimen for the mobilization of peripheral blood stem cells in patients with multiple myeloma. Ann Hematol. 2006;85:394–399. doi: 10.1007/s00277-005-0058-0. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez JM, Ocio EM, San Miguel JF, Pandiella A. Pemetrexed acts as an antimyeloma agent by provoking cell cycle blockade and apoptosis. Leukemia. 2007;21:797–804. doi: 10.1038/sj.leu.2404599. [DOI] [PubMed] [Google Scholar]
- 5.Habuchi T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol. 2005;12:709–716. doi: 10.1111/j.1442-2042.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 6.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Van Der Wal JE, Snow GB, Leemans CR, et al. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol. 2002;161:1051–1060. doi: 10.1016/S0002-9440(10)64266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horii A, Han HJ, Shimada M, Yanagisawa A, Kato Y, Ohta H, et al. Frequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers. Cancer Res. 1994;54:3373–3375. [PubMed] [Google Scholar]
- 8.Ares SL, Polo S, Ezcurdia L, Tognelli F, Mussini S, Gercovich N, et al. Multiple primary cancer in adults (MPCA) J Clin Oncol. 2006;24(18S):16027. [Google Scholar]
- 9.Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26:79–83. doi: 10.1097/00000421-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Wotherspoon AC, Isaacson PG. Synchronous adenocarcinoma and low grade B-cell lymphoma of mucosa associated lymphoid tissue (MALT) of the stomach. Histopathology. 1995;27:325–331. doi: 10.1111/j.1365-2559.1995.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 11.Lovell MO, Valente PT. Unique collision of hodgkin lymphoma and adenosquamous carcinoma in the uterine cervix: synchronous malignant neoplasms of the cervix. J Low Genit Tract Dis. 2003;7:307–310. doi: 10.1097/00128360-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, Cebulski W, Slodkowski M, Wasiutynski A, et al. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–5362. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura M, Shinagawa M, Funaki Y. Synchronous triple early cancers occurring in the stomach, colon and gallbladder. Asian J Surg. 2003;26:46–48. doi: 10.1016/S1015-9584(09)60216-5. [DOI] [PubMed] [Google Scholar]
- 14.Van Dalen R, Church J, McGannon E, Fay S, Burke C, Clark B. Patterns of surgery in patients belonging to amsterdam-positive families. Dis Colon Rectum. 2003;46:617–620. doi: 10.1007/s10350-004-6619-9. [DOI] [PubMed] [Google Scholar]