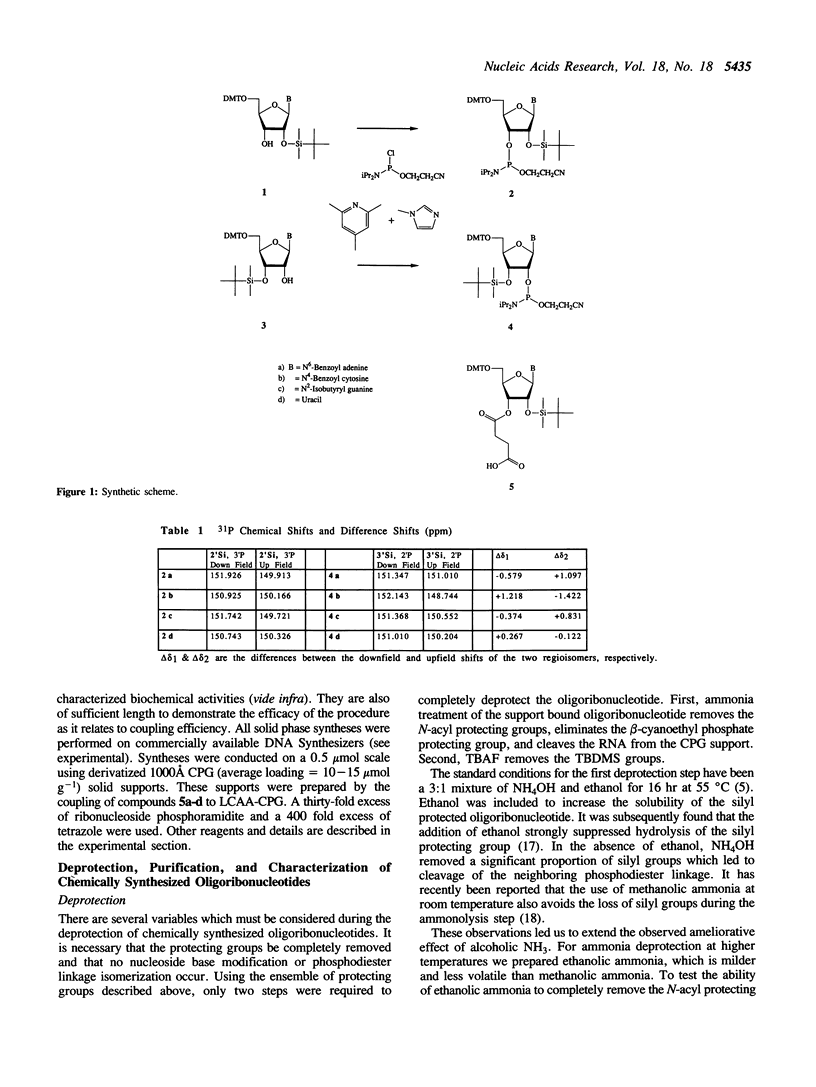
Abstract
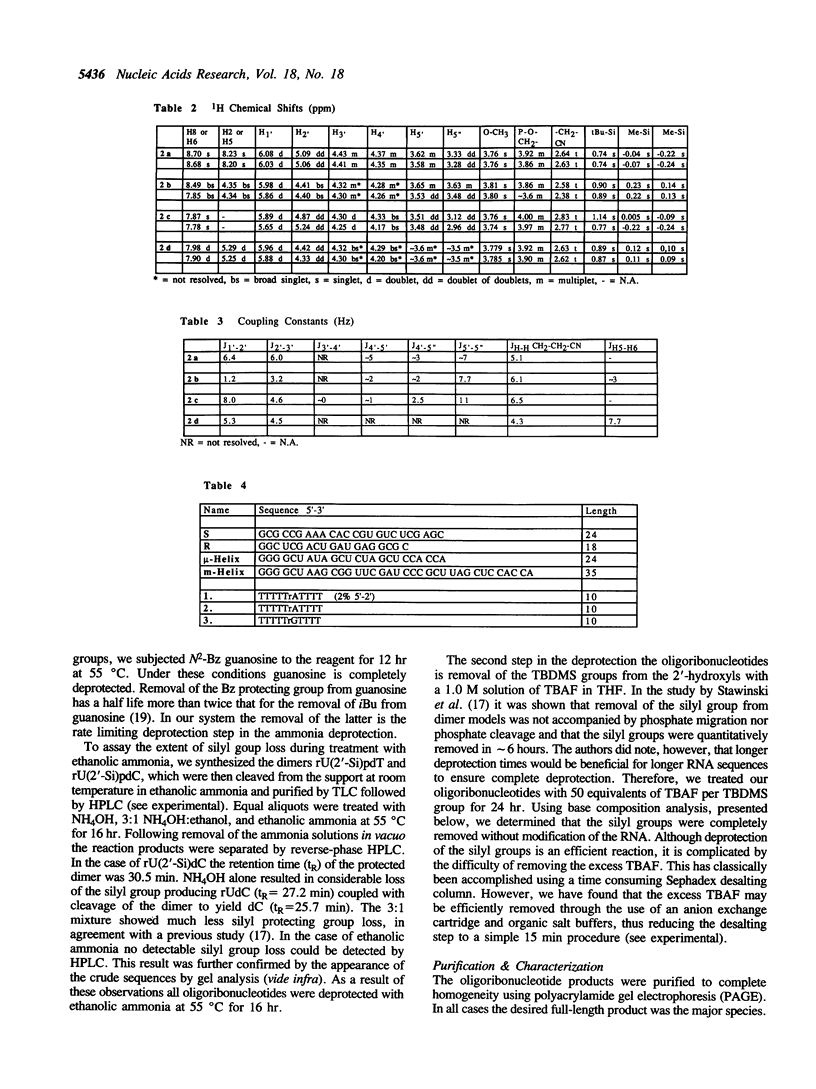
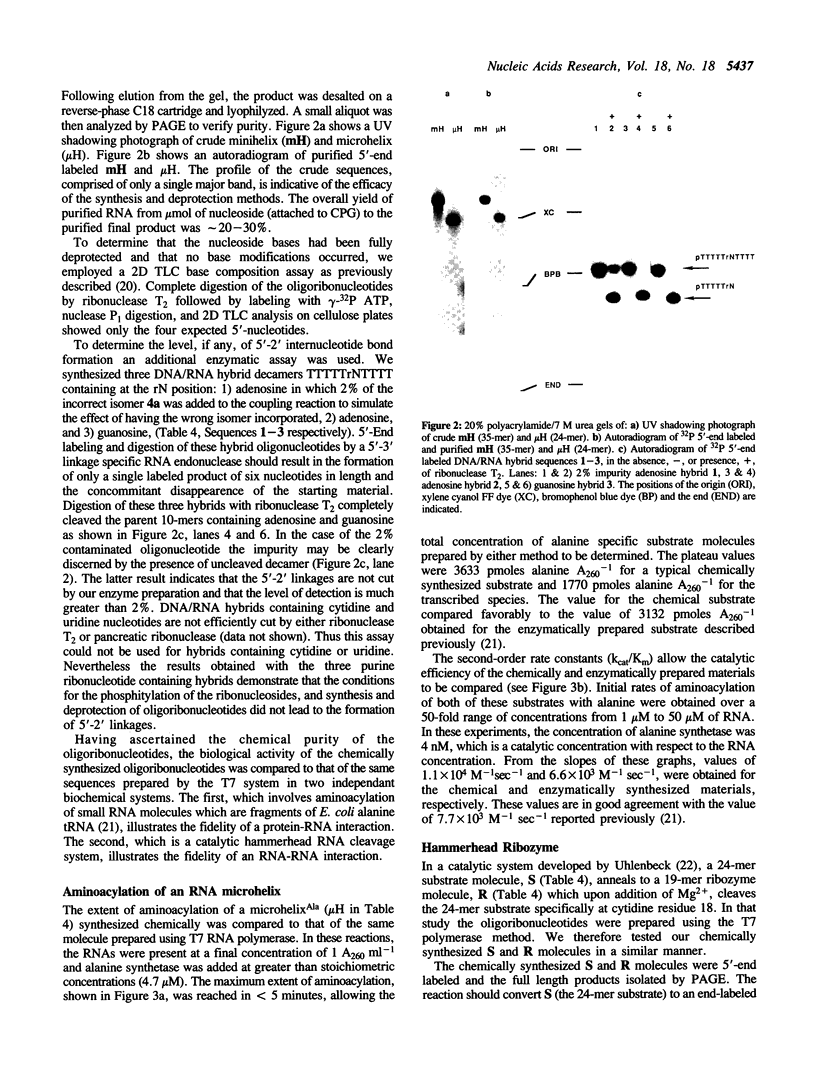
The preparation of fully protected diisopropylamino-beta-cyanoethyl ribonucleoside phosphoramidites with regioisomeric purity greater than 99.95% is described. It is demonstrated that the combination of standard DNA protecting groups, 5'-O-DMT, N-Bz (Ade and Cyt), N-iBu (Gua), beta-cyanoethyl for phosphate, in conjunction with TBDMS for 2'-hydroxyl protection, constitutes a reliable method for the preparation of fully active RNA. Average stepwise coupling yields in excess of 99% were achieved with these synthons on standard DNA synthesizers. Two steps completely deprotect the oligoribonucleotide and workup is reduced to a fifteen minute procedure. Further, it is shown that the deprotected oligoribonucleotides are free from 5'-2' linkages. This methodology was applied to the chemical synthesis of a 24-mer microhelix, a 35-mer minihelix and two halves of a catalytic 'Hammerhead Ribozyme'. These oligoribonucleotides were directly compared in two distinct biochemical assays with enzymatically (T7 RNA polymerase) prepared oligoribonucleotides and shown to possess equal or better activity.
Full text
PDF




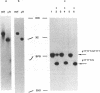
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Aminoacylation of RNA minihelices with alanine. Nature. 1989 Feb 2;337(6206):478–481. doi: 10.1038/337478a0. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Lumelsky N., Altman S. Specific interactions in RNA enzyme-substrate complexes. Science. 1989 Dec 22;246(4937):1578–1584. doi: 10.1126/science.2480641. [DOI] [PubMed] [Google Scholar]
- Iwai S., Ohtsuka E. 5'-Levulinyl and 2'-tetrahydrofuranyl protection for the synthesis of oligoribonucleotides by the phosphoramidite approach. Nucleic Acids Res. 1988 Oct 25;16(20):9443–9456. doi: 10.1093/nar/16.20.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzek R., Caruthers M. H., Longfellow C. E., Swinton D., Turner D. H., Freier S. M. Polymer-supported RNA synthesis and its application to test the nearest-neighbor model for duplex stability. Biochemistry. 1986 Dec 2;25(24):7840–7846. doi: 10.1021/bi00372a009. [DOI] [PubMed] [Google Scholar]
- Lehmann C., Xu Y. Z., Christodoulou C., Tan Z. K., Gait M. J. Solid-phase synthesis of oligoribonucleotides using 9-fluorenylmethoxycarbonyl (Fmoc) for 5'-hydroxyl protection. Nucleic Acids Res. 1989 Apr 11;17(7):2379–2390. doi: 10.1093/nar/17.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]
- Puglisi J. D., Wyatt J. R., Tinoco I., Jr A pseudoknotted RNA oligonucleotide. Nature. 1988 Jan 21;331(6153):283–286. doi: 10.1038/331283a0. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Strömberg R., Thelin M., Westman E. Studies on the t-butyldimethylsilyl group as 2'-O-protection in oligoribonucleotide synthesis via the H-phosphonate approach. Nucleic Acids Res. 1988 Oct 11;16(19):9285–9298. doi: 10.1093/nar/16.19.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using o-nitrobenzyl protection of 2'-hydroxyl via a phosphite triester approach. Nucleic Acids Res. 1986 Aug 11;14(15):6265–6279. doi: 10.1093/nar/14.15.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]