Abstract
We report a patient who underwent gamma knife radiosurgery to treat recurrent meningioma after microsurgery and thereafter developed secondary malignancy adjacent to the original tumor. A 47-year-old woman had underwent resection of the olfactory groove meningioma. Then radiosurgery was done three times over 4 year period for the recurrent tumor. After 58 months from the initial radiosurgery, she presented with headache and progressive mental dullness. Huge tumor in bifrontal location was revealed in MRI. Subsequent operation and pathological examination confirmed diagnosis of glioblastoma. This case fits the criteria of radiation-induced tumor and the clinical implication of the issue is discussed.
Keywords: Glioblastoma, Radiosurgery
INTRODUCTION
Cranial irradiation has been widely used as a therapeutic method treating a wide variety of lesions, particularly neoplasms. Though radiation therapy of central nervous system is usually well-tolerated, it does occasionally cause clinically significant long-term toxicity such as late delayed radiation necrosis and irradiation-related arteriopathy with stroke14). Among other complications induction of neoplasm is a rare but well-documented serious sequela of therapeutic irradiation. Radiosurgery is not free from this problem although there are only a few reported cases14). We report a case of glioblastoma which was developed after gamma knife radiosurgery (GKS) for meningioma.
CASE REPORT
A 47-year-old woman presented with headache, anosmia, and visual dimness. Large extra-axial mass originating from olfactory groove and both frontal base was found on MRI (Fig. 1). She underwent gross total tumor removal, and the pathological diagnosis of meningotheliomatous meningioma was confirmed. Approximately 1 year after surgery, the follow-up MRI scan revealed tumor recurrence. She underwent GKS with 16 Gy of marginal dose at 50% isodose line (Fig. 2A). Three years after initial radiosurgery, the subsequent MRI scan revealed tumor progression at a location different from the previous recurrence. She underwent radiosurgery with 13 Gy of marginal dose and she was subjected to third GKS with 15 Gy of marginal dose on local recurrent lesion after 2 years from second GKS (Fig. 2B, C). At 58 months from the initial GKS, which is also 72 months from the first operation the patient experienced severe headache with vomiting and a huge new lesion was found in the area of the previous radiosurgery (Fig. 3). She underwent the second craniotomy and tumor removal. On this occasion, the pathological finding was consistent with glioblastoma (Fig. 4). She was treated with concomitant chemo-radiotherapy (total dose of irradiation 6000 cGy over 30 fractions and oral temozolomide 75 mg/m2/day during the radiotherapy period) followed by adjuvant chemotherapy with oral temozolomide. First cycle was consisted of 150 mg/m2/day temozolomide for 5 days, and subsequent 5 cycles were maintained with 200 mg/m2/day for 5 days with 4 weeks interval between each cycle. The tumor progression was significant at 11 months from the diagnosis of glioblastoma with neurological deterioration. Ventriculoperitoneal shunt was done for progressive hydrocephalus. Currently, she is alive at the time of this writing (15 months after diagnosis of glioblastoma) with only supportive management.
Fig. 1.
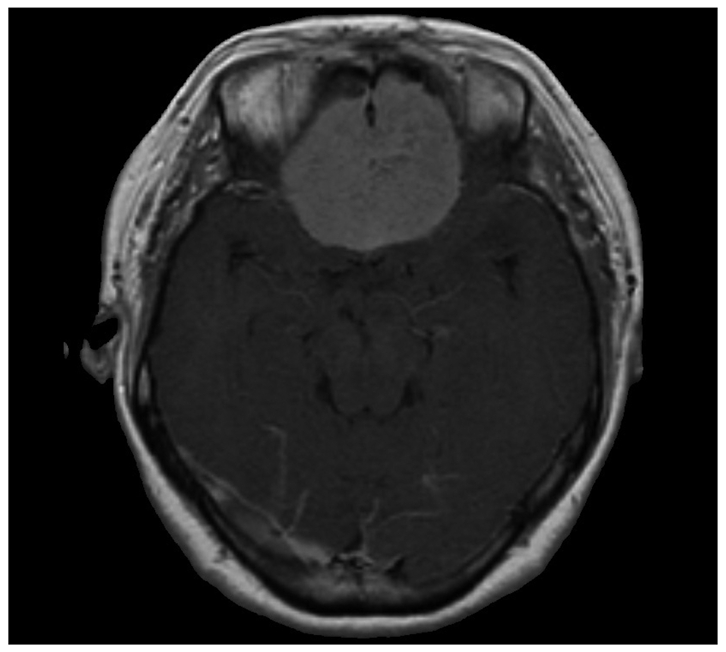
T1-weighted contrast enhanced magnetic resonance image showing well enhanced frontal meningioma upon the olfactory groove.
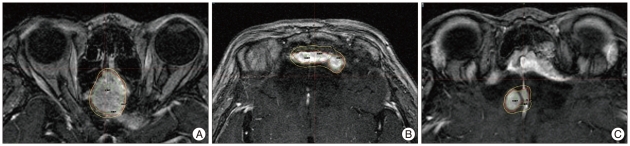
Fig. 2.
First Gamma Knife Surgery planning for meningioma which maximum radiating doses were 30 Gy on July 2004 (A) and 25 Gy on May 2007 (B) and 28 Gy on May 2009 (C).
Fig. 3.
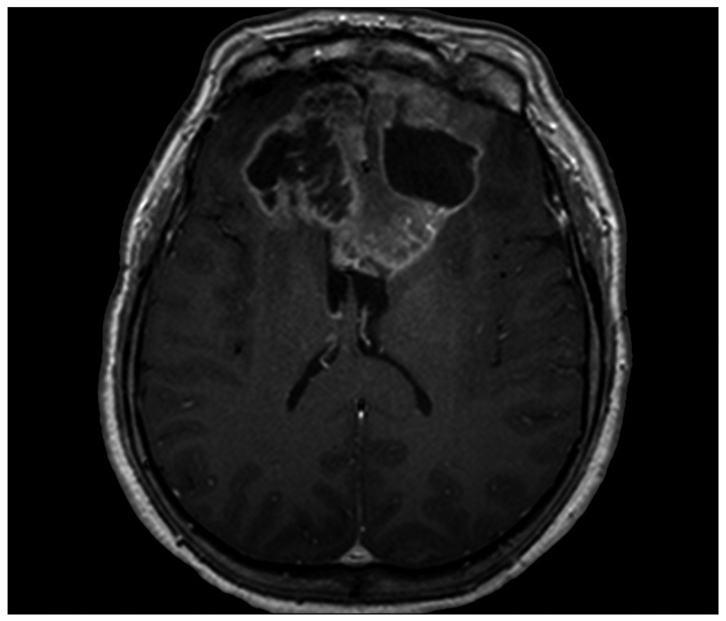
T1-weighted contrast enhanced magnetic resonance image demonstrating a mass with peripheral enhancement and central necrosis involving both frontal lobes with extension into the corpus callosum after 58 months from diagnosis of GM.
Fig. 4.
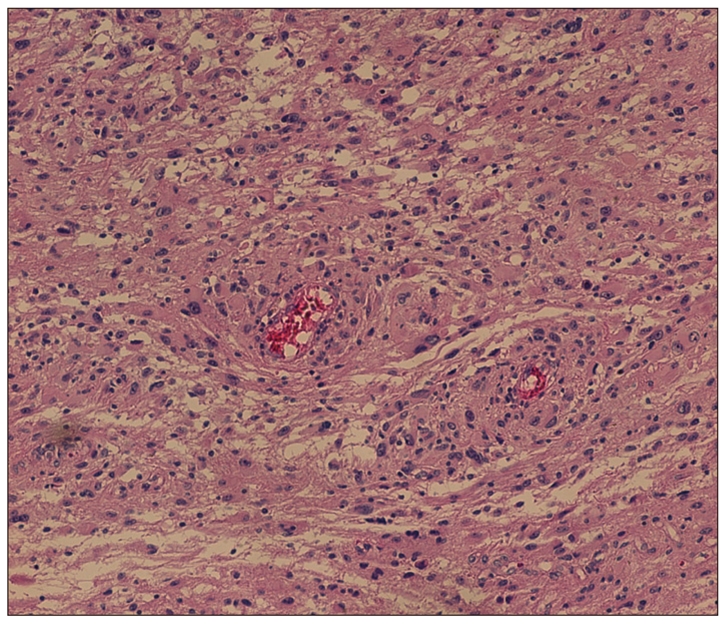
Photomicrographs of the lesion demonstrate an infiltrating glial neoplasm comprised of highly atypical cells with pleomorphic angular nuclei and modest eosinophilic cytoplasm consistent with astrocytic lineage. Numerous mitoses and tumoral necrosis are present. This presentation is compatible with a glioblastoma.
DISCUSSION
There is now increasing concern regarding the potential for radiation-induced neoplasm, because of the intensive use of radiotherapy under a wide range of doses and treatment conditions resulting in a longer survival of brain tumor patients2,14,24). Despite the reported documents about the safety of radiosurgery, additional reports of glioma induction after radiosurgery may temper its use in the treatment of benign lesions, such as meningiomas, particularly in younger patients10,12). The following criteria for radiation-induced neoplasms have been previously described7,11) : 1) a second tumor occurs within the field of irradiation used to treat the primary disease; 2) the tumor is not present prior to irradiation, and there has been a reasonable interval between radiotherapy and the detection of the second tumor (usually several years); 3) a histological difference exists between the primary and subsequent tumor; and 4) no known genetic or predisposing conditions to secondary malignancy are observed. In other series, the criterion of latent period in radiation induced tumor was set to 5 years5,6,13). However, the "5 year" period is not accepted as an absolute standard due to insufficient biologic evidence. Therefore, the present case could be considered as radiation induced tumor. In this way, the present case fits the all criteria and deserves to be diagnosed as radiation-induced malignancy.
The mechanism of radiation-induced neoplasms is multifaceted and not well understood. However, radiation is now well recognized to be a trigger, not only a treatment, for cancer. When the cells are irradiated, the probability of malignancy increases with dose, most likely with no threshold. That is, no dose is too small to be effective. This view is based on experimental data showing that even a single photon can cause a base change leading to mutations that eventually can cause malignancy. In contrast, the severity of malignancy is not dose related. That is, a cancer induced by a small dose of radiation is not less harmful than a cancer induced by a large dose. Furthermore, the absolute value of radiation dose in stereotactic radiosurgery is low. But, in the concept of biological equivalent dose, it is not a low-dose treatment, thus it could cause radiation induced tumor. The lower incidence of radiation induced tumor in radiosurgery, could be explained by relatively smaller volume of normal tissue exposed to radiation compared to conventional radiation. Brada et al.4) reported the risk of second brain tumor formation in 334 patients treated for pituitary tumors with surgery and fractionated small-field irradiation (median dose, 45 Gy). With a cumulative follow-up period of 3760 years, five patients developed second tumors (two astrocytomas, two meningiomas, and one meningeal sarcoma). The latency period is similar to what has been seen with fractionated therapy in the several years period after radiosurgery, and malignant tumors occur earlier than their benign tumor counterparts. The cumulative risk of developing second brain tumors was 1.3% at 10 years and 1.9% at 20 years. The relative risk of a second brain tumor was 9.38 compared with the normal population4).
Concerning the risk of developing a secondary neoplasm, several factors such as patient age, tissue vulnerability, radiation type and dose, underlying disease, and additional chemotherapy may all play an important role2,4,17,24,25). The relative risk of a secondary tumor following cranial irradiation in children has been estimated to be from 2.6 to 38.8 compared with the risk in the standard population8,15,22) while in the adult population it was estimated to be from 9.38 to 164,24). In some cases, younger patients have been reported more vulnerable for second tumor development. Sadetzki et al.16) described 253 patients who developed meningiomas after radiation for tinea capitis. The mean time from exposure to meningioma diagnosis was 36 years (range, 12-49 year). The authors found a higher incidence of multiple lesions, a younger age at diagnosis, and a higher percentage of calvarial lesions than in control patients who developed meningiomas without previous exposure to ionizing radiation16).
The toxicity of therapeutic doses of radiation is primarily acute. However, the carcinogenic effects are not restricted to those tissues manifesting clinical toxicity. In fact, the opposite may be more accurate, because high dose of radiation may sterilize the carcinogenic potential of a tissue by killing cells. Only in those cells that are not killed deoxyribonucleic acid repair errors may lead to transforming mutations. The efficiency of tumor induction varies inversely with repair capacity, which in turn depends on the integrity of cell cycle check-points3,9). The general form of the dose-response curve for radiation associated second tumors is not clear, but several experiments on small animals suggest that the incidence increases with dose up to a maximum usually occurring between 3 and 10 Gy, followed by a subsequent monotonic decrease3). There are also studies reporting that the highest incidence of radiation associated second tumors occurs at field peripheries, where the dose is less than at the field center9). Most of the reported cases of radiation-associated tumor were related to conventional radiotherapy. However, malignant tumors after GKS of benign tumors have rarely been discussed. There were small number of malignant neoplasms after treatment of vestibular schwannoma, meningioma or arteriorvenous malformation (Table 1). Considering that carcinogenetic potential is not necessarily dose-dependent and sublethal damage and repair errors are required for development of secondary tumors, single session radiosurgery may be relatively safer than fractionated radiotherapy with repeated chance of sublethal damage. However, it seems that radiation-induced malignancy can occur within very low-dose peripheral regions as well as the full-dose regions. Because the number of the patients who underwent radiosurgery and long-term follow up is much smaller than that treated with fractionated radiotherapy, relative safety of radiosurgery needs to be proven by further accumulation of clinical data. We draw a conclusion that the number of GKS performed, is not important. As cumulative level of radiation increases by the repeated GKS, so does the oncogenic opportunity. In the present study, the cumulative radiation dose of three repeated GKS is higher than that of conventional radiotherapy, leading to oncogenic change. In our series, clinical outcome was not different and as poor as primary glioblastoma treated with standard treatment of surgery, radiotherapy and chemotherapy. Although malignancy after radiosurgery is very rare, its impact is not acceptable considering the benign nature of the primary disease. No one knows for sure that how much dose of the radiation causes oncogenic transformation. Therefore, the possibility of this fatal complication should be balanced always in case of radiosurgery.
Table 1.
Summary of the radiosurgery induced malignant brain tumor described in literature (including our case)
Date of treatment : at time of the first radiosurgery, Dose : marginal doses or cumulative doses, Interval : time from radiosurgery to second tumor diagnosis, VS : vestibular schwannoma, AVM : ateriovenous malformation, MNG : meningioma, GBM : glioblastoma multiforme, MS : malignant schwannoma, MPNST : malignant peripheral nerve sheath tumor, GKS : gamma knife radiosurgery
CONCLUSION
We experienced a case of radiosurgery-associated glioblastoma that developed after GKS for meningioma. Although the risk of radiosurgery-associated tumor is very low, application of radiosurgery for benign tumors should be very cautious because its occurrence is fatal to the patients.
References
- 1.Bari ME, Forster DM, Kemeny AA, Walton L, Hardy D, Anderson JR. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg. 2002;16:284–289. doi: 10.1080/02688690220148888. [DOI] [PubMed] [Google Scholar]
- 2.Bliss P, Kerr GR, Gregor A. Incidence of second brain tumours after pituitary irradiation in Edinburgh 1962-1990. Clin Oncol (R Coll Radiol) 1994;6:361–363. doi: 10.1016/s0936-6555(05)80187-6. [DOI] [PubMed] [Google Scholar]
- 3.Boice JD, Jr, Land CE, Shore RE, Norman JE, Tokunaga M. Risk of breast cancer following low-dose radiation exposure. Radiology. 1979;131:589–597. doi: 10.1148/131.3.589. [DOI] [PubMed] [Google Scholar]
- 4.Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304:1343–1346. doi: 10.1136/bmj.304.6838.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahan WG. Radiation-induced sarcoma--50 years later. Cancer. 1998;82:6–7. doi: 10.1002/(sici)1097-0142(19980101)82:1<6::aid-cncr2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Chang SM, Barker FG, 2nd, Larson DA, Bollen AW, Prados MD. Sarcomas subsequent to cranial irradiation. Neurosurgery. 1995;36:685–690. doi: 10.1227/00006123-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Dodick DW, Mokri B, Shaw EG, Miller GM, Unni KK. Sarcomas of calvarial bones : rare remote effect of radiation therapy for brain tumors. Neurology. 1994;44:908–912. doi: 10.1212/wnl.44.5.908. [DOI] [PubMed] [Google Scholar]
- 9.Epstein R, Hanham I, Dale R. Radiotherapy-induced second cancers : are we doing enough to protect young patients? Eur J Cancer. 1997;33:526–530. doi: 10.1016/s0959-8049(97)00056-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaido T, Hoshida T, Uranishi R, Akita N, Kotani A, Nishi N, et al. Radiosurgery-induced brain tumor. Case report. J Neurosurg. 2001;95:710–713. doi: 10.3171/jns.2001.95.4.0710. [DOI] [PubMed] [Google Scholar]
- 11.Liwnicz BH, Berger TS, Liwnicz RG, Aron BS. Radiation-associated gliomas : a report of four cases and analysis of postradiation tumors of the central nervous system. Neurosurgery. 1985;17:436–445. doi: 10.1227/00006123-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 12.McIver JI, Pollock BE. Radiation-induced tumor after stereotactic radiosurgery and whole brain radiotherapy : case report and literature review. J Neurooncol. 2004;66:301–305. doi: 10.1023/b:neon.0000014497.28981.4b. [DOI] [PubMed] [Google Scholar]
- 13.Muracciole X, Régis J. Radiosurgery and carcinogenesis risk. Prog Neurol Surg. 2008;21:207–213. doi: 10.1159/000157166. [DOI] [PubMed] [Google Scholar]
- 14.Nishio S, Morioka T, Fukui M. Radiation injury of the brain. Crit Rev Neurosurg. 1997;7:408–415. [Google Scholar]
- 15.Ron E, Modan B, Boice JD, Jr, Alfandary E, Stovall M, Chetrit A, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 16.Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D. Radiation-induced meningioma : a descriptive study of 253 cases. J Neurosurg. 2002;97:1078–1082. doi: 10.3171/jns.2002.97.5.1078. [DOI] [PubMed] [Google Scholar]
- 17.Salvati M, Cervoni L, Puzzilli F, Bristot R, Delfini R, Gagliardi FM. High-dose radiation-induced meningiomas. Surg Neurol. 1997;47:435–441. doi: 10.1016/s0090-3019(96)00360-6. discussion 441-442. [DOI] [PubMed] [Google Scholar]
- 18.Salvati M, Frati A, Russo N, Caroli E, Polli FM, Minniti G, et al. Radiation-induced gliomas : report of 10 cases and review of the literature. Surg Neurol. 2003;60:60–67. doi: 10.1016/s0090-3019(03)00137-x. discussion 67. [DOI] [PubMed] [Google Scholar]
- 19.Sanno N, Hayashi S, Shimura T, Maeda S, Teramoto A. Intracranial osteosarcoma after radiosurgery--case report. Neurol Med Chir (Tokyo) 2004;44:29–32. doi: 10.2176/nmc.44.29. [DOI] [PubMed] [Google Scholar]
- 20.Shamisa A, Bance M, Nag S, Tator C, Wong S, Norén G, et al. Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. Case report and review of the literature. J Neurosurg. 2001;94:816–821. doi: 10.3171/jns.2001.94.5.0816. [DOI] [PubMed] [Google Scholar]
- 21.Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after gamma knife radiosurgery. Lancet. 2002;360:309–310. doi: 10.1016/S0140-6736(02)09521-1. [DOI] [PubMed] [Google Scholar]
- 22.Soffer D, Pittaluga S, Feiner M, Beller AJ. Intracranial meningiomas following low-dose irradiation to the head. J Neurosurg. 1983;59:1048–1053. doi: 10.3171/jns.1983.59.6.1048. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen J, Mirz F, Wetke R, Astrup J, Bojsen-Møller M, Nielsen E. Intracranial sarcoma in a patient with neurofibromatosis type 2 treated with gamma knife radiosurgery for vestibular schwannoma. Am J Otol. 2000;21:364–370. doi: 10.1016/s0196-0709(00)80046-0. [DOI] [PubMed] [Google Scholar]
- 24.Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993;72:2227–2233. doi: 10.1002/1097-0142(19931001)72:7<2227::aid-cncr2820720727>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Tucker MA, D'Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Rockhill J, Born DE, Sekhar LN. A case of high-grade undifferentiated sarcoma after surgical resection and stereotactic radiosurgery of a vestibular schwannoma. Skull Base. 2010;20:179–183. doi: 10.1055/s-0029-1242195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu JS, Yong WH, Wilson D, Black KL. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576–1577. doi: 10.1016/S0140-6736(00)03134-2. [DOI] [PubMed] [Google Scholar]