Abstract
Background
Intralipid, a brand name for the first safe fat emulsion for human use, has been shown to be cardioprotective. However, the mechanism of this protection is not known. Here we investigated the molecular mechanism(s) of Intralipid-induced cardioprotection against ischemia/reperfusion injury, particularly the role of GSK-3β and mitochondiral permeability transition pore in this protective action.
Methods
In-vivo rat hearts or isolated Langendorff-perfused mouse hearts were subjected to ischemia followed by reperfusion with Intralipid (1% in ex-vivo and one bolus of 20% in in-vivo) or vehicle. The hemodynamic function, infarct size, threshold for the opening of mitochondiral permeability transition pore and phosphorylation levels of Akt/ERK/GSK-3β were measured.
Results
Administration of Intralipid at the onset of reperfusion resulted in ~70% reduction in infarct size in the in-vivo rat model. Intralipid also significantly improved functional recovery of isolated Langendorff-perfused mouse hearts as the rate pressure product was increased from 2999±863mmHg*beats/min in control to 13676±611 mmHg*beats/min (Mean±SEM) and the infarct size was markedly smaller (18.3±2.4% vs. 54.8±2.9% in control, P<0.01). The Intralipid-induced cardioprotection was fully abolished by LY294002, a specific inhibitor of PI3K, but only partially by PD98059, a specific ERK inhibitor. Intralipid also increased the phosphorylation levels of Akt/ERK1/GSK-3β by 8, 3 and 9 fold, respectively. The opening of mitochondiral permeability transition pore was inhibited by Intralipid as calcium retention capacity was higher in Intralipid group (274.3±8.4nM/mg vs. 168.6±9.6nM/mg control).
Conclusions
Postischemic treatment with Intralipid inhibits the opening of mitochondiral permeability transition pore and protects the heart through GSK-3β via PI3K/Akt/ERK pathways.
Introduction
Coronary heart disease remains the leading cause of morbidity and mortality in western countries. The best hope of salvaging viable myocardium after a coronary occlusion is by rapid reperfusion of the ischemic myocardium, either by thrombolysis or primary percutaneous coronary intervention1. Although reperfusion restores blood flow, oxygen, and nutrients to the cardiac muscle, it also has the potential to induce reperfusion injury. Postconditioning of the heart with brief episodes of reperfusion/occlusion at the onset of reflow has been shown to limit infarct size2;3. However, this approach is not practical for patients treated with thrombolytic agents and therefore a more generic pharmacological postconditioning is still needed. The ideal pharmacological candidates need to be safe and effective when administrated during the first few minutes of reperfusion by inducing cellular protection or enhancing myocardial tolerance to ischemia/reperfusion injury. Several drugs have yielded encouraging results in animals and a few have been tested in humans, however, none of these modalities have been widely accepted4–6.
Lipids and in particular polyunsaturated fatty acids (PUFAs) have received special cardiovascular research attention, as PUFA rich diets are associated with a decreased risk of coronary artery disease7;8. Acute application of PUFA to cardiomyocytes has also been shown to shorten action potential duration and this could be accounted for anti-arrhythmic mechanism of the PUFAs9. Intralipid, a brand name for the first safe fat emulsion for human use; Intralipid 20% is an emulsion of soy bean oil (20%), egg yolk phospholipids (1.2%) and glycerol (2.2%). Intralipid has been widely used in patients who need total parenteral nutrition and as a vehicle for different drugs like propofol. It has been shown recently that post-ischemic administration of Intralipid protects the isolated rat heart against ischemia/reperfusion injury10. However, the molecular mechanism in which Intralipid mediates cardioprotection is completely unknown.
Here we found that post-ischemic administration of Intralipid protects the heart both in the in-vivo rat model as well as in isolated mouse hearts. We then investigated the mechanism of Intralipid-induced cardioprotection. Our data revealed that postischemic treatment of Intralipid inhibits the opening of the mitochondrial permeability transition pore (mPTP) and protects the heart by recruiting the reperfusion injury salvage kinases (RISK) pathway PI3K/Akt/ERK1 leading to phosphorylation of GSK-3β.
Materials and Methods
Animals
Male Sprague-Dawley rats 250–300 g and male mice (C57BL/6) 2–3 months old were used. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH Publication No. 85–23, revised 1996). Animal protocol was approved Animal Research Committee, University of California, Los Angeles, California.
Left anterior descending coronary artery occlusion and measurement of infarct size
Male Sprague-Dawley rats were anesthetized with ketamine (80 mg/kg i.p.) and xylazine (8 mg/kg i.p.). The rats were intubated and ventilated with a ventilator (CWE SAR-830/P). The hearts were exposed through a left thoracotomy in the fourth intercostal space. The pericardium was opened, and a 5.0 Prolene suture was tightened around the proximal left anterior descending coronary artery. Ischemia was confirmed by ST elevation in Electrocardiograph. The heart was subjected to 30 min of ischemia, following by 180 min of reperfusion, which was achieved by releasing the tension on the ligature. An Intralipid bolus (20%, 5ml/kg body weight) was applied via the femoral vein 5 minutes before reperfusion. Same volume of PBS was given in the control group (see Fig. 1A). At the end of the experiment, 2.5 ml of 1% Evans Blue dye was injected into the femoral vein and the myocardial ischemic area at risk (AAR) was identified as the region lacking blue staining. The ventricles of the hearts were sliced transversely into 2mm thick slices. The slices were incubated in 1% triphenyltetrazolium chloride (TTC) at 37°C for 15 min to identify the non-infarcted and infarcted areas. The infarcted area was displayed as the area unstained by TTC. Infarct size was expressed as a percentage of the AAR.
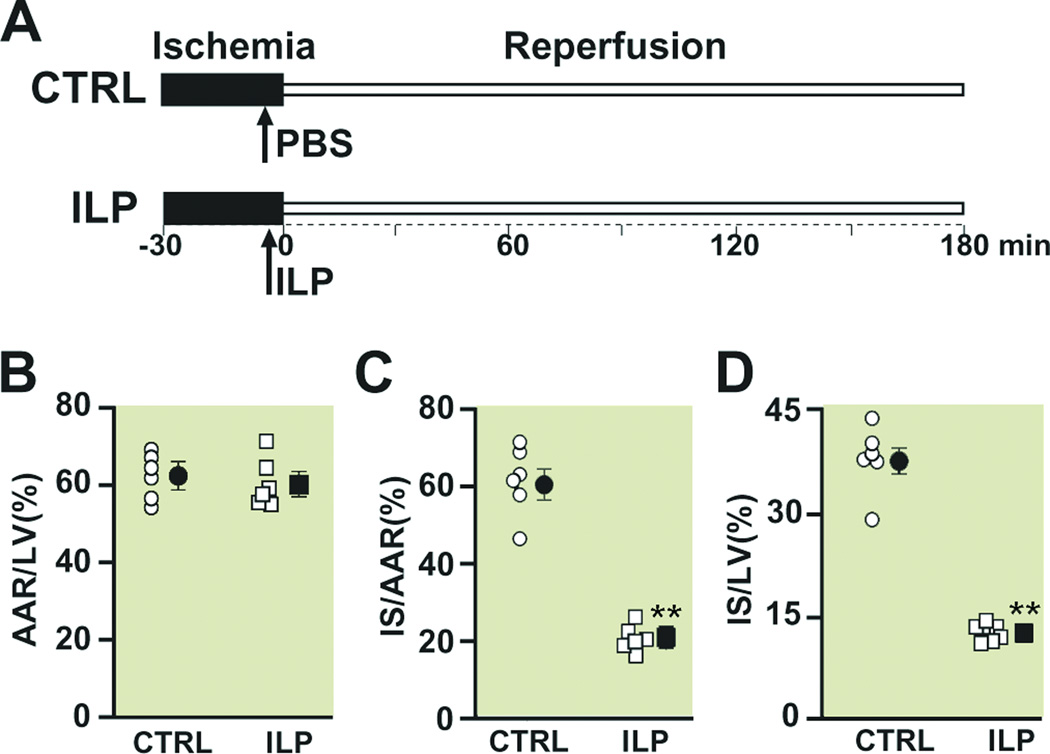
Figure 1. Intralipid administration reduces the infarct size in the in vivo ischemia/reperfusion rat model.
A. experimental protocol, the left coronary artery was occluded for 30 minutes followed by 3 hr of reperfusion. One single IV bolus of Phosphate Buffered Saline (PBS) (control group, CTRL) or 20% Intralipid (5ml/kg body weight, ILP) was administered 5 min before reperfusion. Percentage of area at risk (AAR) divided by left ventricle (LV) (B), infarct size (IS) divided by AAR (C), and infarct size (IS) divided by left ventricle in CTRL (circles) and Intralipid (squares). The individual measurements (n=6 in ILP and n=6 in CTRL) are shown in open shapes whereas the averages (Mean±SEM) are shown in filled shapes **P<0.01 vs. CTRL.
Langendorff preparation
Mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and heparin (200 IU/kg) was injected to prevent blood coagulation. The heart was quickly removed and perfused through the aorta in a Langendorff apparatus with Krebs Henseleit bicarbonate buffer solution (KH) (mM): glucose 11.1, NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, CaCl2 2 at pH 7.4 bubbled with 95% O2/5% CO2 at 37 °C. Once the equilibration was achieved, the aorta was clamped for 20 min to induce global normothermic (37°C) ischemia (the heart was immersed in the 37°C Krebs solution during ischemia), followed by reperfusion with KH alone (control) or with additional 1% Intralipid. The duration of reperfusion was 40 min for heart functional measurements and 10 min for calcium retention capacity and signaling pathways.
Heart functional measurements
A catheter (1.4F Millar SPR-671) connected to a pressure transducer (Power Lab, ADInstruments Inc. Colorado Springs, CO) was directly inserted into the left ventricle (LV) to measure left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and heart rate (HR). The (LVDP) was calculated as LVDP = LVSP−LVEDP and the rate pressure product (RPP) as RPP = HR×LVDP. The maximum rate of LV pressure rise of (dP/dtmax) and decline (−dP/dtmin) were directly calculated from the selected stable recordings.
Myocardial necrosis
At the end of the reperfusion, the hearts were cut into four transverse slices and myocardial necrosis was assessed by measurement of the infarct size using triphenyltetrazolium chloride (TTC) staining11. Since the heart was immersed in the 37°C Krebs solution during ischemia, 40 min reperfusion was sufficient to induce necrosis in the mouse model as used by our group as well as other groups11;12. In fact, the size of necrosis was similar for reperfusion of 40 and 90 min (data not shown). The slices were fixed in 4% paraformaldehyde, and the area of necrosis was quantified by Photoshop and expressed as the percentage of total ventricular area.
Ca2+-induced mitochondrial permeability transition
2.5.1. Preparation of isolated mitochondria
Mitochondria is prepared as previously described3. Briefly, myocardial sections of ex-vivo hearts reperfused for 10 min (approximately 0.15–0.22 g) were placed in isolation buffer A containing (mM: 70 sucrose, 210 mannitol, 1 EDTA and 50 Tris-HCl, pH 7.4 at 4°C. The tissue was finely minced with scissors and homogenized in the same buffer A (1 ml buffer/0.1g of tissue) using Kontes and Potter-Elvehjem tissue grinders. The homogenate was centrifuged at 1,300 g for 3 min; the supernatant was filtered through a cheesecloth and centrifuged at 10,000 g for 10 min. The mitochondrial pellet was resuspended in isolation buffer B containing in mM 70 sucrose, 210 mannitol, 0.1 EDTA and 50 Tris-HCl, pH 7.4. Mitochondrial protein concentration was measured using the Bradford assay.
2.5.2. Calcium Retention Capacity (CRC)
The onset of the mPTP opening was assessed following in-vitro Ca2+ overload as previously described.13 Free Ca2+ concentration outside the mitochondria was recorded with 0.5 µM calcium green-5N (Invitrogen, Carlsbad, CA) using excitation and emission wavelengths set at 500 and 530 nm, respectively. Isolated mitochondria (500 µg of protein) were suspended in 2 ml buffer C (mM, 150 sucrose, 50 KCl, 2 KH2PO4, 5 succinic acid and 20 Tris/HCl, pH 7.4). Samples were pre-incubated for 90 sec in the spectrofluorometer cuvette, and CaCl2 pulses (2 µl of 10 mM stock solution) were applied every 60 sec in the spectrofluorometer. The Ca2+ pulses induced a peak of extra-mitochondrial Ca2+ concentration that returned to near-baseline level as Ca2+entered the mitochondrial matrix via the Ca2+ uniporter. With increasing Ca2+ loading, the extra-mitochondrial Ca2+ concentration started accumulating, reflecting a lower capacity for mitochondria Ca2+ uptake, which was followed by a sustained Ca2+ increase indicating a massive release of the mitochondria Ca2+ by the mPTP opening. The Ca2+ retention capacity (CRC) was defined as the amount of Ca2+ required to trigger this massive Ca2+ release which was used here as an indicator of the mPTP sensitivity to Ca2+. CRC was expressed as µmolar of CaCl2 per mg of mitochondrial protein.
Western Blot analysis
The entire ex-vivo hearts were used for making whole heart lysates, since in this model the whole heart is considered to be area at risk. Hearts were homogenized at 4°C in (mM): 150 NaCl, 50 Tris-HCl, 1 EGTA. 1 EDTA, 1 NaF, 1 PMSF, 1 Na3VO4, 1% NP-40, 0.1% SDS and 0.5% Sodium Deoxycholate (pH 7.4) supplemented with Protease and Phospatase Inhibitor cocktails (Roche, San Francisco, CA). The samples were centrifuged at 12,000 g for 10 min and the supernatants were collected. Protein concentration was measured and 100 µg of total protein was loaded on a 4–20% gradient Tris/HCl SDS polyacrylamide gel, electrotransferred to nitrocellulose paper, blocked with 5% nonfat dry milk in 20 mM of TBS with 0.1% Tween and 0.5% Triton-X100 and incubated with primary antibodies. Blots were then indirectly labeled using infrared fluorophore-conjugated secondary antibodies for 1 h at room temperature, and visualized with the Odyssey™ Imaging System (Li-Cor, Lincoln, NE). Equal loading of protein onto each lane in the gel was confirmed with Vinculin. The proteins were first normalized to their corresponding Vinculin and then the phosphorylated proteins were normalized to their corresponding total protein levels.
Pharmacological Agents and antibodies
Intralipid 20% was purchased from Sigma (St. Louis, MO). Intralipid was used at 5ml/kg in the in-vivo rat model of ischemia/reperfusion injury based on the previous work by Weinberg et al14 and 1% in ex-vivo. The dose in ex-vivo was calculated based on the dose that the heart is exposed in the in-vivo model by taking into account dilution of Intralipid in blood stream15. The LY294002 was purchased from GIBCO/Invitrogen (Carlsbad, CA) and was used at 45 µM according to the data sheet provided by GIBCO stating that Ly294002 at 50 µM completely abolishes the PI3k activity without apparent cell toxicity. PD98059 was purchased from Invitrogen and was used 10 µM, a concentration that has been used previously by many investigators in isolated langendorff perfused hearts to explore the role of ERK signal transduction pathway16-18. The primary antibodies used were anti-ERK1/2, (rabbit polyclonal), anti-phospho ERK1/2 (Thr202/Tyr204, mouse monoclonal), anti-AKT (rabbit polyclonal), phospho AKT (Ser 473, rabbit polyclonal), anti-GSK-3β-phospho GSK-3β Mouse monoclonal anti-Vinculin. All antibodies were purchased from Cell Signaling (Danvers, MA), except anti-vinculin which was from Sigma (St. Louis, MO). The secondary antibodies were goat anti-rabbit Alexa 680 from Invitrogen (Carlsbad, CA) and goat anti mouse IR Dye 800 CW from Odyssey™, Li-Cor.
Statistical Analysis
For the in vivo study which has only 2 groups, means were compared using the t-test. For the ex vivo studies with four or five groups, mean profiles over time were compared across groups using repeated measure analysis of variance (ANOVA) methods. Under the ANOVA model, pairwise mean comparisons were judged significant using the Tukey studentized range criterion. This criteria prevents the type I error from exceeding the nominal alpha=0.05 level for the outcome. SPSS, version 13.0, (SPSS Inc, Chicago Ill) was used to carry out the computations. As all outcomes were continuous, results were summarized with Means ± standard errors of the mean (SEM).
Results
Intralipid protects the heart against ischemia/reperfusion injury in the in-vivo rat model
It has been previously shown that post-ischemic administration of Intralipid can protect the heart against ischemia/reperfusion injury in isolated rat hearts10. Here we first examined whether Intralipid can also protect the heart against ischemia/reperfusion injury in the in-vivo rat model. The left coronary artery was occluded for 30 min followed by 3 hr of reperfusion. One single bolus of PBS or Intralipid was applied through the femoral vein 5 min before the reperfusion (Fig. 1A). The area at risk (AAR) to LV ratio was similar in both groups (62.1±2.4 in control (n=6) vs. 60.1±2.6 in Intralipid (n=6)), indicating that the two groups were subjected to a comparable degree of ischemic risk (Fig. 1B). However, the infarct size was significantly smaller in the Intralipid group compared to control; the ratio of infarct size to AAR was 20.7±1.3 in Intralipid vs. 61.8±3.1 in control(P<0.01), and the ratio of infarct size to LV was 12.4±0.6 in Intralipid vs. 38.1±1.5 in control (P<0.01) (Fig. 1C, D). These data demonstrate that only one bolus of Intralipid right before reperfusion is sufficient to protect the heart against ischemia/reperfusion injury in-vivo.
Post-ischemic administration of Intralipid protects isolated mice hearts against ischemia/reperfusion injury
To explore the molecular mechanism in which Intralipid mediates protection against ischemia/reperfusion injury, we used the Langendorff-perfused isolated mouse heart rather than the in-vivo model of ischemia/reperfusion injury as the former can be manipulated easier to unravel the key mechanisms mediating Intralipid-induced cardioprotection. To this end, we first examined if Intralipid could also protect mouse hearts against ischemia/reperfusion injury as in rats. We used the well-established protocol to induce ischemia/reperfusion injury in isolated mouse hearts11;12(Fig. 2A). Similar to rats, postischemic administration of Intralipid significantly improved the functional recovery (RPP= 13676±611mmHg*beats/min (n=6) vs. 2999±863 mmHg*beats/min in control (n=6) (P<0.01) at 40 min of reperfusion, Fig. 2B). Intralipid group also showed a much better LV dP/dtmax and LV dP/dtmin, and LVDP compared to control hearts (Fig. 2C,D and Table-1). The infarct size was also significantly smaller in Intralipid group (18.3±2.4% in Intralipid (n=9) vs. 54.9±2.9% in control (n=10), P<0.01, Fig. 2E–J). These data suggest that Intralipid protects both mice and rats from ischemia/reperfusion injury.
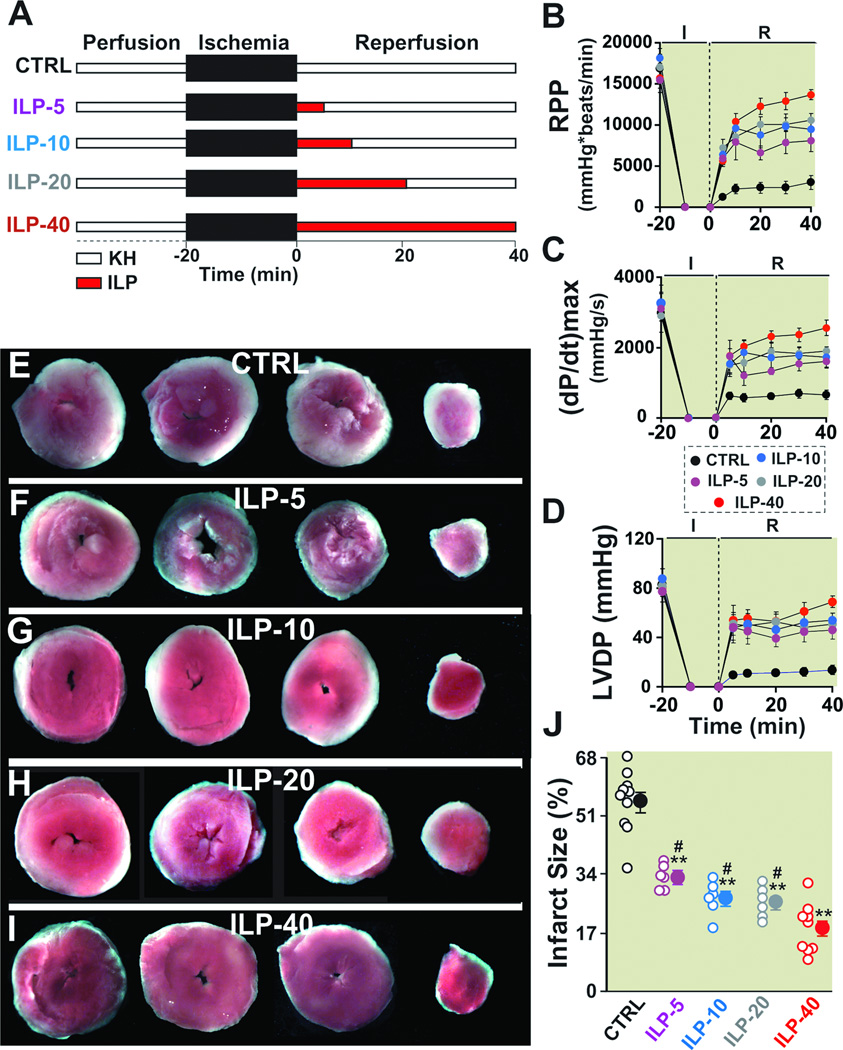
Figure 2. Administration of Intralipid at reperfusion improves heart functional recovery and reduces infarct size against reperfusion injury.
A. Experimental protocol. The isolated mouse hearts were reperfused with Krebs Henseleit (KH, control group, CTRL), or 1% Intralipid for 5 min (ILP-5), 10 min (ILP-10), 20 min (ILP-20) or 40 min (ILP-40), followed by reperfusion with KH for the remainder of 40 min. Rate pressure product (RPP, B), the maximum rate of left ventricle (LV) pressure rise (dP/dtmax) and decline (−dP/dtmin, C) and left ventricular developed pressure (LVDP, D) as a function of time in CTRL (black, n=6), ILP-5 (purple, n=6), ILP-10 (blue, n=6), ILP-20 (gray, n=6) and ILP-40 (red, n=6). Four slices of the same heart after 2,3,5-triphenyltetrazolium chloride (TTC) staining in CTRL (E), ILP-5 (F), ILP-10 (G), ILP-20 (H), and ILP-40 (I). The white area represents the infarct zone and the red shows the viable area. J. The area of necrosis as the percentage of total left ventricular (LV) area in CTRL (black, n=10), ILP-5 (purple, n=6), ILP-10 (blue, n=6), ILP-20 (gray, n=6) and ILP-40 (red, n=9). The individual measurements in each groups are shown in open circles whereas the averages (Mean±SEM) are shown in filled circles **P < 0.01 vs. CTRL, #P<0.05 vs. ILP-40.
Table-1.
Intralipid improves heart functional recovery against reperfusion injury through PI3K/Akt pathways
| LVSP mmHg |
LVEDP mmHg |
LVDP mmHg |
HR Beats/min |
|
|---|---|---|---|---|
| Baseline | ||||
| CTRL | 99±10 | 4±2 | 95±11 | 188±44 |
| ILP | 96±6 | 2±1 | 94±6 | 161±32 |
| ILP+LY | 88±4 | 3±1 | 85±5 | 197±12 |
| LY | 77±2 | 4±2 | 73±2 | 241±17 |
| Reperfusion | ||||
| 10 min | ||||
| CTRL | 15±2 | 4±1 | 11±3 | 135±28 |
| ILP | 80±16** | 3±1 | 77±5** | 139±32 |
| ILP+LY | 21±4 | 9±3 | 12±2 | 207±26 |
| LY | 33±6 | 12±6 | 21±3 | 108±7 |
| 20 min | ||||
| CTRL | 16±3 | 4±2 | 12±3 | 209±28 |
| ILP | 77±12** | 8±3 | 69±6** | 169±25 |
| ILP+LY | 21±5 | 7±2 | 14±3 | 222±22 |
| LY | 21±5 | 4±2 | 17±3 | 133±12 |
| 30 min | ||||
| CTRL | 16±2 | 5±1 | 11±2 | 223±39 |
| ILP | 73±13** | 6±2 | 67±7** | 177±18 |
| ILP+LY | 25±7 | 8±2 | 17±2 | 221±28 |
| LY | 23±3 | 2±1 | 21±4 | 194±13 |
| 40 min | ||||
| CTRL | 19±4 | 6±2 | 13±3 | 219±36 |
| ILP | 80±16** | 5±2 | 75±8** | 157±14 |
| ILP+LY | 22±4 | 4±1 | 18±3 | 221±23 |
| LY | 27±6 | 6±4 | 21±3 | 189±23 |
Cardiac functional recovery parameters of left ventricular systolic pressure (LVSP); left ventricular end-diastolic pressure (LVEDP); left ventricular developed pressure (LVDP) and heart rate (HR) before ischemia (baseline) and at different times of reperfusion in control group (CTRL), 1% Intralipid (ILP), 1% Intralipid+LY294002 (ILP+LY) and LY294002 alone (LY).
Values are Mean ± SEM;
P < 0.01 vs CTRL (n = 6).
Administration of Intralipid during the first few minutes of reperfusion is sufficient to induce protection and the protection is maintained after removal of Intralipid
As the first few minutes of reperfusion are critical in myocardial protection, we investigated whether administration of Intralipid for shorter durations than 40 min could protect the heart and whether the functional recovery is maintained upon removal of Intralipid. The isolated mouse hearts were therefore treated with Intralipid for only the first 5, 10, 20 min of reperfusion followed by KH for the remaining of 40 min as shown in Fig 2A. When Intralipid was only administered for 5 minutes (ILP-5), the hemodynamic indexes at the end of 40 min reperfusion were all significantly higher than control group that did not receive any Intralipid at reflow (Fig. 2B–D, Table-1). It was also interesting to note that hemodynamic indexes were maintained when Intralipid was switched to KH at 5 min reperfusion, as these values were not significantly different between 5, 10 and 20 min in Intralipid group. When Intralipid was applied for longer times of 10 min (ILP-10) or 20 min (ILP-20), the hemodynamic indexes at the end of 40 min reperfusion were not significantly higher than ILP-5 (RPP=8058±1297 in ILP-5 (n=6); RPP=9468±1272 in ILP- 10 (n=6) and 10571±797mmHg*beats/min in ILP-20 (n=6).
Consistent with the higher functional recovery in Intralipid treated groups, the infarct sizes were also significantly smaller compared to the control group (33.1±1.4% in ILP-5 (n=6); 26.8±2.1% in ILP-10 (n=6), 25.7±1.8% in ILP-20 (n=6) and 18.3±2.4% in ILP-40 (n= 9) vs. 54.9±2.9% in control (n=10), P<0.01, Fig. 2E–I). In fact, administration of Intralipid during the first even 5 minutes of reperfusion was sufficient to reduce the myocardial infarct size by ~50%. The infarct sizes between ILP-5, ILP-10 and ILP-20 were similar, but were significantly larger than ILP-40 (P<0.05). These data strongly support the view that administration of Intralipid during even the first 5 minutes of reperfusion is sufficient to improve significantly the functional recovery and reduce the infarct size compared to control, although the cardioprotection achieved by 40 min of Intralipid treatment (ILP-40) was significantly higher than ILP-5, ILP-10 and ILP-20.
Intralipid-induced cardioprotection is fully abolished in the presence of a specific inhibitor of PI3K, LY294002
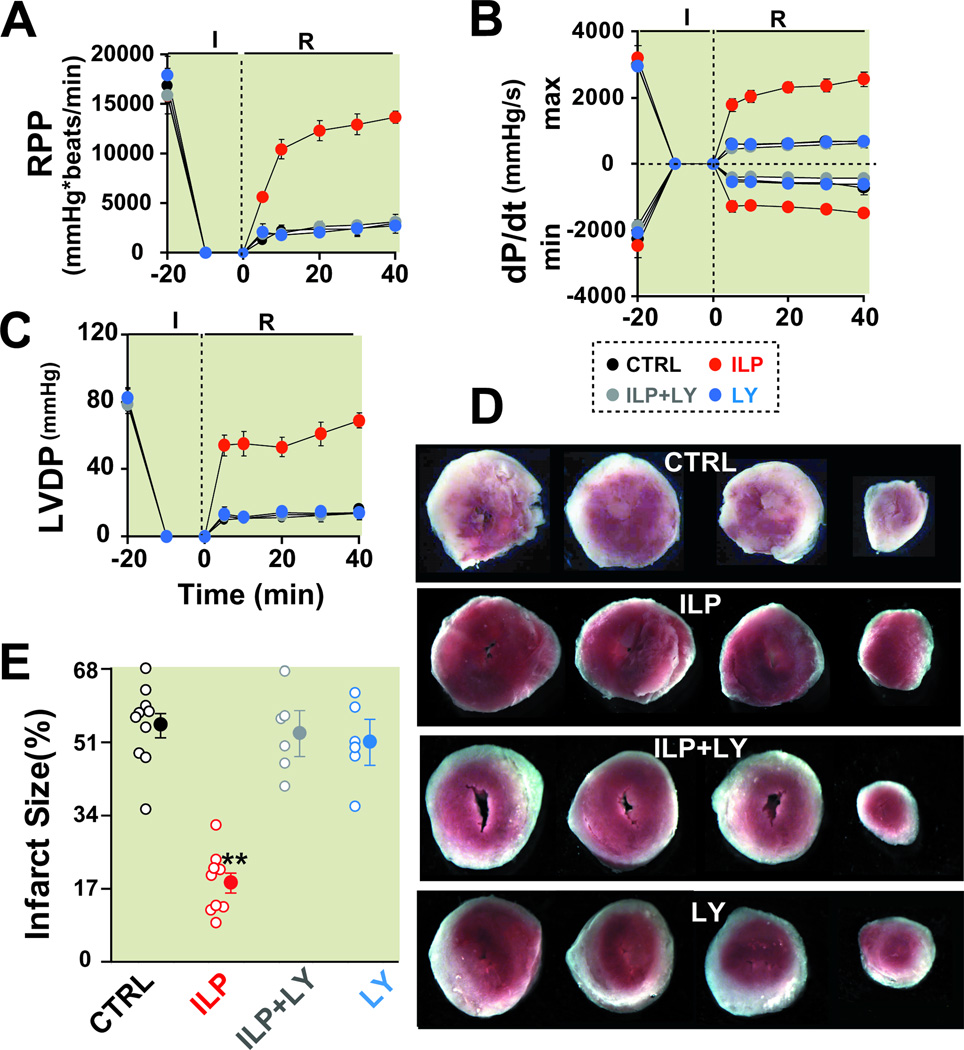
To explore the molecular mechanism in which Intralipid can mediate the protection against ischemia/reperfusion injury, the potential involvement of the well-known prosurvival PI3K-AKT pathway in Intralipid-induced cardioprotection was first examined. The isolated mouse hearts were reperfused for 40 min with Intralipid alone or together with PI3K inhibitor LY294002 (LY, 45 µM). The Intralipid-induced cardioprotection was fully abolished when Intralipid+LY294002 (ILP+LY) was applied, as RPP was significantly lower in the presence of LY294002 (Fig. 3A and Table-1). LY294002 also prevented fully the improvement of LV dP/dtmax, LV dP/dtmin and LVDP observed with Intralipid (Fig. 3B, C). In fact, all of the hemodynamic indexes in ILP+LY were not significantly different from control. LY alone had no effect on the hemodynamic parameters as these values were similar to control (Table-1). The infarct size was also significantly larger in ILP+LY group when compared to Intralipid alone (52.9±5.4% in ILP+LY (n=6) vs. 18.3±2.4% in Intralipid (n=9), P<0.01, Fig. 3D,E). The infarct size in LY group was not significantly different from control (50.9±5.4% in LY (n=6) vs. 54.9±2.9% in control (n=10)). The reduced myocardial functional recovery together with increased infarct size demonstrates PI3K signaling is directly involved in Intralipid-induced cardioprotection against ischemia/reperfusion injury.
Figure 3. PI3K inhibitor abolishes Intralipid-induced cardioprotection against ischemia/ reperfusion injury.
Rate pressure product (RPP, A), the maximum rate of left ventricle (LV) pressure rise (dP/dtmax) and decline (−dP/dtmin, B) and left ventricular developed pressure (LVDP, C) as a function of time in control group (CTRL, black, n=6), 1% Intralipid (ILP, red, n=6), 1% Intralipid+LY294002 (ILP+LY, gray, n=6) and LY294002 alone (LY, blue, n=6). D. Four slices of the same heart in CTRL, ILP, ILP+LY and LY alone after 2,3,5-triphenyltetrazolium chloride (TTC) staining. The white area represents the infarct zone and the red shows the viable area. E. The area of necrosis as the percentage of total left ventricular (LV) area in CTRL (black, n=10), ILP (red, n=9), ILP+LY (gray, n=6) and in LY (blue, n=6). The individual measurements in each group are shown in open circles whereas the averages (Mean±SEM) are shown in filled circles **P<0.01 vs. CTRL.
ERK inhibitor PD98059 partially abolishes Intralipid-induced cardioprotection against ischemia/ reperfusion injury
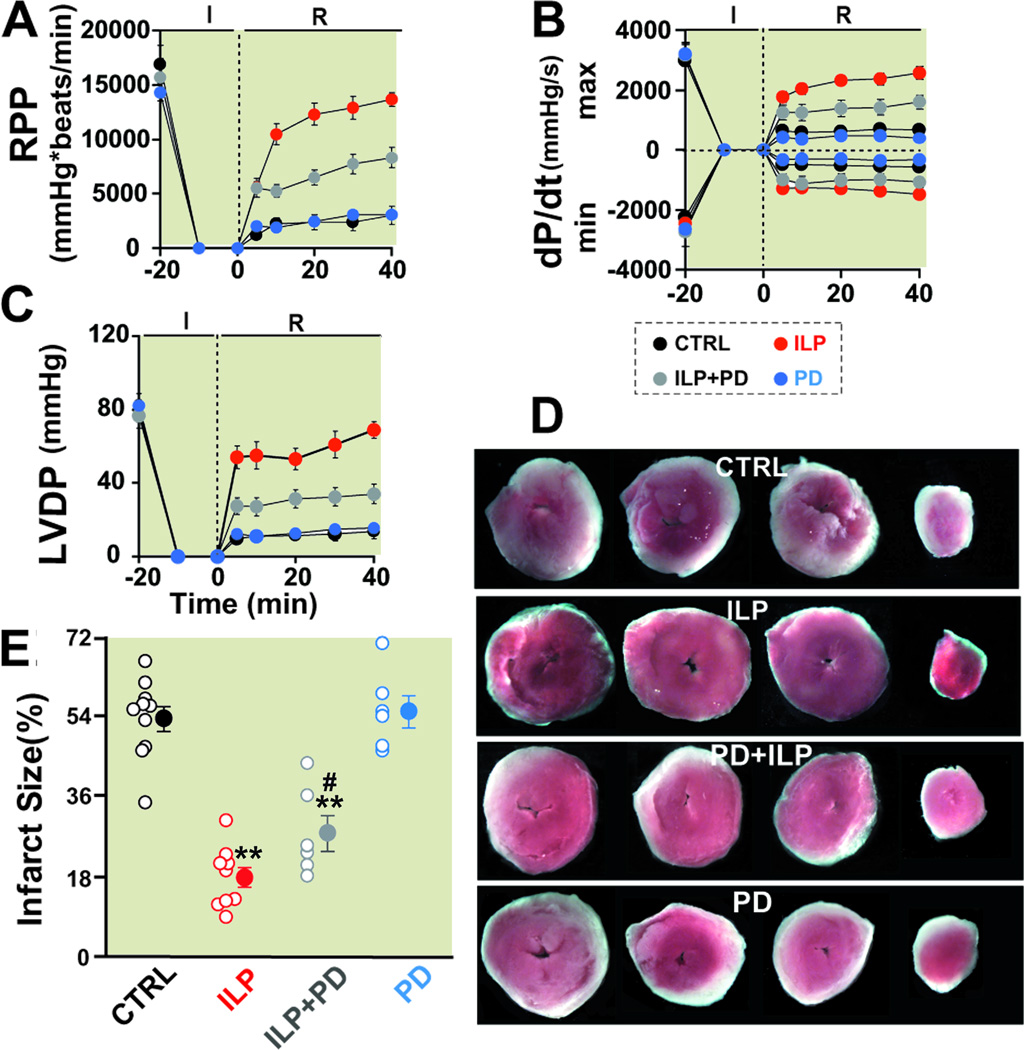
We then examined the involvement of ERK pathway in Intralipid-induced cardioprotection using ERK inhibitor PD98059 (10 µM). The Intralipid-induced cardioprotection was partially abolished when Intralipid+PD98059 (ILP+PD) was applied, as at the end of 40 min reperfusion the RPP, dP/dtmax, dP/dtmin as well as LVDP were all significantly lower compared to the group treated with Intralipid alone, but still significantly higher than control (Fig 4A–C, Table-2). The infarct size was also larger when compared to Intralipid alone (28.5±4.1% in ILP+PD (n=6) vs. 18.3±2.4% in Intralipid (n=9), P<0.01, Fig. 4D, E). PD98059 alone had no effect on the hemodynamic parameters (Table-2) nor on the infarct size as these values were not significantly different from control (56.5±3.6% in PD98059 (n=6) vs. 54.9±2.9% in control (n=10)). The reduction in Intralipid-induced cardioprotection in the presence of PD98059 demonstrates that ERK signaling is also participating in the protection offered by Intralipid against ischemia/reperfusion injury.
Figure 4. ERK inhibitor abolishes partially Intralipid-induced cardioprotection against ischemia/ reperfusion injury.
Rate pressure product (RPP, A), the maximum rate of LV pressure rise (dP/dtmax) and decline (−dP/dtmin, B) and left ventricular developed pressure (LVDP, C) as a function of time in control group (CTRL, black, n=6), 1% Intralipid (ILP, red, n=6), 1% Intralipid+PD98059 (ILP+PD, gray, n=6) and PD98059 alone (PD, blue, n=6). D. Four slices of the same heart in CTRL, ILP, ILP+PD and PD after TTC staining. The white area represents the infarct zone and the red shows the viable area. E. The area of necrosis as the percentage of total left ventricular (LV) area in CTRL (black, n=10), ILP (red, n=9), ILP+PD (gray, n=6)) and in PD (blue, n=6)). The individual measurements in each group are shown in open circles whereas the averages (Mean±SEM) are shown in filled circles. **P < 0.01 vs. CTRL and #P<0.05 vs. ILP).
Table-2.
ERK inhibitor PD98059 partially abolishes Intralipid-induced cardioprotection against ischemia/ reperfusion injury
| LVSP mmHg |
LVEDP mmHg |
LVDP mmHg |
HR Beats/min |
|
|---|---|---|---|---|
| Baseline | ||||
| CTRL | 99±10 | 4±2 | 95±11 | 188±44 |
| ILP | 96±6 | 2±1 | 94±6 | 161±32 |
| PD+ILP | 80±6 | 2±1 | 78±6 | 193±25 |
| PD | 92±10 | 2±1 | 90±10 | 179±36 |
| Reperfusion | ||||
| 10 min | ||||
| CTRL | 15±2 | 4±1 | 11±3 | 135±28 |
| ILP | 80±16** | 3±1 | 77±5** | 139±32 |
| PD+ILP | 35±6## | 3±1 | 32±6## | 184±43 |
| PD | 32±9 | 15±6 | 17±3 | 150±18 |
| 20 min | ||||
| CTRL | 16±3 | 4±2 | 12±3 | 209±28 |
| ILP | 77±12** | 8±3 | 69±6** | 169±25 |
| PD+ILP | 41±7## | 3±2 | 38±5## | 222±35 |
| PD | 19±3 | 4±2 | 15±2 | 166±18 |
| 30 min | ||||
| CTRL | 16±2 | 5±1 | 11±2 | 223±39 |
| ILP | 73±13** | 6±2 | 67±7** | 177±18 |
| PD+ILP | 42±8## | 2±1 | 40±7## | 249±47 |
| PD | 23±4 | 5±3 | 18±1 | 169±31 |
| 40 min | ||||
| CTRL | 19±4 | 6±2 | 13±3 | 219±36 |
| ILP | 80±16** | 5±2 | 75±8** | 157±14 |
| PD+ILP | 47±7## | 2±1 | 45±6## | 221±29 |
| PD | 23±1 | 4±2 | 19±2 | 165±27 |
Cardiac functional recovery parameters of left ventricular systolic pressure (LVSP); left ventricular end-diastolic pressure (LVEDP); left ventricular developed pressure (LVDP) and heart rate (HR) before ischemia (baseline) and at different times of reperfusion in control group (CTRL), 1% Intralipid (ILP), 1% Intralipid+PD98059 (ILP+PD) and PD98059 alone (PD).
Values are Mean ± SEM;
P < 0.01 vs. CTRL and
P < 0.01 vs. ILP (n = 6).
Intralipid induces Akt, ERK and GSK-3βphosphorlyation
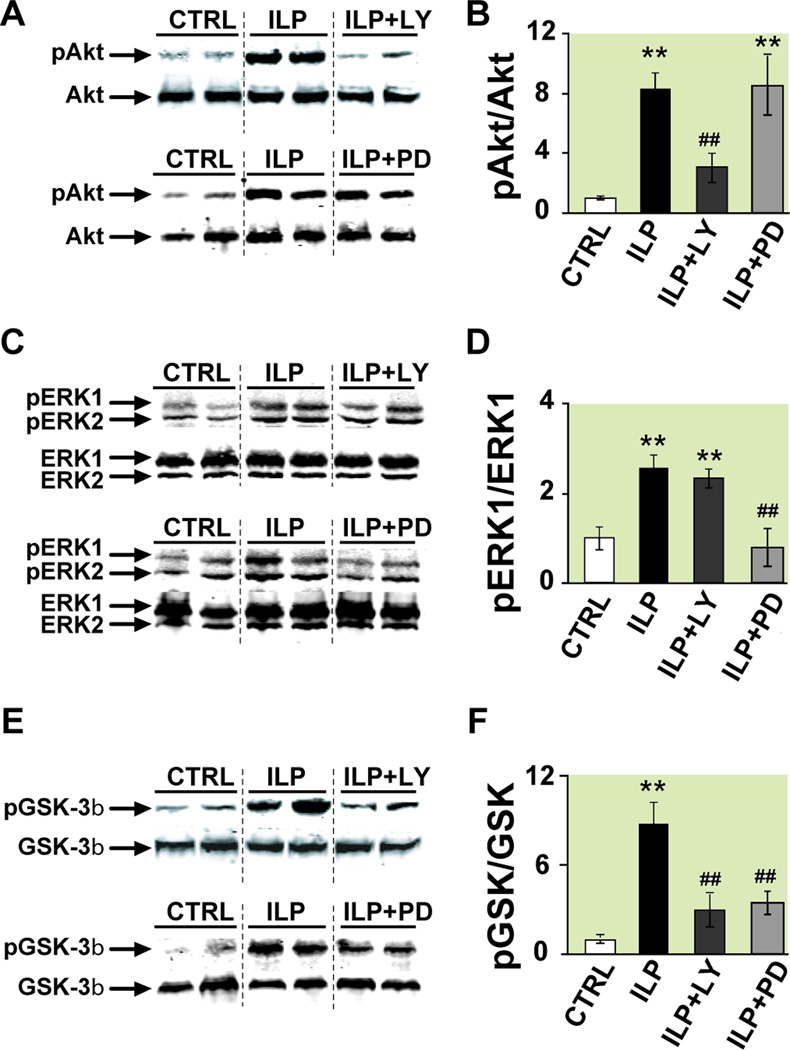
To further confirm the involvement of RISK pathway in Intralipid-induced cardioprotection against ischemia/reperfusion injury, Western Blot analysis of whole heart lysates (ex-vivo) which were reperfused for 10 min with KH (control), Intralipid, ILP+LY or ILP+PD were performed. Intralipid-induced cardioprotection was associated with significant increase in phosphorylation of Akt (~8 fold) which was reversed when Intralipid was applied together with LY294002, but not with PD (in arbitrary units normalized to control (n=6): 8.3±1.2 in Intralipid (n=4), vs. 3.2±0.8 in ILP+LY (n=4) and 8.5±2.2 in ILP+PD (n=4)), Fig. 5A, B). These data further confirm our findings that the protection by Intralipid is mediated via the prosurvival PI3K-Akt pathway. Phospho-ERK1 levels were also significantly increased in Intralipid group (~3 fold) compared with control. PD completely abolished phosphorylation of ERK induced by Intralipid (in arbitrary units normalized to control (n=6): 2.5±0.3 in Intralipid (n=4) vs. 0.8±0.4 in ILP+PD (n=4) and 2.3±0.2 in ILP+LY (n=4), Fig. 5C, D). These data clearly demonstrate that in addition to PI3K/Akt pathway, the protection by Intralipid is also mediated via the ERK pathway. GSK-3β phosphorylation was also significantly increased (~9 fold) by Intralipid and the administration of both LY294002 and PD significantly reduced the level of phosphorylated GSK-3β in the myocardium treated with Intralipid (in arbitrary units normalized to control (n=6): 8.5±1.5 (n=4) vs. 2.9±1.1 in ILP+LY (n=4) and 3.3±0.7 in ILP+PD (n=4), Fig. 5E, F). These data strongly support the role of Akt/ERK/GSK-3β in Intralipid-induced cardioprotection against ischemia/reperfusion injury.
Figure 5. Involvement of PI3K-Akt and ERK pathways and their downstream target GSK-3β in Intralipid -induced protection.
A, C, E. representative immunoblots of pAkt and total Akt (A), pERK1,2 and total ERK1,2 (C), and pGSK-3β and total GSK-3β (E) in heart homogenates subjected to ischemia/reperfusion from control group (CTRL), Intralipid group (ILP) and 1% Intralipid+LY294002 (ILP+LY) top panels or CTRL, ILP and 1% Intralipid+PD98059 (ILP+PD) lower panels. B, D, F. Western Blot quantification (Mean±SEM) of pAkt protein to total Akt (B), pERK1 to total ERK (D) and pGSK-3β to total GSK-3β (E) ratios in CTRL (white bars, n=6), ILP (black bars, n=4), ILP+LY (dark gray bars, n=4) and ILP+PD (light gray bars, n=4). **P<0.01 vs. CTRL; ##P<0.01 vs. ILP.
Intralipid inhibits the opening of the mitochondrial permeability transition pore and this inhibition is abolished by PI3K inhibitor
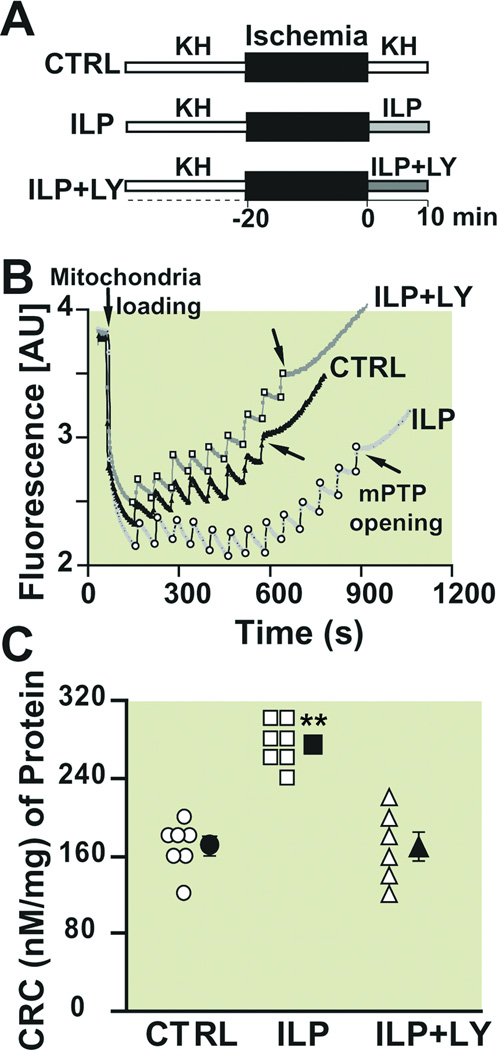
Since the inhibition of the mPTP opening during reperfusion has been shown to induce cardioprotection19, we investigated whether Intralipid-induced cardioprotection is mediated by inhibition of the mPTP opening. We compared the threshold for opening of mPTP in response to calcium overload in isolated mitochondria from hearts reperfused with KH (control) or Intralipid for 10 min (Fig. 6A). An example of the time course of Ca2+ concentration in the mitochondrial external medium is shown in Fig. 6B. In control group, 7 pulses of 20 nM Ca2+ were sufficient to trigger the opening of mPTP. Interestingly, the calcium load significantly increased in mitochondria isolated from Intralipid group as the number of calcium pulses required for opening of mPTP was increased to 14 pulses. The bar plot in Fig. 6C summarizes the calcium retention capacity (CRC); CRC was significantly higher in the Intralipid group compared to control (274.3±8.4 in Intralipid (n=7) vs. 168.6±9.6 nM/mg mitochondrial protein in control (n=7), p<0.01). These data strongly suggest that inhibition of mitochondrial permeability transition pore opening by Intralipid is one of the key events in the Intralipid-induced cardioprotection against I/R. In the presence of LY (45 µM), Intralipid effect was prevented as the CRC was reduced to 170.0±15.3 nM/mg mitochondrial protein (n=6), which was not significantly different from control. These data demonstrate clearly that Intralipid inhibits the opening of mPTP and this inhibition is abolished in the presence of LY.
Figure 6. Intralipid inhibits the opening of the mitochondrial permeability transition pore and this inhibition is abolished by PI3K inhibitor.
A. Experimental protocol for measuring calcium retention capacity. At the onset of reperfusion, isolated hearts are perfused with Krebs Henseleit (KH, control group, CTRL), 1% Intarlipid (ILP) or 1% Intralipid+LY294002 (ILP+LY) for 10 min. B. Typical recording of the mitochondria permeability transition pore opening in isolated mitochondria from control (black trace), ILP (light gray trace) and ILP+LY (dark gray trace). Fourteen pulses (gray arrows) of 20 nM calcium were required to trigger the opening of mitochondria permeability transition pore in ILP group compared to 7 pulses (black arrows) in CTRL and 8 pulses in ILP+LY. C. Calcium retention capacity (CRC) in CTRL (circles, n=7), ILP (squares, n=7) and ILP+LY (triangles, n=6). The individual measurements in each groups are shown in open shapes whereas the averages (Mean±SEM) are shown in filled shapes.**P<0.01 vs. CTRL and ILP+LY.
Discussion
We show here that administration of Intralipid right before the onset of reperfusion results in ~70% reduction in infarct size in the in-vivo rat model. Intralipid application at reperfusion also improves the functional recovery of isolated Langendorff-perfused mouse hearts by ~4 fold and significantly reduces the infarct size. Administration of Intralipid during even the first 5 minutes of reperfusion is sufficient to induce protection and the protection is maintained after removal of Intralipid. These data strongly indicate that Intralipid treatment has a cardioprotective effect against ischemia/reperfusion injury both in mice and rats. In this study, we provided the underlying mechanism of Intralipid-induced cardioprotection which is mediated by inhibition of the mPTP and the recruitment of the RISK pathway leading to phosphorylation of GSK-3β.
The Ovize group was the first to demonstrate a direct link between PI3K activation and opening of mPTP in the postconditioning3. Here we show that the cardioprotection provided by Intralipid at reperfusion is associated with inhibition of the mPTP opening, as the mitochondrial calcium uptake required for the opening of the mPTP was significantly higher in Intralipid-treated hearts compared to control (Fig. 6). We propose that Intralipid enhances the homeostasis of cardiomyocytes to better regulate calcium overload and therefore increase the threshold for opening of the mPTP. To see if the Intralipid-induced inhibition of the mPTP opening was through the PI3K pathway, LY294002, a specific inhibitor of PI3K was used. The Intralipid-induced cardioprotection was fully abolished in the presence of LY294002, and the infarct size was also significantly larger compared to Intralipid alone (Fig. 3). The calcium retention capacity was much lower when Intralipid was applied together with PI3K inhibitor LY, suggesting that PI3K-Akt pathway is one of the key pathways in this protection (Fig. 6). Our data also show Intralipid increased phosphorylation of Akt and ERK. This Intralipid-induced phosphorylation of Akt, was completely abolished in the presence of LY294002 (Fig. 6), and Intralipid-induced phosphorylation of ERK1, was completely abolished in the presence of PD (Fig. 5). These data strongly support the vital role of the prosurvival PI3K/Akt as well as ERK pathway in Intralipid-induced cardioprotection and are in agreement with previous work showing that activation of these two pathways have a cardioprotective effect in ischemia/reperfusion experiments in rodents20. In pigs, however, the activation of the RISK pathway does not seem to be crucial for postconditioning21. GSK-3β phosphorylation has emerged as an end effecter step where multiple protective signaling pathways converge. Here we show that GSK-3β mediates Intralipid-induced cardioprotection via PI3/Akt and MEK/ERK pathways. These are the two major pathways that have been demonstrated to be involved in cardioprotection against I/R injury. However, other upstream kinases such as PKC, PKA, PKG, p70S6 could also phosphorylate GSK-3β to induce cardioprotection (see review22). Whether Intralipid-induced cardioprotection is mediated through these kinases remain to be seen in future studies. Using genetic manipulation and pharmacological agents, GSK-3β phosphorylation has also been shown to lead to inhibition of the mPTP opening and therefore inducing cardioprotection13;23. Here we found that Intralipid treatment increased phosphorylation of GSK-3β (Fig. 5). These observations are in agreement with other studies that have also implicated the increased phosphorylation of GSK-3β to be a common feature of different cardioprotective agents22;24, although the requirement of GSK-3β inactivation to induce the inhibition of mPTP opening has been challenged recently13;25.
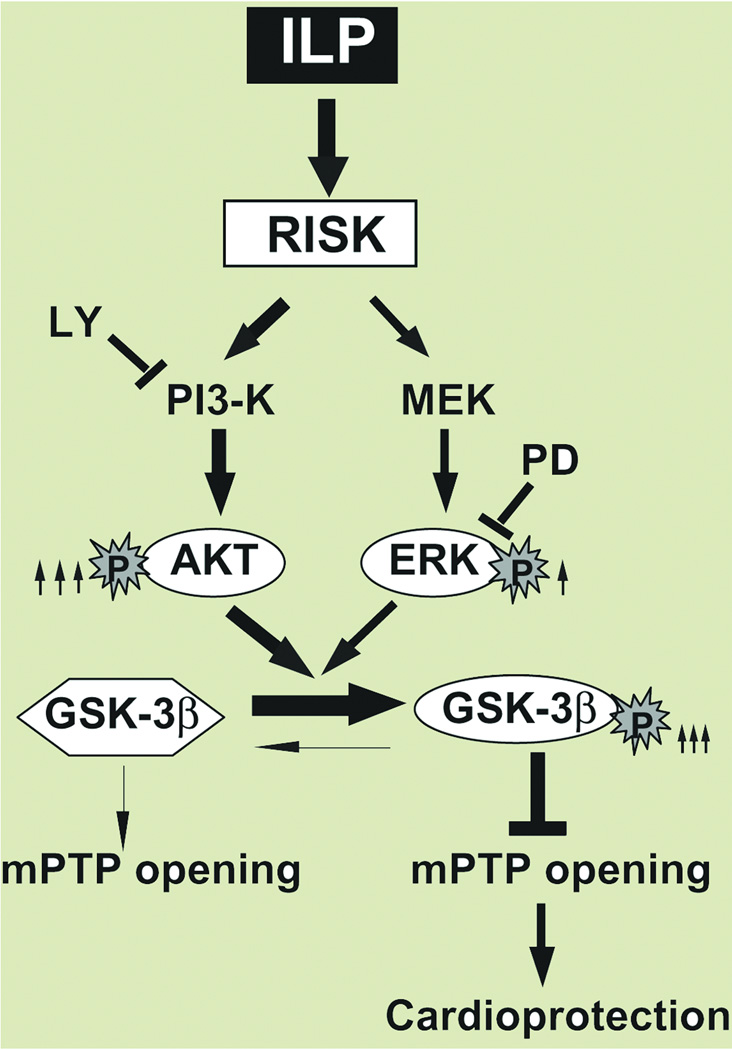
Fig. 7 summarizes our hypothetical scheme of the mechanism of protection by Intralipid against ischemia/reperfusion injury. Activation of the RISK pathway by Intralipid increases the phosphorylated levels of Akt and ERK. These two pathways converge to shift the equilibrium of GSK-3β from active form (not phosphorylated) toward the GSK-3β inactive form (phosphorylated). Once GSK-3β is phosphorylated, it inhibits the opening of the mPTP, which results in cardioprotection.
Figure 7. Proposed mechanisms underlying Intralipid-induced cardioprotection against ischemia/reperfusion injury.
The Reperfusion Injury Salvage Kinases (RISK) pathway is activated in the presence of Intralipid (ILP), resulting in increased phosphorylation of both Akt and ERK, although the degree of activation is much more prounnced in Akt (8 fold, shown by thick arrow) than in ERK (3 fold, shown by thin arrow). Both pathways converge to phosphorylate GSK-3β (inactive form), which in turn inhibits the opening of the mPTP and induces protection against reperfusion injury. The protection provided by Intralipid is fully abolished by the PI3K specific inhibitor, LY294002 (LY) and partially by PD98059 (PD).
Our data strongly indicate that Intralipid is a very powerful postischemic pharmacological agent. The role of Intralipid in preconditioning, however seems to be controversial10;26. Hu et al reported that Intralipid has no effect on the infarct size in in-vivo model of I/R injury26 whereas in another work from the same group, Intralipid was shown to reduce the infarct size in ex-vivo model of I/R in rats10. These conflicting results could be due the fact that only male rats were used in one study10, whereas in the other study both male and females rats were used26. It is now well accepted that female rats are better protected against I/R injury compared to their male counterparts27;28. It was also not clear whether an equal number of males and females were used in each group26. Therefore, the fact that Intralipid administration prior to ischemia failed to reduce the infarct size in the in-vivo model of I/R injury could be simply due to the higher numbers of males in the Intralipid group26.
Intralipid has also been proposed as a rescue therapy for severe local anesthetic-induced cardiovascular collapse and certain drugs cardiotoxicity29–33. In fact several case reports demonstrates the effectiveness of Intralipid in treating amlodipine poisoning30 and diltiazem poisoning29, but the only recommended therapeutic use for Intralipid as a "rescue agent" is for Bupivacaine-induced cardiac arrest14;34;35. However, the beneficial effect of Intralipid seems to be restricted to drug-induced cardiac arrest as Intralipid has been shown to be detrimental in a rabbit model of hypoxia-induced cardiac arrest36. Less than adequate myocardial coronary perfusion pressure during resuscitation in this model probably plays a key factor in negative results obtained. More studies are required to evaluate effect of Intralipid in hypoxic cardiac arrest.
The effect of Intralipid on vasculature is also controversial37–39. Intralipid has been shown to promote peripheral vasodilation in humans37. Osanai et al., however showed that Intralipid impaired postischemic endothelium-dependent vasodilatation in the canine iliac artery39. Decreased endothelial nitric oxide production or deactivation of nitric oxide by oxidative stress was suggested to be the main mechanism for impairment of vasodilatation by Intralipid. However, in the same species, stimulation of the nitric oxide pathway by Intralipid has been reported38. Further studies are required to clarify the role of Intralipid on vasculature in health and in various disease models.
Conclusions
We show here that only one bolus of Intralipid (20%) right before reperfusion is sufficient to protect the heart against ischemia/reperfusion injury in-vivo. Intralipid application at the reperfusion also improves the functional recovery of isolated Langendorff-perfused mouse hearts by ~4 fold and significantly reduces the infarct size. Postischemic administration of Intralipid inhibits the opening of the mitochondrial permeability transition pore. Phosphorylation of GSK-3β, which is involved in the cardioprotective action of Intralipid against ischemia/reperfusion injury Intralipid has already been in clinical use for almost 4 decades for patients who need total parenteral nutrition and it has been shown to be safe and well tolerated. Here we propose that Intralipid could be a clinically safe compound for targeting GSK-3β at the time of reperfusion to protect the myocardium against ischemia/reperfusion injury and certainly warrant further investigation in human heart.
Final Box Summary.
What we already know about this topic:
-
--
Activation of the Reperfusion-Injury-Salvage-Kinase (RISK) pathway protects against myocardial infarction and delays opening of the mitochondrial permeability transition pore (mPTP)
-
--
Intralipid may have cardioprotective effects, but, the magnitude of and mechanisms responsible for these actions are unclear
What this article tells us that is new:
-
--
Intralipid substantially protects against myocardial infarction and inhibits mPTP opening by activating the RISK pathway and by increasing (inhibitory) phosphorylation of glycogen synthase kinase 3 beta
Acknowledgment
The authors would like to thank Dr. Jeffrey Gornbein, DrPH Biostatistics, Senior Statistician, from Department of Biomathematics at UCLA, Los Angeles, CA, for the statistical assistance.
Funding
Mansoureh Eghbali is supported by National Institutes of Health grants HL089876 and HL089876S1 (Bethesda, Maryland), and Jean C. Bopassa is supported by an American Heart Association fellowship 09POST2190008 (Dallas, Texas).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992;86:81–90. doi: 10.1161/01.cir.86.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am.J.Physiol Heart Circ.Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovas Res. 2006;69:178–185. doi: 10.1016/j.cardiores.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: Staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 5.Tamareille S, Ghaboura N, Treguer F, Khachman D, Croue A, Henrion D, Furber A, Prunier F. Myocardial reperfusion injury management: Erythropoietin compared with postconditioning. Am.J.Physiol Heart Circ.Physiol. 2009;297:H2035–H2043. doi: 10.1152/ajpheart.00472.2009. [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur.J.Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Gylling H, Miettinen TA. A review of clinical trials in dietary interventions to decrease the incidence of coronary artery disease. Curr.Control Trials Cardiovasc.Med. 2001;2:123–128. doi: 10.1186/cvm-2-3-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EB, Skou HA, Christensen JH, Dyerberg J. N-3 fatty acids from fish and coronary artery disease: Implications for public health. Public Health Nutr. 2000;3:91–98. doi: 10.1017/s1368980000000112. [DOI] [PubMed] [Google Scholar]
- 9.Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic Rabbits. J.Nutr. 2004;134:3250–3256. doi: 10.1093/jn/134.12.3250. [DOI] [PubMed] [Google Scholar]
- 10.Liu SL, Wang Y, Wang RR, Chai YF, Wu W, Huang H, Liu J. Protective effect of intralipid on myocardial ischemia/reperfusion injury in isolated rat heart. Zhongguo Wei Zhong.Bing.Ji.Jiu.Yi.Xue. 2008;20:227–330. [PubMed] [Google Scholar]
- 11.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. J.Physiol Heart Circ.Physiol. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 13.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg GL, Di GG, Ripper R, Kelly K, Massad M, Edelman L, Schwartz D, Shah N, Zheng S, Feinstein DL. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 15.Lee HB, Blaufox MD. Blood volume in the rat. J.Nucl.Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 16.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler.Thromb.Vasc.Biol. 2006;26:e108–e113. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 17.Bell RM, Clark JE, Hearse DJ, Shattock MJ. Reperfusion kinase phosphorylation is essential but not sufficient in the mediation of pharmacological preconditioning: Characterisation in the bi-phasic profile of early and late protection. Cardiovasc Res. 2007;73:153–163. doi: 10.1016/j.cardiores.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Milano G, von Segesser LK, Morel S, Joncic A, Bianciardi P, Vassalli G, Samaja M. Phosphorylation of phosphatidylinositol-3-kinase-protein kinase B and extracellular signal-regulated kinases 1/2 mediate reoxygenation-induced cardioprotection during hypoxia. Exp.Biol.Med.(Maywood.) 2010;235:401–410. doi: 10.1258/ebm.2009.009153. [DOI] [PubMed] [Google Scholar]
- 19.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ.Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 21.Skyschally A, van CP, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ.Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 22.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ.Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamareille S, Ghaboura N, Treguer F, Khachman D, Croue A, Henrion D, Furber A, Prunier F. Myocardial reperfusion injury management:erythropoietin compared with postconditioning. Am.J.Physiol Heart Circ.Physiol. 2009;297:H2035–H2043. doi: 10.1152/ajpheart.00472.2009. [DOI] [PubMed] [Google Scholar]
- 24.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ.J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 25.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ.Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 26.Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can.J.Anaesth. 2009;56:115–125. doi: 10.1007/s12630-008-9016-3. [DOI] [PubMed] [Google Scholar]
- 27.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol and Exp Ther. 2005;315:1125–1135. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 28.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovas Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Montiel V, Gougnard T, Hantson P. Diltiazem poisoning treated with hyperinsulinemic euglycemia therapy and intravenous lipid emulsion. Eur.J.Emerg.Med. 2011;18:121–123. doi: 10.1097/MEJ.0b013e32834130ab. [DOI] [PubMed] [Google Scholar]
- 30.West PL, McKeown NJ, Hendrickson RG. Iatrogenic lipid emulsion overdose in a case of amlodipine poisoning. Clin.Toxicol.(Phila) 2010;48:393–396. doi: 10.3109/15563651003670843. [DOI] [PubMed] [Google Scholar]
- 31.Lin EP, Aronson LA. Successful resuscitation of bupivacaine-induced cardiotoxicity in a neonate. Paediatr.Anaesth. 2010;20:955–957. doi: 10.1111/j.1460-9592.2010.03406.x. [DOI] [PubMed] [Google Scholar]
- 32.Foxall G, McCahon R, Lamb J, Hardman JG, Bedforth NM. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516–518. doi: 10.1111/j.1365-2044.2007.05065.x. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth.Pain Med. 2003;28:198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg G, Hertz P, Newman J. Lipid, not propofol, treats bupivacaine overdose. Anesth and Analg. 2004;99:1875–1876. doi: 10.1213/01.ANE.0000138550.57760.22. [DOI] [PubMed] [Google Scholar]
- 35.Onyuksel H, Sethi V, Weinberg GL, Dudeja PK, Rubinstein I. Bupivacaine, but not lidocaine, disrupts cardiolipin-containing small biomimetic unilamellar liposomes. Chem.Biol.Interact. 2007;169:154–159. doi: 10.1016/j.cbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Harvey M, Cave G, Kazemi A. Intralipid infusion diminishes return of spontaneous circulation after hypoxic cardiac arrest in rabbits. Anesth and Analg. 2009;108:1163–1168. doi: 10.1213/ane.0b013e31819367ba. [DOI] [PubMed] [Google Scholar]
- 37.Riksen NP, Bosselaar M, Bakker SJ, Heine RJ, Rongen GA, Tack CJ, Smits P. Acute elevation of plasma non-esterified fatty acids increases pulse wave velocity and induces peripheral vasodilation in humans in vivo. Clin.Sci.(Lond) 2007;113:33–40. doi: 10.1042/CS20060365. [DOI] [PubMed] [Google Scholar]
- 38.Doursout MF, Joseph PM, Liang YY, Hartley CJ, Chelly JE. Role of propofol and its solvent, intralipid, in nitric oxide-induced peripheral vasodilatation in dogs. Br.J.Anaesth. 2002;89:492–498. [PubMed] [Google Scholar]
- 39.Osanai H, Okumura K, Hayakawa M, Harada M, Numaguchi Y, Mokuno S, Murase K, Matsui H, Toki Y, Ito T, Hayakawa T. Ascorbic acid improves postischemic vasodilatation impaired by infusion of soybean oil into canine iliac artery. J.Cardiovasc.Pharmacol. 2000;36:687–692. doi: 10.1097/00005344-200012000-00001. [DOI] [PubMed] [Google Scholar]