Abstract
Fatty acids and/or isoprenoids are covalently attached to a variety of disease-related proteins. The distinct chemical properties of each of these hydrophobic moieties allow lipid modification to serve as a mechanism to regulate protein structure, localization and function. This review highlights recent progress in identifying inhibitors of protein lipidation and their effects on human disease. Myristoylation inhibitors have shown promise in blocking the action of human pathogens. Although inhibitors that block prenylation of Ras proteins have not yet been successful for cancer treatment, they may be efficacious in the rare premature aging syndrome progeria. Agents that alter the palmitoylation status of Ras, Wnt and Hh proteins have recently been discovered, and represent the next generation of potential chemotherapeutics.
Protein lipidation: What’s the big fat deal?
A wide range of proteins, including many proteins involved in human diseases, are modified by covalent linkage of fatty acids and/or isoprenoid groups. Attachment of these hydrophobic groups plays a major role in regulating protein structure and function. Myristate and palmitate are the two fatty acids typically linked to proteins (Table 1) [1]. Approximately 150 human proteins are known to be myristoylated, including Src family tyrosine kinases (SFKs) and c-Abl. Myristate is attached to the N-terminal glycine of protein substrates in a reaction catalyzed by myristoyl-CoA:protein N-myristoyltransferase (NMT) (Glossary) [2]. The fatty acid forms a stable, amide linkage with the protein. By contrast, cysteine residues within proteins can be acylated with the 16-carbon fatty acid palmitate, as well as other longer chain fatty acids, both saturated and unsaturated. This process is generically termed palmitoylation and occurs on several hundred mammalian proteins. In humans, a family of 23 palmitoyl acyltransferases (PATs) containing a conserved Asp-His-His-Cys, or “DHHC”, sequence mediate palmitoylation of intracellular proteins, whereas secreted proteins such as Wnt and Hedgehog are palmitoylated by PATs of the membrane bound O-acyl transferase (MBOAT) family [3]. Palmitoylation is a reversible reaction; the thioester linkage between palmitate and cysteine can be readily broken by thioesterases. Regulated cycles of palmitoylation and depalmitoylation lead to reversible membrane association, allowing palmitoylated proteins to signal from multiple subcellular locations [3, 4]. The saturated nature of the fatty acid promotes insertion of palmitoylated proteins into liquid ordered domains of the membrane, known as lipid rafts. Multiple signaling proteins are enriched in lipid rafts, and raft association promotes intracellular signal transduction [1, 4].
Table I.
Lipid modification of proteins
| Lipid | Structure | Linkage | Dynamic or Stable | Modified Residue | Enzyme |
|---|---|---|---|---|---|
| Myristate |
|
Amide | Stable | H2N-Glyh | NMTa |
| Palmitate |
|
Thioester | Dynamic | Cys | DHHC PATsb |
| Palmitate |
|
Amide | Stable | H2N-Cysh | Hhatc |
| Palmitoleic |
|
Oxyester | Stable | Ser | Porcnd |
| Farnesyl |
|
Thioether | Stable | Cys | FTasee |
| GeranylGeranyl |
|
Thioether | Stable | Cys | GGTaseIf |
| GeranylGeranyl |
|
Thioether | Stable | Cys | GGTaseII/REPg |
N-myristoyl transferase
DHHC motif containing palmitoyl acyltransferases
Hedgehog acyltransferase
Porcupine
Farnesyl Transferase
Geranylgeranyl Transferase I
Geranylgeranyl Transferase II/Rab Escort Protein
The modified residue is the N-terminal amino acid of the protein. The fatty acid is attached to the N-terminal amino group of the polypeptide backbone, unlike the remaining modifications in this table where the lipid is attached to the amino acid side-chain.
Prenyl transferases catalyze attachment of farnesyl (FTase) or geranylgeranyl (GGTase) isoprenoids onto a Cys at or near the C-terminus of small G and other target proteins (Table 1). A characteristic “CAAX” box (Cys-aliphatic-aliphatic-X) is found at the C-terminus of many prenylated proteins, where “X” determines whether the protein will be farnesylated or geranylgeranylated. Following prenylation, the “AAX” sequence is cleaved, and the C-terminal, prenylated Cys is carboxymethylated.
Many proteins involved in normal and malignant cell growth require lipidation for their signaling functions (Table 2). Thus, the enzymes that regulate lipid attachment and removal have emerged as potential drug targets in a wide variety of disease states. Several novel inhibitors of fatty acyl transferases, thioesterases, and prenyl transferases have recently been identified. Moreover, our understanding of the dynamics of intracellular trafficking of lipid modified proteins has dramatically expanded over the past few years. This review focuses on a set of intracellular and secreted proteins that are lipidated (Table 2), and reports the latest advances in targeting the lipid modification enzymatic machinery for disease treatment.
Table II.
Examples of lipid modified proteins involved in disease
| Protein | Modification(s) | Disease States |
|---|---|---|
| Src family kinases | Myristate; myristate + palmitate | breast and colon carcinomas |
| HIV-1 Gag | Myristate | AIDS |
| c-Abl | Myristate | Breast cancer; neutropenia |
| K-Ras | Farnesyl | Pancreatic cancer; colorectal cancer |
| H-Ras, N-Ras | Farnesyl + palmitate | Bladder cancer, AML |
| LaminA | Farnesyl | Progeria |
| Wnt proteins | Palmitate + palmitoleate | Colorectal cancer; lung cancer |
| Shh | Palmitate + cholesterol | Pancreatic cancer; sarcomas |
NMT inhibitors to treat human pathogen infections
Studies of SFKs reveal that myristoylation is critical for promoting membrane binding and cellular transformation [1, 5]. Likewise, myristoylation is required for membrane binding of the HIV-1 Gag polyprotein, and inhibition of myristoylation prevents formation of HIV-1 virions [6]. Given these essential functions, one might advocate developing NMT inhibitors for treating diseases such as cancer and AIDS. However, this approach is flawed as inhibition of NMT in human cells would also affect more than 100 other N-myristoylated proteins required for normal cellular signaling, viability and growth.
There is a silver lining in this strategy if one considers that N-myristoylation is essential for the growth and survival of Candida albicans, Trypanosoma brucei, and Leishmania major, human pathogens responsible for fungal infections, African sleeping sickness, and leishmaniasis, respectively [7, 8]. There is relatively high homology between human NMT and fungal and parasitic NMTs on the sequence level, and the binding sites for myristoylCoA within NMT are also highly conserved. However, the binding sites for the protein substrates are divergent between human and fungal or parasitic protozoan NMTs. These differences have been exploited to create NMT inhibitors that are pathogen-specific. NMT inhibitors that work as anti-fungal agents have been developed [8], and there is promising data to suggest that Leishmania parasites would be susceptible to NMT inhibition [9]. Moreover, Frearson et al [10] recently identified a set of lead compounds that interact with the peptide binding pocket of T. brucei NMT and inhibit its activity. These compounds kill trypanosomes at nM concentrations, and cure trypanosomiasis in mice. Further optimization is required to achieve better selectivity for T. brucei NMT over the human homolog and for the ability to diffuse into the brain and CNS.
Myristoylation regulates tyrosine kinase activity
Myristate plays a key regulatory role in the c-Abl tyrosine kinase. Binding of myristate within a hydrophobic pocket of c-Abl induces bending of the αI helix that helps to latch c-Abl into an autoinhibitory state. Recently, two types of small molecules that bind to the myristoyl binding site in c-Abl have been described. DPH binds to the myristoyl binding pocket, prevents bending of the αI helix, and induces c-Abl activation [11]. GNF-2 binds to the same pocket in Bcr-Abl, but instead stabilizes the bent conformation of the αI helix, resulting in kinase inhibition [12]. Importantly, a derivative of GNF-2 is effective at inhibiting Bcr-Abl activity in cells that are resistant to treatment with the tyrosine kinase inhibitor imatinib [12].
A hydrophobic pocket is predicted to exist in the kinase c-Src, but access of myristate to this pocket is blocked and c-Src does not undergo a myristoyl switch. Unlike c-Abl, myristoylation enhances c-Src tyrosine kinase activity. In addition, myristoylation increases ubiquitination and degradation of activated c-Src [13]. This serves to keep levels of activated membrane-bound c-Src in check.
Targeting Ras Prenylation with FTIs and GGTIs
Ras is one of the most commonly mutated genes in human cancer (Table II). All three major Ras isoforms – H-Ras, N-Ras and K-Ras4B – are modified by FTase. Farnesylation is required for Ras to bind membranes, transform cells and induce tumorigenesis in animals. Over the past decade, intensive efforts have been devoted to developing FTase inhibitors (FTIs) as anti-cancer agents [14, 15]. Genetic models that induce conditional loss of either FTase or GGTase1 in mice validate the importance of these enzymes in promoting K-Ras induced lung cancer and myeloproliferative diseases [16–18]. To date, four types of FTIs have been developed: peptidomimetics, farnesylpyrophosphate analogs, bisubstrate analogs that combine the two former features, and small molecule inhibitors. In cells, FTIs inhibit H-Ras farnesylation and membrane association, block the activation of MAP kinase and PI3 kinase/Akt pathways [19], and reduce the growth of a wide variety of human cancer cell lines. FTIs are also effective at blocking tumor growth in xenograft mouse models. However, results obtained from nearly 75 clinical trials of four different FTIs (lonafarnib, tipifarnib, BMS-214662 and L-778123) reveal these agents to have no or minimal efficacy in the treatment of hematopoietic cancers or solid tumors [14]. Moreover, the extent of any clinical response was not correlated with the dose of the drug, Ras mutation status, or the extent of FTase inhibition.
Several molecular mechanisms have been invoked to explain why FTIs fail to block cancer in the clinic. First, when FTase is inhibited, K-Ras becomes alternatively geranylgeranylated, thereby retaining membrane binding and the ability to transform cells [20]. Thus, current efforts are directed towards testing GGTase1 inhibitors. GGTIs block the geranylgeranylation of the small G proteins RalA and RalB, induce apoptosis, and inhibit tumor cell growth in human cancer cells both in vitro and in animals [21]. Second, there is a subset of patients who do respond to FTIs. Response to tipifarnib in AML patients can be predicted by a 2-gene expression signature profile that measures the ratio of the RasGRP:APTX genes [22]. Third, the lack of correlation between response to FTIs and Ras mutational status, whether measured in cells, mouse xenografts or human patients [14], implies that other targets for FTIs are involved. At least 50–100 proteins encoded by the human genome are predicted to be farnesylated, and the identities of only a subset have been determined. Of note, the RHEB (Ras homolog enriched in brain) protein has been shown to be exclusively farnesylated and to be a target for FTIs [23]. Farnesylation of Rheb is required for its ability to stimulate mTOR [24], a protein kinase involved in regulation of normal and malignant cell growth. Identification of tumors that are addicted to proteins that are exclusively prenylated by either FTase or GGTase1 might open a new avenue for therapeutic intervention by FTIs and GGTIs. Alternatively, the enzymes that catalyze proteolysis and carboxymethylation of the CAAX box might also serve as potential therapeutic targets in tumors driven by Ras and other small GTPases [25, 26].
FTIs as agents to treat Progeria
There is a clear target for FTIs in the treatment of the human premature aging syndrome HGPS (Hutchinson-Gilford progeria syndrome), a rare genetic disorder caused by a point mutation in the laminA gene [27]. The laminA protein is normally farnesylated in its C-terminal CAAX box. This modification triggers proteolytic cleavage of the C-terminal farnesylated tail by ZMPSTE24, a Zn2+ metalloprotease, releasing mature lamin A, which then inserts into the nuclear lamina. The HGPS mutation alters mRNA splicing, leading to deletion of a 50 residue region that contains the ZMPSTE24 cleavage site. As a result, the mutant lamin, termed progerin, is not cleaved and retains the farnesylated C-terminal tail. Insertion of progerin into the nuclear envelope causes severe nuclear structural abnormalities that are believed to be ultimately responsible for the disease phenotype [28]. Homozygous deletion of the ZMPSTE24 gene or targeted introduction of the HGPS mutation in lamin A recapitulates the progeria phenotype in transgenic mice [29, 30]. Treatment of these mice with an FTI normalizes nuclear morphology and improves disease symptoms and survival [29, 30]. Clinical trials of lonafarnib, as either monotherapy or in combination with a statin, are currently in progress.
Palmitoylation regulates Src family kinases
In order to signal efficiently, SFKs must be membrane bound. Two motifs are required for membrane binding: Src uses myristate in combination with a basic sequence, while nearly all other SFKs use myristate plus palmitate (Box 1). Differential fatty acylation is a critical determinant of intracellular trafficking and SFK signaling. This is best illustrated by comparing Src, which is myristoylated, with Fyn, which is both myristoylated and palmitoylated. Inactive Src is primarily localized to the perinuclear region of cells and, upon activation, uses RhoB positive endosomes to traffic to the plasma membrane. By contrast, translocation and activation of Fyn is dependent on RhoD enriched endosomes [31]. Mutations that prevent palmitoylation of Fyn or introduce palmitoylation sites into Src reverse the differential dependence of the modified proteins on RhoB versus RhoD [31]. Src and Fyn exhibit differential potency in initiating prostate cancer. Src is much more effective than Fyn in promoting transformation in an FGF10-induced model of prostatic carcinoma, and this hierarchical order is reversed when Fyn palmitoylation sites are eliminated by mutation and palmitoylation sites are introduced into Src [32].
Text Box 1. Two signals for membrane binding of myristoylated or farnesylated proteins.
The hydrophobicity of a single myristate or farnesyl moiety alone is not sufficient to stably anchor a protein to a lipid bilayer, thus
A second signal is required for stable membrane binding.
The second signal is typically a polybasic cluster of amino acids (lysines and arginines) or a palmitate modification.
For c-Src, myristate provides hydrophobic interactions with the lipid bilayer core, while a polybasic cluster in the N-terminal SH4 region of c-Src interacts electrostatically with negatively charged phospholipids on the cytoplasmic leaflet of the plasma membrane. Other proteins that use a “myristate plus basic” motif for membrane binding include MARCKS, HIV-1 Gag, and hisactophilin.
Myristate plus palmitate motifs anchor SFKs, Gα subunits of heterotrimeric G proteins, eNOS, and AKAP (A Kinase anchoring protein) to the membrane.
Farnesyl plus basic motifs anchor K-Ras4B and lamin B3 to the membrane.
Farnesyl plus palmitate motifs anchor H-Ras and N-Ras to the membrane.
Different combinations of the two signals provide distinct modes of membrane interaction that are differentially regulated, thereby allowing the modified proteins to undergo reversible membrane association bilayer [1]. For example, basic domain containing proteins can be released from the membrane by phosphorylation within the polybasic cluster (e.g. K-Ras4B, MARCKs). Some myristoylated proteins undergo an intramolecular myristoyl switch, resulting in sequestration of myristate within a hydrophobic cleft and release from the membrane (e.g. recoverin). Likewise, geranylgeranyl groups of Rho and Rab proteins bind to hydrophobic grooves within guanine nucleotide dissociation inhibitors (GDIs) that sequester the prenyl group and promote release of the prenylated protein from the membrane. Palmitoylated proteins undergo reversible cycles of palmitoylation/depalmitoylation, resulting in reversible membrane binding.
The presence of two saturated fatty acids, such as myristate and palmitate or two palmitates, drives SFKs into membrane rafts. In the immune system, raft association promotes signaling by enhancing interactions of SFKs with other lipid-raft associated signaling proteins [3]. However, in tumor cells, the situation appears to be reversed. Okada’s group has provided extensive evidence to suggest that raft association inhibits transforming activity by SFKs [33]. This has been largely attributed to SFK binding to a palmitoylated adaptor protein that resides in lipid rafts, Cbp [33, 34]. When Cbp is overexpressed, transformation and tumorigenesis by activated c-Src is suppressed [33]. Conversely, Cbp levels are downregulated in colon cancer and lung cancer cells or when activated c-Src is overexpressed [35]. At least two mechanisms have been proposed to account for the ability of Cbp to suppress SFK activity. Cbp functions as a membrane-bound adaptor for Csk, the negative regulatory tyrosine kinase that phosphorylates SFKs and keeps them in an inactive state. Cbp binding recruits Csk from the cytosol to the membrane, where it can access and inactivate membrane-bound SFKs. However, Cbp binding and inactivation of SFKs can occur even in the absence of Csk (i.e. in Csk −/− mice), and is presumably due to the ability of Cbp to sequester SFKs in lipid rafts and inhibit access to critical substrates.
The kinetics of SFK palmitoylation have recently been explored using total internal reflection microscopy to follow membrane association of Lck [36]. It takes approximately 400 msec for membrane proximal Lck molecules to bind the plasma membrane; this likely represents the time required for a palmitoyl acyltransferase to attach the first of two palmitates. Lck remains in the membrane for 50 sec, which is sufficient time to traverse the entire plasma membrane of a T cell. Depalmitoylation is also fast, occurring within 7–9 sec [36]. Another study using tandem fluorescent imaging with biorthogonal probes revealed that palmitate turnover on Lck occurs with a half-life of less than 5 min and that depalmitoylation is increased by T cell activation [37]. Thus, reversible cycles of palmitoylation/depalmitoylation occur on a time scale consistent with the dynamics of SFK signaling.
Regulation of Ras palmitoylation
A dynamic cycle of palmitoylation/depalmitoylation/repalmitoylation plays a major role in regulating the localization and signaling activity of Ras proteins (Figure 1). Following prenylation by FTase, H-Ras and N-Ras are palmitoylated in the Golgi [38] and routed to the plasma membrane via the secretory pathway. Removal of palmitate by thioesterases results in dissociation of depalmitoylated H-Ras and N-Ras from the plasma membrane and retrograde trafficking to the Golgi, where H-Ras and N-Ras accumulate as a result of being re-palmitoylated [39, 40]. Continuous cycling establishes two locations for Ras signaling, the plasma membrane and the Golgi, each with different effectors and downstream signaling outputs as well as different temporal regulation [41].
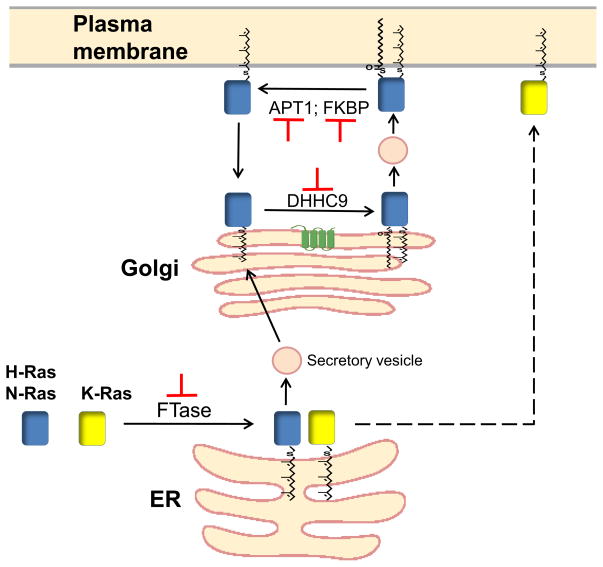
Figure 1. Trafficking and Processing of Ras.
H-, N-, and K-Ras are first farnesylated by FTase in the cytosol and bind to the ER membrane, where further processing of the CAAX box occurs. K-Ras (yellow box) travels by a non-vesicular, undefined pathway directly to the plasma membrane (dotted line). Farnesylated H- and N-Ras (blue box) traffic to the Golgi via secretory vesicles and are palmitoylated there by a Golgi-associated PAT, DHHC9. Transit of farnesylated, palmitoylated Ras to the plasma membrane is mediated by the secretory pathway. Removal of palmitate from plasma membrane-localized Ras is accelerated by binding of FKBP, and can be catalyzed by APT1. Depalmitoylated Ras dissociates from the membrane, and accumulates in the Golgi as a result of repalmitoylation. Inhibitors have recently been described for each of the above modification reactions (in red) [14, 43, 44, 47, 48].
Non-palmitoylated mutants of H-Ras and N-Ras are mislocalized and have reduced signaling activity. Thus, agents that disrupt the palmitoylation/depalmitoylation cycle might be efficacious in blocking Ras activity. A palmitoyl acyltransferase complex with specificity for H-Ras and N-Ras has been identified, GCP16/DHHC9 [42], and its activity can be blocked in vitro by a small molecule inhibitor of palmitoylation [43, 44]. Although this agent reduces the growth of human tumor cells, it has not yet been established whether the anti-proliferative effect is due to direct inhibition of Ras palmitoylation.
An alternative approach to regulating Ras has been to tackle the depalmitoylation reaction. Acyl protein thioesterase 1 (APT1) is a non-specific thioesterase that can remove fatty acyl groups from lysophospholipids as well as a wide variety of proteins, including H-Ras [45, 46]. A small molecule inhibitor of APT1, termed palmostatin B, has been developed and shown to inhibit depalmitoylation of H-Ras [47]. One might expect that Ras proteins that cannot be depalmitoylated would remain at the plasma membrane. However, in cells treated with palmostatin B, N-Ras is redistributed randomly to all endomembranes [47]. Likewise, a synthetic N-Ras protein with palmitate attached via a non-cleavable thioether bond is also nonspecifically localized to all internal membranes [39]. Random distribution is likely due to constant and rapid fusion and mixing of intracellular membranes with the plasma membrane. Treating cells expressing the oncogenic H-RasGly12Val with palmostatin partially reverts the transformed phenotype, and this effect is rescued by expression of activated K-Ras, which is not palmitoylated. The caveats to these experiments are that the effects of palmostatin B could be due to inhibition of other thioesterases and/or that APT1 is not the only relevant thioesterase for depalmitoylation of Ras. Nonetheless, these findings suggest that agents that interfere with Ras depalmitoylation may have therapeutic value.
New insight into the mechanisms that regulate Ras acylation status has been provided by Ahearn et al [48], who showed that depalmitoylation is accelerated by binding of the prolyl isomerase FKBP12 to palmitoylated forms of H-Ras and N-Ras. Depalmitoylation induced by FKBP12 depends on Pro-179, which is immediately upstream of the palmitoylated Cys in the H-Ras tail. FK506, which binds to FKBP12 and inhibits its prolyl isomerase activity, causes an increase in the steady-state levels of palmitate in H-Ras [48]. Treatment of cells with FK506 prevents relocalization of H-Ras to the Golgi, increases the amount of GTP-loaded, activated H-Ras at the plasma membrane, and enhances Ras signaling.
Palmitoylation inhibitors to block Wnt and Shh driven cancers
Dysregulation of Wnt signaling pathways induces oncogenesis [49]. Wnt proteins are dually acylated with palmitate and palmitoleate (Figure 2), and attachment of these fatty acids is required for Wnt protein secretion and signaling [50, 51]. A multipass membrane protein termed Porcupine (Porcn) has been implicated in fatty acid modification of Wnt proteins [50, 52, 53]. Porcn is a member of the MBOAT family of membrane bound O-acyl transferases. Deletion of Porcn in mice results in an embryonic lethal phenotype due to failure to secrete functional Wnt proteins and defective gastrulation [54]. Mutations in the Porcn gene in humans cause Goltz syndrome or focal dermal hypoplasia, an X-linked dominant disorder characterized by multiple skin malformations [55]. The symptoms of Goltz syndrome can be recapitulated in mice with conditional disruption of Porcn [56].
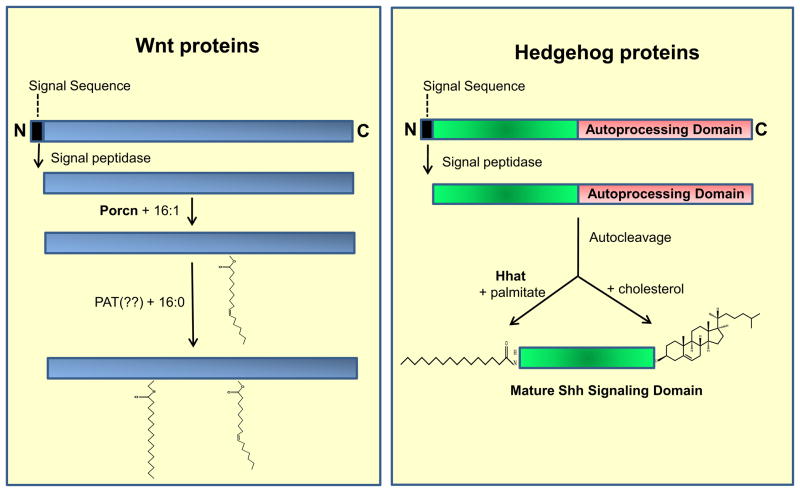
Figure 2. Processing and Modifications of Wnt and Hedgehog Proteins.
Left panel: Wnt proteins are synthesized with an N-terminal signal sequence that is removed by signal peptidase. Porcn catalyzes attachment of palmitoleate (16:1) to Ser209 in Wnt3a. A second site of modification with palmitate (16:0) has been identified as Cys77 in Wnt3a, but the PAT responsible for this reaction has not yet been identified. The equivalent Cys and Ser residues are conserved in nearly all other Wnt family members. Right panel: Sonic hedgehog is synthesized as a precursor polypeptide. The N-terminal signal sequence is removed by signal peptidase. The C-terminal autoprocessing domain catalyzes autocleavage, and during this reaction a molecule of cholesterol is added to the C-terminus of ShhN, the N-terminal Shh fragment. Hhat catalyzes attachment of palmitate to the N-terminal Cys of ShhN. The mature signaling molecule, ShhNp, is secreted from the cell.
Mass spectrometric analysis of purified Wnt3a revealed that two fatty acids are attached at two sites: palmitate is attached via a thioester linkage to Cys77 and palmitoleate is bound to Ser209 via an oxyester linkage [50, 53]. Ser209 mutation prevents both Wnt3a secretion and palmitoylation of Cys77, whereas mutation of Cys77 abolishes Wnt3a signaling but has no effect on its secretion. These findings imply that Ser209acylation occurs first and is a prerequisite for Cys77 modification.
Several recent studies have explored the relationships among Wnt protein glycosylation, fatty acylation, secretion and signaling and have established the following model. The N-terminal signal sequence is removed and Wnts are glycosylated in the ER. Glycosylation is required for modification of Ser209 (Wnt3a numbering) by ER-associated Porcn [57]. Wnts then traffic to the Golgi, where they bind to Wntless, a transmembrane protein that promotes Wnt trafficking to the plasma membrane. The ability of Wnt to functionally interact with Wntless is dependent on Ser209 acylation [58, 59], explaining why Ser209 mutation prevents Wnt secretion. Of note, one of the Wnt family members, Wnt8/WntD, lacks the equivalent of Ser209; it is secreted, but in a Porcn- and Wntless-independent fashion [60].
Modification of the two lipidation sites appears to be differentially regulated and has different biological consequences [61]. Cys77 palmitoylation occurs in a Porcn-independent manner, and the acyltransferase responsible for Cys77 modification has not yet been identified. Mutation of the Cys77 equivalent site in Wnt1 affects non-canonical (that is β-catenin independent) Wnt signaling pathways. Attachment of palmitoleic acid to Ser209 is mediated by Porcn and regulates β-catenin dependent signaling [61]. A series of small molecule inhibitors that target Porcn has been described and these compounds inhibit Wnt/β-catenin pathway activation [62]. Given its critical role in Wnt biogenesis and signaling activity, Porcn is an appealing target for treating diseases driven by Wnt ligand overexpression.
In adult tissues, aberrant Shh overexpression or signaling drives the growth of multiple human cancers, including medulloblastoma, basal cell carcinoma, liver, pancreatic and urogenital tumors. Shh signaling is mediated by a 19kDa polypeptide generated by autocleavage of the Shh precursor polypeptide in the ER [63, 64]. Two independent lipid modification reactions then occur (Figure 2). Cholesterol is covalently attached to the C-terminus during the autocleavage reaction. Palmitate is linked to the cysteine residue at the N-terminus of the polypeptide chain backbone. A stable amide bond is formed between palmitate and the N-terminal amine of the N-terminal cysteine. The dually lipidated protein, termed ShhNp, represents the mature, active signaling molecule. Following secretion, lipidated ShhNp can adhere to the cell surface, thereby sharpening the concentration gradient, and can also form multimeric ShhNp containing complexes that participate in long and short range signaling [65].
Cholesterol modification contributes to Shh signaling activity, primarily in long-range interactions during embryonic patterning. Palmitoylation of Shh is essential for effective long- and short-range signaling. However, palmitoylation of Shh is independent of cholesterol attachment [66], and non-palmitoylated Shh exhibits extreme signaling defects regardless of whether the molecule is also cholesteroylated. A recent study suggested that cleavage of a short N-terminal palmitoylated peptide is required to generate activated Shh, and that this occurs as part of a shedding and solubilization process [67]. This finding is difficult to reconcile with the multiple reports demonstrating that fatty acylated, mature Shh added exogenously to cells exhibits signaling activity that is nearly 200-fold higher than the equivalent non-acylated Shh [68, 69].
Attachment of palmitate to Shh is catalyzed by the multipass membrane protein Hhat (hedgehog acyltransferase) [66, 70]. Loss of Hhat reproduces the phenotype observed with either loss of Shh or expression of non-palmitoylated Shh. Definitive biochemical evidence that Hhat is a palmitoyl acyltransferase for Shh was achieved by purifying the enzyme to homogeneity [66] and identifying active site residues within Hhat that are critical for its ability to catalyze Shh palmitoylation [71]. Hhat is a promising target for drug development in human cancers driven by aberrant overexpression of Shh, such as pancreatic cancer, and small molecule inhibitors of Hhat have recently been developed (Petrova, Glickman and Resh, unpublished data). Of note, Shh plays a prominent role in paracrine signaling in pancreatic cancer [72], suggesting that Hhat inhibitors might also be effective in blocking Shh-activated stromal cells in the tumor microenvironment.
Concluding Remarks
Several important points are raised in this review. First, protein lipidation is often used to promote membrane localization, an essential step for many disease-related proteins such as SFKs and Ras. Second, lipidation can directly influence protein activity. This can occur as a result of intramolecular protein interactions, as in the case of c-Abl, or by regulating the signaling activity of secreted proteins such as the Wnts and Shh. Third, inhibitors targeting the enzymes that catalyze protein fatty acylation or prenylation have potential clinical utility in the treatment of a wide variety of human ailments, including parasitic diseases, premature aging syndrome, and cancer. Finally, recent studies of SFKs and Ras proteins have highlighted the dynamic nature and functional importance of both palmitoylation and depalmitoylation reactions. Nevertheless, a number of outstanding questions remain and are summarized in Box 2. Future progress could be accelerated as three-dimensional structures of lipid modifying enzymes are modeled with their respective inhibitors bound. Moreover, a more complete inventory of all lipid modified proteins in the cell is essential. To this end, several recent studies have used large-scale proteomic profiling to identify palmitoylated proteins [73–80]. Proteomic based approaches have led to the identification of both known and novel palmitoylated proteins, and have shed light on their subcellular localization and distribution within specific cell types. This information will be extremely useful for the design of more specific agents that target the pathophysiologic functions of lipidated proteins.
Text Box 2. Outstanding questions.
Can pathogen-specific NMT inhibitors be sufficiently optimized to treat parasitic infections without inhibiting human NMT function?
Will combination therapy with FTase and GGTase inhibitors be effective for treating activated Ras-driven tumors, and what are the critical prenylated protein targets?
How do we explain both the positive and negative regulatory effects on SFK signaling activity by lipid rafts? Is this dichotomy cell-type or pathway dependent?
Can agents that interfere with Ras depalmitoylation be developed with sufficient specificity to block the growth of activated H-Ras and N-Ras-driven tumors?
Why are Wnt and Shh, secreted morphogens that signal over multiple distances, synthesized with two lipophilic groups, and how are these signaling proteins transported in the extracellular milieu?
Are there other targets for Porcn and Hhat, in addition to Wnt proteins and hedgehog proteins, and can Porcn and Hhat inhibitors be optimized for therapeutic intervention in tumors driven by Wnt or Shh ligand overexpression?
Acknowledgments
Research in the author’s laboratory was supported by NIH grants GM57966, CA72309, and CA158474 and by Cycle for Survival of Memorial Sloan-Kettering Cancer Center.
Glossary
- DHHC
A family of palmitoyl acyltransferases that catalyze attachment of palmitate to intracellular proteins
- Farnesyl
a 15-carbon isoprenoid lipid that is attached to target proteins by the enzyme farnesyl transferase (FTase; see Table 1 for the structure of farnesyl). The farnesyl group, in combination with a second signal (see Text Box 1), helps to anchor modified proteins to membranes, facilitating localization and signal transduction
- Geranylgeranyl
a 20 carbon isoprenoid lipid covalently attached to proteins by the enzyme geranylgeranyl transferase (GGTase; see Table 1 for the chemical structure). The geranylgeranyl group is more hydrophobic than farnesyl, and is sufficient to anchor the modified protein to membranes
- Hedgehog protein
Vertebrates express three hedgehog family proteins: Sonic (Shh), Indian (Ihh), and Desert (Dhh) hedgehog, secreted proteins that regulate growth and patterning during development. These protein morphogens act in a concentration-dependent manner to form short and long range signaling gradients. Shh plays an essential role in regulating brain and limb development, cell proliferation and cell differentiation in both mice and humans
- Hedgehog acyltransferase (Hhat)
a multi-pass membrane protein responsible for attaching palmitate to Shh, thereby regulating its activity
- Lipid rafts
Subdomains of the plasma membrane enriched in cholesterol, glycosphingolipids, and fatty acylated signaling proteins. These rafts can help concentrate and segregate membrane-associated proteins to facilitate signal transduction cascades
- Membrane bound O-acyl transferase (MBOAT)
a family of multipass membrane proteins implicated in attachment of fatty acids to secreted proteins (such as Hedgehog, Wnts, and ghrelin) and lipids (cholesterol, diacylglycerol)
- Myristate
a 14-carbon saturated fatty acid that is attached co-translationally by the enzyme N-myristoyltransferase to the N-terminal glycine of specific proteins (see Table 1 for the chemical structure of myristate). Myristate and a second signal (see Text Box 1), promotes membrane binding of the modified protein
- Myristoyl-CoA:protein N-myristoyltransferase (NMT)
a cytosolic enzyme that catalyzes the covalent attachment of myristate to the N-terminal glycine of target proteins. Inhibitors against this enzyme show promise in treating human pathogen infections
- Palmitate
a 16-carbon saturated fatty acid (see Table 1 for the chemical structure of palmitate) that is attached to a wide variety of membrane bound proteins. Palmitate is typically attached to a cysteine side chain within a protein via thioester linkage (S-palmitate). Thioester bonds are readily hydrolyzed by thioesterases, allowing S-palmitoylated proteins to undergo reversible membrane binding. In a small subset of proteins (e.g. hedgehog), palmitate is attached to the amine of an N-terminal cysteine via a stable amide linkage
- Palmitoleate
a 16-carbon mono-unsaturated fatty acid, most commonly found as 16:1Δ9 with the double bond between carbons 9 and 10 (see Table 1 for the chemical structure of palmitoleate). This modification plays an important role in regulating the Wnt signaling pathways, which are crucial for regulating cell growth
- Palmitoyl acyltransferase (PAT)
a family of enzymes that catalyzes attachment of palmitate and other long chain fatty acids to protein targets
- Porcn
a member of the MBOAT family implicated in promoting palmitoleate attachment to Wnt proteins
- Ras
a family of small GTPases that regulate normal and malignant cell growth. Lipidation of Ras proteins is a central component of Ras signaling, and dysregulation of this process affects disease development
- Rheb
Ras homolog enriched in brain, a small GTPase that is exclusively farnesylated. Farnesylation of Rheb is required for its ability to stimulate mTOR, a kinase that regulates cell growth and is a target of the immunosuppressant rapamycin
- Shh
Sonic hedgehog, a member of the hedgehog family of secreted proteins that regulate growth and development
- Wnt
A family of 19 secreted proteins that regulate cell fate and tissue development during development, and tissue homeostasis and stem cell maintenance in adults
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 2.Farazi TA, et al. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 3.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 4.Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 5.Sigal CT, et al. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc Natl Acad Sci U S A. 1994;91:12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, et al. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright MH, et al. Protein myristoylation in health and disease. J Chem Biol. 2009 doi: 10.1007/s12154-009-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masubuchi M, et al. Synthesis and biological activities of benzofuran antifungal agents targeting fungal N-myristoyltransferase. Bioorg Med Chem. 2003;11:4463–4478. doi: 10.1016/s0968-0896(03)00429-2. [DOI] [PubMed] [Google Scholar]
- 9.Brannigan JA, et al. N-myristoyltransferase from Leishmania donovani: structural and functional characterisation of a potential drug target for visceral leishmaniasis. J Mol Biol. 2010;396:985–999. doi: 10.1016/j.jmb.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frearson JA, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–732. doi: 10.1038/nature08893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, et al. Discovery and characterization of a cell-permeable, small-molecule c-Abl kinase activator that binds to the myristoyl binding site. Chem Biol. 2011;18:177–186. doi: 10.1016/j.chembiol.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patwardhan P, Resh MD. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol Cell Biol. 2010;30:4094–4107. doi: 10.1128/MCB.00246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berndt N, et al. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11:775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsimberidou AM, et al. Farnesyltransferase inhibitors: where are we now? Expert Opin Investig Drugs. 2010;19:1569–1580. doi: 10.1517/13543784.2010.535516. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, et al. Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc Natl Acad Sci U S A. 2010;107:6471–6476. doi: 10.1073/pnas.0908396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjogren AK, et al. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J Clin Invest. 2007;117:1294–1304. doi: 10.1172/JCI30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjogren AK, et al. Inactivating GGTase-I reduces disease phenotypes in a mouse model of K-RAS-induced myeloproliferative disease. Leukemia. 2011;25:186–189. doi: 10.1038/leu.2010.242. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, et al. Both farnesyltransferase and geranylgeranyltransferase I inhibitors are required for inhibition of oncogenic K-Ras prenylation but each alone is sufficient to suppress human tumor growth in nude mouse xenografts. Oncogene. 1998;16:1467–1473. doi: 10.1038/sj.onc.1201656. [DOI] [PubMed] [Google Scholar]
- 20.Whyte DB, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 21.Falsetti SC, et al. Geranylgeranyltransferase I inhibitors target RalB to inhibit anchorage-dependent growth and induce apoptosis and RalA to inhibit anchorage-independent growth. Mol Cell Biol. 2007;27:8003–8014. doi: 10.1128/MCB.00057-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raponi M, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111:2589–2596. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]
- 23.Basso AD, et al. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280:31101–31108. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- 24.Castro AF, et al. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 25.Winter-Vann AM, et al. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc Natl Acad Sci U S A. 2005;102:4336–4341. doi: 10.1073/pnas.0408107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manandhar SP, et al. Small-molecule inhibitors of the Rce1p CaaX protease. J Biomol Screen. 2007;12:983–993. doi: 10.1177/1087057107307226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young SG, et al. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria--new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res. 2005;46:2531–2558. doi: 10.1194/jlr.R500011-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Yang SH, et al. Assessing the efficacy of protein farnesyltransferase inhibitors in mouse models of progeria. J Lipid Res. 2010;51:400–405. doi: 10.1194/jlr.M002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SH, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandilands E, et al. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J Cell Sci. 2007;120:2555–2564. doi: 10.1242/jcs.003657. [DOI] [PubMed] [Google Scholar]
- 32.Cai H, et al. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci U S A. 2011;108:6579–6584. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oneyama C, et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426–436. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Oneyama C, et al. Transforming potential of Src family kinases is limited by the cholesterol-enriched membrane microdomain. Mol Cell Biol. 2009;29:6462–6472. doi: 10.1128/MCB.00941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanou T, et al. The transmembrane adaptor Cbp/PAG1 controls the malignant potential of human non-small cell lung cancers that have c-src upregulation. Mol Cancer Res. 2011;9:103–114. doi: 10.1158/1541-7786.MCR-10-0340. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann L, et al. Direct observation and quantitative analysis of Lck exchange between plasma membrane and cytosol in living T cells. J Biol Chem. 2010;285:6063–6070. doi: 10.1074/jbc.M109.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang MM, et al. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci U S A. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocks O, et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141:458–471. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Rocks O, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin JS, et al. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170:261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorentzen A, et al. Regulation of Ras localization by acylation enables a mode of intracellular signal propagation. Sci Signal. 2010;3:ra68. doi: 10.1126/scisignal.20001370. [DOI] [PubMed] [Google Scholar]
- 42.Swarthout JT, et al. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 2005;280:31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 43.Jennings BC, et al. 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J Lipid Res. 2009;50:233–242. doi: 10.1194/jlr.M800270-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducker CE, et al. Discovery and characterization of inhibitors of human palmitoyl acyltransferases. Mol Cancer Ther. 2006;5:1647–1659. doi: 10.1158/1535-7163.MCT-06-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel G, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satou M, et al. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151:4765–4775. doi: 10.1210/en.2010-0412. [DOI] [PubMed] [Google Scholar]
- 47.Dekker FJ, et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol. 2010;6:449–456. doi: 10.1038/nchembio.362. [DOI] [PubMed] [Google Scholar]
- 48.Ahearn IM, et al. FKBP12 binds to acylated H-ras and promotes depalmitoylation. Mol Cell. 2011;41:173–185. doi: 10.1016/j.molcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Doubravska L, et al. Fatty acid modification of Wnt1 and Wnt3a at serine is prerequisite for lipidation at cysteine and is essential for Wnt signalling. Cell Signal. 2011;23:837–848. doi: 10.1016/j.cellsig.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Galli LM, et al. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- 53.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 54.Biechele S, et al. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 56.Barrott JJ, et al. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komekado H, et al. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 58.Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Coombs GS, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ching W, et al. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galli LM, Burrus LW. Differential Palmit(e)oylation of Wnt1 on C93 and S224 Residues Has Overlapping and Distinct Consequences. PLoS One. 2011;6:e26636. doi: 10.1371/journal.pone.0026636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, et al. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J Cell Biol. 2011;192:825–838. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grover VK, et al. Lipid modifications of Sonic hedgehog ligand dictate cellular reception and signal response. PLoS One. 2011;6:e21353. doi: 10.1371/journal.pone.0021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buglino JA, Resh MD. Hhat is a palmitoylacyl transferase with specificity for N-palmitoylation of sonic hedgehog. J Biol Chem. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohlig S, et al. Sonic hedgehog shedding results in functional activation of the solubilized protein. Dev Cell. 2011;20:764–774. doi: 10.1016/j.devcel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Kohtz JD, et al. N-terminal fatty-acylation of sonic hedgehog enhances the induction of rodent ventral forebrain neurons. Development. 2001;128:2351–2363. doi: 10.1242/dev.128.12.2351. [DOI] [PubMed] [Google Scholar]
- 69.Taylor FR, et al. Enhanced potency of human Sonic hedgehog by hydrophobic modification. Biochemistry (Mosc) 2001;40:4359–4371. doi: 10.1021/bi002487u. [DOI] [PubMed] [Google Scholar]
- 70.Chamoun Z, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 71.Buglino JA, Resh MD. Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog. PLoS One. 2010;5:e11195. doi: 10.1371/journal.pone.0011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian H, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang R, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yount JS, et al. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol. 2010;6:610–614. doi: 10.1038/nchembio.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin BR, et al. Global profiling of dynamic protein palmitoylation. Nat Methods. 2011;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dowal L, et al. Proteomic analysis of palmitoylated platelet proteins. Blood. 2011;118:e62–73. doi: 10.1182/blood-2011-05-353078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merrick BA, et al. Proteomic profiling of S-acylated macrophage proteins identifies a role for palmitoylation in mitochondrial targeting of phospholipid scramblase 3. Mol Cell Proteomics. 2011;10:M110 006007. doi: 10.1074/mcp.M110.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang W, et al. Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mol Cell Proteomics. 2010;9:54–70. doi: 10.1074/mcp.M800448-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Forrester MT, et al. Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res. 2011;52:393–398. doi: 10.1194/jlr.D011106. [DOI] [PMC free article] [PubMed] [Google Scholar]