Abstract
During asymmetric stem cell division, polarization of the cell cortex targets fate determinants unequally into the sibling daughters, leading to regeneration of a stem cell and production of a progenitor cell with restricted developmental potential. In mitotic neural stem cells (neuroblasts) in fly larval brains, the antagonistic interaction between the polarity proteins Lethal (2) giant larvae (Lgl) and atypical Protein Kinase C (aPKC) ensures self-renewal of a daughter neuroblast and generation of a progenitor cell by regulating asymmetric segregation of fate determinants. In the absence of lgl function, elevated cortical aPKC kinase activity perturbs unequal partitioning of the fate determinants including Numb and induces supernumerary neuroblasts in larval brains. However, whether increased aPKC function triggers formation of excess neuroblasts by inactivating Numb remains controversial. To investigate how increased cortical aPKC function induces formation of excess neuroblasts, we analyzed the fate of cells in neuroblast lineage clones in lgl mutant brains. Surprisingly, our analyses revealed that neuroblasts in lgl mutant brains undergo asymmetric division to produce progenitor cells, which then revert back into neuroblasts. In lgl mutant brains, Numb remained localized in the cortex of mitotic neuroblasts and failed to segregate exclusively into the progenitor cell following completion of asymmetric division. These results led us to propose that elevated aPKC function in the cortex of mitotic neuroblasts reduces the function of Numb in the future progenitor cells. We identified that the acyl-CoA binding domain containing 3 protein (ACBD3) binding region is essential for asymmetric segregation of Numb in mitotic neuroblasts and suppression of the supernumerary neuroblast phenotype induced by increased aPKC function. The ACBD3 binding region of Numb harbors two aPKC phosphorylation sites, serines 48 and 52. Surprisingly, while the phosphorylation status at these two sites directly impinged on asymmetric segregation of Numb in mitotic neuroblasts, both the phosphomimetic and non-phosphorylatable form of Numb suppressed formation of excess neuroblasts triggered by increased cortical aPKC function. Thus, we propose that precise regulation of cortical aPKC kinase activity distinguishes the sibling cell identity in part by ensuring asymmetric partitioning of Numb into the future progenitor cell where Numb maintains restricted potential independently of regulation by aPKC.
Keywords: Asymmetric division, cell polarity, intermediate neural progenitor, neuroblast, Lethal (2) giant larvae, Numb
Introduction
During asymmetric stem cell divisions, polarization of the cell cortex allows unequal partitioning of the cell fate determinants that instruct the daughter progeny to either self-renew as a stem cell or adopt the progenitor cell identity (Neumüller and Knoblich, 2009; Prehoda, 2009). Progenitor cells possess restricted developmental potential and undergo limited rounds of cell division that give rise to differentiated progeny. Mis-regulation of cortical polarity in asymmetrically dividing stem cells can impinge on the accumulation and/or function of fate determinants in the intended recipient cell. Such defects can lead to generation of progenitor cells that possess stem cell-like properties, perturbing homeostasis and contributing to tumor initiation. Thus, insight into the mechanisms that distinguish sibling cell identity during normal tissue development will likely improve our understanding of aberrant processes from congenital birth defects to tumorigenesis.
In fly larval brains, two classes of neuroblast lineages can be unambiguously identified based on expression of the cell fate markers and properties of their daughter progeny (Chia et al., 2008; Doe, 2008; Egger et al., 2008; Knoblich, 2008; Knoblich, 2010; Weng and Lee, 2011) (Figure S1A). A type I neuroblast expresses Deadpan (Dpn) and Asense (Ase) and divides asymmetrically to self-renew a neuroblast and to generate a progenitor cell called a ganglion mother cell (GMC). In contrast, a type II neuroblast (Dpn+Ase−) divides asymmetrically to self-renew and to generate an immature intermediate neural progenitor cell (INP) that lacks the expression of Dpn and Ase and is transiently arrested in the cell cycle while acquiring INP identity (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). Following maturation, an INP (Dpn+Ase+) undergoes limited rounds of asymmetric divisions to regenerate and to produce GMCs. A key functional property that distinguishes these two neuroblast lineages rests on their dependence on Notch signaling for maintenance of their identity (Bowman et al., 2008; Song and Lu, 2011; Weng et al., 2011). While dispensable for maintenance of a type I neuroblast, Notch signaling is crucial in maintaining type II neuroblasts (Figure S1B–E).
The mutually antagonistic interaction between Lgl and aPKC in mitotic neuroblasts ensures that Numb segregates exclusively into the cortex of the presumptive progenitor cell where Numb functions to specify progenitor cell identity (Lee et al., 2006; Rolls et al., 2003; Wang et al., 2006). In lgl mutant brains, increased cortical aPKC function disrupts asymmetric segregation of Numb in mitotic neuroblasts and triggers formation of supernumerary neuroblasts. Consistent with Numb acting as a conserved inhibitor of Notch signaling, neuroblasts lacking numb function or expressing constitutively active Notch generate supernumerary neuroblasts at the expense of progenitor cells (Bowman et al., 2008; Frise et al., 1996; Guo et al., 1996; Lee et al., 2006; Rhyu et al., 1994; San-Juán and Baonza, 2011; Wang et al., 2006; Zhong et al., 1997). Thus, elevated cortical aPKC kinase activity induces supernumerary neuroblast formation likely by attenuating Numb-dependent regulation of Notch signaling. The fly Numb protein contains five evolutionarily conserved aPKC phosphorylation sites, and the non-phosphorylatable form of the Numb transgenic protein at these sites (Numb5A) fails to segregate asymmetrically in mitotic sensory organ precursor cells (Dho et al., 2006; Nishimura and Kaibuchi, 2007; Smith et al., 2007). aPKC can indeed directly phosphorylate Numb through these sites and render Numb non-functional (Dho et al., 2006; Nishimura and Kaibuchi, 2007; Smith et al., 2007; Wirtz-Peitz et al., 2008). Together, these results led to the hypothesis that increased cortical aPKC kinase activity induces supernumerary neuroblasts by perturbing the localization and the function of Numb. Thus far, evidence supporting this proposed mechanism appears largely correlative. First, direct evidence linking aPKC kinase activity to the de-localization of Numb from the cortex of mitotic neuroblasts is absent. Second, whether phosphorylation by aPKC indeed renders Numb inactive in progenitor cells has never been tested. Finally, type II neuroblasts require Notch signaling for maintenance of their identity; therefore, over-expression of Numb or Numb5A most likely induces supernumerary type II neuroblasts in lgl mutant brains to undergo premature differentiation rather than restoring proper specification of INP identity (Wirtz-Peitz et al., 2008) (Figs. S1B–G). As such, whether increased cortical aPKC kinase activity induces supernumerary neuroblasts by impinging on the localization and the function of Numb remains an open question
In this study, we show that despite failing to segregate Numb asymmetrically, neuroblasts in lgl mutant brains reproducibly undergo asymmetric division to generate progenitor cells. This result suggests that increased cortical aPKC kinase activity impinged on the segregation but not the function of Numb. Surprisingly, the non-phosphorylatable Numb5A at the five conserved aPKC phosphorylation sites exclusively partitioned in mitotic neuroblasts, indicating that Numb contains additional aPKC phosphorylation sites required for asymmetric segregation. Indeed, the two aPKC phosphorylation sites, serines 48 and 52, in the ACBD3 binding region played a pivotal role in asymmetrically segregating Numb into the cortex of the future progenitor cell. Most unexpectedly, Numb suppressed supernumerary neuroblasts induced by increased cortical aPKC function regardless of the phosphorylation status at serines 48 and 52. Thus, we propose that the antagonistic interaction between Lgl and aPKC ensures that sufficient Numb reaches the future progenitor cells where Numb maintains their limited potential irrespective of its phosphorylation by aPKC.
Materials and Methods
Fly Stocks and Transgenes
The novel lgl mutants were generated by EMS mutagenesis following standard procedures. The numb deletion constructs were generated by site-directed mutagenesis of the numb cDNA, sequenced, and cloned in the pUAST-HA vector for germline transformation. The UAS-numbS2A and UAS-numbS2D flies were generated using the pUAST-attB-HA vector for insertion into an identical docking site in the fly genome via the φC31 integrase-mediated transgenesis (Bischof and Basler, 2008). Erm-lacZ flies were generated by cloning the R9D11 enhancer element upstream of a minimal promoter and the lacZ gene followed by φC31 integrase-mediated transgenesis. Drosophila cultures were maintained at 25°C under standard conditions. Other mutant and transgenic flies used in the study include lgl334 (Peng et al., 2000), aPKC06403 (Rolls et al., 2003), UAS-aPKCcaax (Sotillos et al., 2004), Erm-GAL4 (Pfeiffer et al., 2008), Wor-GAL4, Ase-GAL4 (Zhu et al., 2006), UAS-numb (Knoblich et al., 1997), UAS-numb5A (Smith et al., 2007), UAS-numbΔN (Knoblich et al., 1997), UAS-Notchintra (Chung and Struhl, 2001), and aph-15072 (Weng et al., 2011). The UAS-NotchRNAi and UAS-spdoRNAi lines were obtained from the Vienna Drosophila Resource Center. Oregon R, Sca-GAL4, UAS-dcr2, aph-1D35, hs-flp, Act-FRT-Stop-FRT-GAL4, UAS-GFP, UAS-flp, Act-FRT-Stop-FRT-lacZ, and tubGal80ts flies were obtained from the Bloomington Drosophila Stock Center.
Immunofluorescent Staining and Antibodies
Antibody staining was performed as previously described. Antibodies used in this study include rat anti-Dpn (1:1), rabbit anti-Ase (1:400), mouse anti-Pros (1:100), sheep anti-Lgl (1:1000, S. Goode), guinea pig anti-Numb (1:2500, J. Skeath), mouse anti-Dlg(1:50, DSHB), mouse anti-β-gal (1:100, Sigma), chicken anti-GFP(1:2000, Aves Labs), rat anti-α-tubulin (1:100, Serotec), rabbit anti-aPKC (1:1000, Sigma), rabbit anti-phospho-HistoneH3 (1:1000, Upstate), mouse anti-HA (1:1000, Covance), and mouse anti-c-Myc (1:50, DSHB). Secondary antibodies were from Invitrogen and Jackson ImmunoResearch (details are available upon request). Fluorescent conjugates of phalloidin (Invitrogen), which stain F-actin, were used to mark the cell cortex. All images are single confocal sections acquired on a Leica SP5 scanning confocal microscope.
Lineage Clone Induction
For neuroblast clone induction, wild-type or lgl mutant larvae containing hs-flp were heat-shocked as follows to induce recombination and marking by the Act-FRT-STOP-FRT-GAL4 driving UAS-GFP expression. After hatching, larvae were cultured for 24 hours at 25°C, subjected to a 1 hour heat-shock at 37°C, and returned to 25°C for 24 or 48 hours as indicated. For INP clone induction, wild-type or lgl mutant larvae containing erm-GAL4, tubGAL80ts, and UAS-flp were cultured at 33°C for 72 hours to induce recombination and marking by the Act-FRT-STOP-FRT-lacZ reporter. For clones over-expressing Notchintra, larvae containing hs-flp were cultured at 25°C and heat-shocked at 24 hours after hatching for 2 hours at 37°C to induce Act-FRT-STOP-FRT-GAL4 recombination and expression of UAS-Notchintra and UAS-GFP. Larval brains were then dissected and stained as described previously.
Over-expression Experiments
For expression of transgenes using wor-GAL4 alone or in the lgl rescue experiments using ase-GAL4, larvae were cultured at 32.5°C for 72 hours. For aPKCcaax overexpression studies, larvae were cultured at 31°C for 72 hours and at 33°C for 96 hours when using Erm-GAL4. Larval brains were then dissected and stained as described previously.
Results
Supernumerary neuroblasts in lgl mutant brains most likely arise from progenitor cells
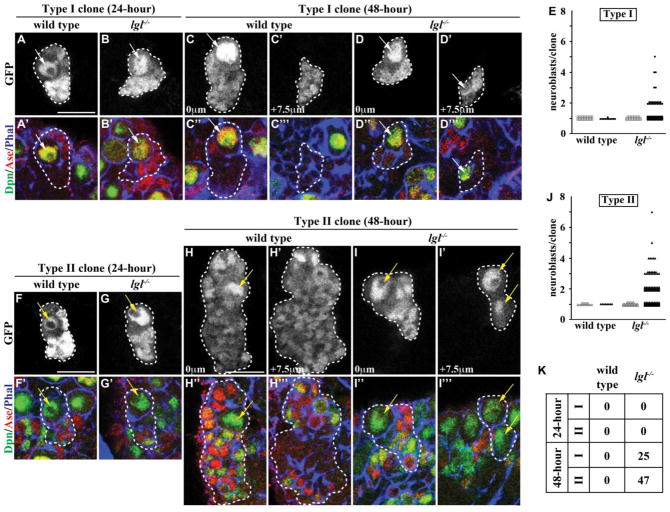
Increased cortical aPKC kinase activity phosphorylates Numb, an evolutionarily conserved protein instrumental for specification of the daughter sibling cell fate during asymmetric cell division, possibly rendering it inactive in lgl mutant brains (Guo et al., 1996; Nishimura and Kaibuchi, 2007; Rhyu et al., 1994; Smith et al., 2007; Wirtz-Peitz et al., 2008; Zhong et al., 1997). In addition, analyses of various lgl mutant alleles showed that both type I and II neuroblasts become aberrantly expanded in lgl mutant brains (Figs. 1E, J and S2). These data led us to hypothesize that supernumerary neuroblasts in lgl mutant brains arise from symmetric neuroblast divisions. We tested this hypothesis by assessing the identity of cells in the lineage clones derived from single type I neuroblasts in wild-type or lgl mutant brains at 24 or 48 hours after clone induction. In wild-type brains, we detected a single neuroblast (Dpn+Ase−) per type I neuroblast clone, and the neuroblast was always surrounded by GMCs (Dpn−Ase+) and their daughter progeny (Dpn−Ase−) (Figs. 1A, C, E, K and S1A). Unexpectedly, all 24-hour type I neuroblast clones in lgl mutant brains also contained a single neuroblast surrounded by GMCs and their daughter progeny (Figs. 1B, E and K). However, 25% of the 48-hour type I neuroblast clones in lgl mutant brains contained more than one neuroblast per clone, and we frequently observed supernumerary neuroblasts formed basally in the clones (Figs. 1D, E and K; n = 91). Thus, we propose that in lgl mutant brains, type I neuroblasts divide asymmetrically to self-renew and to generate GMCs, which revert back into type I neuroblasts.
Figure 1. lgl is required for the maintenance, but not specification, of progenitor cells.
(A–B) At 24 hours after clone induction, type I neuroblast clones in both wild-type and lgl334/3644 mutant brains contained a single neuroblast surrounded by GMCs and neurons. (n = 30 and 28 clones, respectively) (C–D) At 48 hours after clone induction, type I neuroblast clones in wild-type brains still contained a single neuroblast surrounded by GMCs and neurons; however, type I neuroblast clones in lgl334/3644 mutant brains contained a parental neuroblast immediately adjacent to GMCs and neurons with supernumerary neuroblasts located in the basal portion of the clone. (n = 11 and 91 clones, respectively) (E) Quantification of the number of neuroblasts per type I neuroblast clone is shown for wild-type and lgl mutant clones at 24 (open) and 48 (filled) hours after clone induction. (F–G) At 24 hours after clone induction, type II neuroblast clones in both wild-type and lgl334/3644 mutant brains contained a single neuroblast surrounded by immature INPs, INPs, GMCs and neurons. (n = 15 and 25 clones, respectively) (H–I) At 48 hours after clone induction, type II neuroblast clones in wild-type brains still contained a single neuroblast surrounded by immature INPs, INPs, GMCs and neurons; however, type II neuroblast clones in lgl334/3644 mutant brains contained a parental neuroblast isolated from the supernumerary neuroblasts by many immature INPs, INPs, GMCs and neurons. (n = 8 and 144 clones, respectively) (J) Quantification of the number of neuroblasts per type II neuroblast clone is shown for wild-type and lgl mutant clones at 24 (open) and 48 (filled) hours after clone induction. (K) The table shows the frequency of clones containing supernumerary neuroblasts. Brains were stained with the indicated markers. Single neuroblast clones marked by GFP are circled by the dotted line. Arrows indicate the neuroblasts (white, Type I; yellow, Type II). Single confocal planes of the same clone are shown at 0 mm and +7.5 mm (C–D and H–I). All scale bars are 10 μm.
The 24-hour or 48-hour type II neuroblast lineage clones in wild-type brains contain a single neuroblast (Dpn+Ase−) per clone, and the neuroblast was always directly surrounded by immature INPs (Dpn−Ase− or Dpn−Ase+) while INPs (Dpn+Ase+), GMCs and their daughter progeny were typically one or more cells away (Figs. 1F, H, J–K and S1A). Surprisingly, all 24-hour type II neuroblast clones in lgl mutant brains also contained a single neuroblast surrounded by immature INPs, INPs and their daughter progeny (Figs. 1G, J and K). Most importantly, 47% of the 48-hour clones in lgl mutant brains contained more than one neuroblast per clone, and we reproducibly observed supernumerary neuroblasts formed basally in the clones (Figs. 1I, J and K; n = 144). These results led us to conclude that in lgl mutant brains, type II neuroblasts also divide asymmetrically to self-renew and to produce immature INPs that mature into INPs, but INPs revert back into type II neuroblasts. Based on these data, we propose that Lgl functions to maintain restricted potential in progenitor cells including INPs and GMCs.
Increased cortical aPKC kinase activity triggers reversion of INPs back into neuroblasts
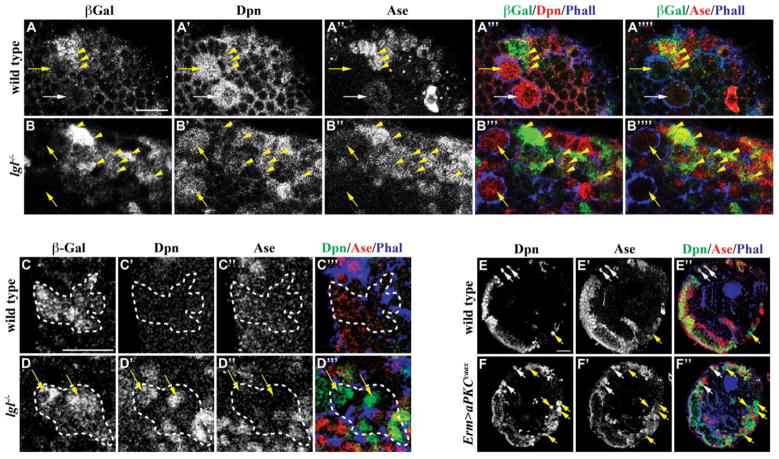
If Lgl indeed functions to maintain restricted potential in progenitor cells, we predict that the genetic clones derived from INPs in lgl mutant brains should contain supernumerary type II neuroblasts. In order to induce the INP lineage clones, we first examined whether INPs in lgl mutant brains show expression of the INP-specific earmuff-lacZ reporter transgene (Weng et al., 2010) (this study). earmuff-LacZ was detected in small Dpn+Ase+ cells surrounding type II neuroblasts but was undetectable in both type I and II neuroblasts in wild-type and lgl mutant brains (Figs. 2A–B). Thus, the INP-specific enhancer element in the earmuff gene remains active in lgl mutant brains, allowing us to induce lineage clones derived from INPs in wild-type or lgl mutant brains by expressing flipase driven by the earmuff-Gal4 (Weng et al., 2010). All INP clones in wild-type brains (n = 31) contained only progeny that lack Dpn and Ase expression but never type II neuroblasts (Fig. 2C). In contrast, lgl mutant brains (86%, n = 21) contained INP clones with one or more type II neuroblasts (Fig. 2D). These aberrant neuroblasts can indeed undergo asymmetric division to self-renew and to produce progenitor cells as indicated by the presence of immature INPs within the clones. Thus, INPs can indeed revert back into type II neuroblasts in lgl mutant brains.
Figure 2. lgl mutant and aPKCcaax overexpressing INPs revert back to type II neuroblasts.
(A–B) Wild-type and lgl334/3644 mutant brains expressed erm-lacZ specifically in INPs, but not in neuroblasts. (C–D) While an INP-derived clone in a wild-type brain only contained neurons, an INP-derived clone in an lgl334/3644 mutant brain contained multiple supernumerary type II neuroblasts. (n = 31 and 21 brains, respectively) (E–F) Overexpression of aPKCcaax driven by Erm-GAL4 leads to supernumerary type II neuroblasts in comparison to a wild-type brain. (n = 8 and 9 brains, respectively) Brains were stained with the indicated markers. Clones marked by β-galactosidase are circled by the dotted line. Arrows indicate the neuroblasts (white, Type I; yellow, Type II) and yellow arrowheads indicate the INPs. All scale bars are 10 μm.
Since Lgl functions with aPKC in mitotic neuroblasts, we examined if reversion of INPs back into type II neuroblasts in lgl mutant brains occurs due to increased cortical aPKC kinase activity. We first tested if reduced function of aPKC can suppress supernumerary type II neuroblasts and INPs in lgl mutant brains. While a wild-type brain lobe contained 8 type II neuroblasts and 58 ± 8 INPs (Dpn+Ase+earmuff-LacZ+), an lgl mutant brain lobe possessed 36 ± 9 type II neuroblasts and 131 ± 25 INPs (Fig. S3B). Consistent with our hypothesis, an lgl mutant brain lobe heterozygous for aPKC contained 13 ± 5 type II neuroblasts and 66 ± 19 INPs (Fig. S3B). We next directly assessed if unrestrained cortical aPKC kinase activity is sufficient to trigger reversion of INPs back into type II neuroblasts. Indeed, INPs ectopically expressing constitutively membrane localized aPKCcaax under the control of earmuff-Gal4 generated supernumerary type II neuroblasts (Figs. 2E–F and S3A). Thus, precise regulation of aPKC kinase activity plays a critical role in maintaining restricted potential in INPs.
Numb requires the ACBD3 binding region for its localization and function in neuroblasts
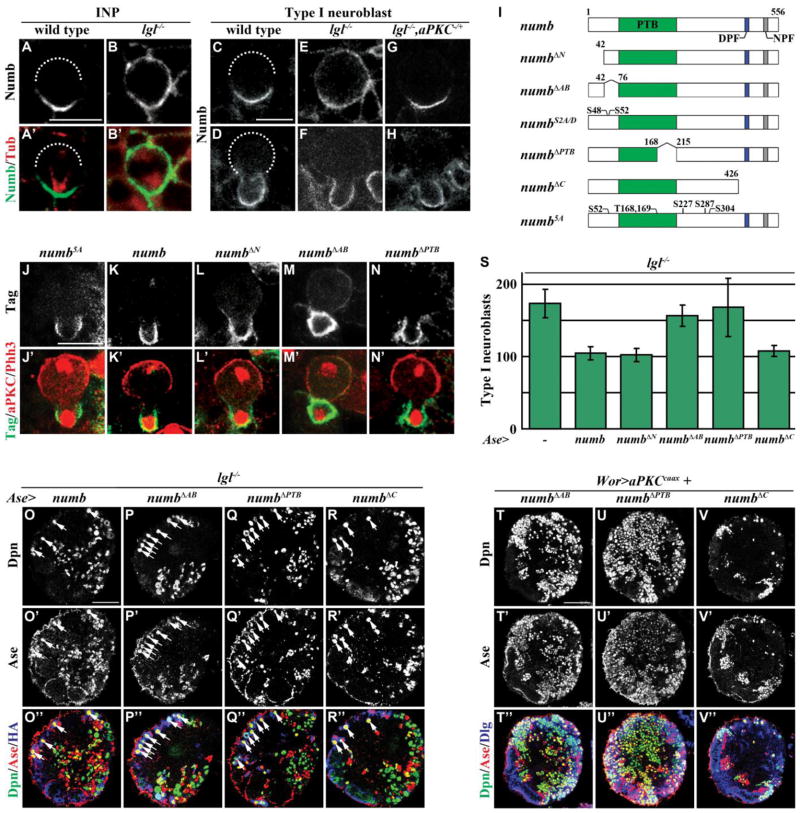
Numb, which localized in the basal cortex of mitotic INPs and type I neuroblasts, is an excellent candidate for acting downstream of Lgl to maintain restricted potential in progenitor cells (Figs. 3A, C–D). In lgl mutant brains, Numb localized uniformly in the cortex of metaphase INPs (100%, n = 9) and type I neuroblasts (100%, n = 46) and became enriched in the cortex of the future GMC in telophase neuroblasts (76%, n = 13) (Figs. 3B, E and F). Furthermore, heterozygosity of aPKC restored asymmetric localization and segregation of Numb in mitotic INPs and type I neuroblasts in lgl mutant brains (Figs. 3G–H; metaphase = 78%, n = 9; telophase = 89%, n = 9). Moreover, neuroblasts ectopically expressing aPKCcaax showed uniform cortical localization of Numb in both metaphase and telophase (Fig. S4) (Wang et al., 2006). These data led us to conclude that increased cortical aPKC kinase activity perturbs asymmetric localization of Numb in the cortex of mitotic neuroblasts and likely reduces the function of Numb in the cortex of the future progenitor cell in lgl mutant brains.
Figure 3. The localization and function of Numb in neuroblasts requires the ACBD3 binding region.
(A–B) Numb localized to the basal cortex of a wild-type INP, but distributed uniformly throughout the cortex of an lgl334/3644 mutant INP. (n = 8 and 9, respectively) (C–H) Wild-type neuroblasts showed asymmetric localization of Numb at metaphase and telophase, while lgl334/3644 mutant neuroblasts showed uniform cortical Numb localization at metaphase and basal enrichment of Numb at telophase. Additionally, the heterozygous mutant aPKC06403/+ restored the asymmetric localization of Numb in lgl334/3644 mutant neuroblasts at metaphase and telophase. (n = 30, 23, 46, 13, 9 and 9, respectively) (I) The diagram shows an illustration of the Numb protein as well as the deletion and mutant constructs used. (J–N) Similar to the full-length Numb transgenic protein, Numb5A, NumbΔN, and NumbΔPTB localized exclusively in the basal cortex of telophase neuroblasts; however, NumbΔAB did not localize exclusively to the basal cortex. (Tag indicates the myc epitope tag in J–L and the HA epitope tag in M–N) (n = 21, 10, 15, 57, and 27, respectively) (O–R) Supernumerary expression of either full-length Numb or NumbΔC using Ase-GAL4 suppressed the supernumerary type I neuroblasts in lgl334/3644 mutant brains, but expression of NumbΔAB or NumbΔPTB failed to rescue the supernumerary neuroblast phenotype. (n = 7, 10, 6, and 5, respectively) (S) Quantification of the number of type I neuroblasts per brain lobe is shown for expression of each transgenic Numb protein by Ase-GAL4 in lgl334/3644 mutant brains. (T–V) Expression of NumbΔC, but not NumbΔAB or NumbΔPTB, suppressed the supernumerary neuroblasts induced by expression of aPKCcaax driven by Wor-GAL4. (n = 6 per genotype) Brains were stained with the indicated markers. Dotted lines mark the location of the apical cortex. White arrows indicate the type I neuroblasts. Scale bars are 5 μm (A–B), 10 μm (C–N), and 25 μm (O–V).
We tested if aPKC regulates asymmetric localization of Numb in mitotic neuroblasts via the five conserved aPKC phosphorylation sites proposed by a previous study (Wirtz-Peitz et al., 2008). Surprisingly, ectopic expression of the non-phosphorylatable Numb5A transgenic protein at these sites in the presence of the endogenous Numb segregated exclusively into the cortex of the future progenitor cell in telophase neuroblasts (Fig. 3J; 100%, n = 21). This result strongly suggested that aPKC regulates Numb via alternative phosphorylation sites prompting us to first identify the domain(s) required for asymmetrically localizing Numb in mitotic brain neuroblasts. We ectopically expressed the UAS-numb transgenes that encode various truncated forms of Numb in type I neuroblasts in the presence of endogenous Numb and examined their localization pattern (Fig. 3I). Identical to the full-length Numb transgenic protein, NumbΔN, NumbΔPTB and NumbΔC segregated exclusively into the cortex of the future GMC in the telophase neuroblasts (Figs. 3K–L and N; data not presented; 100%, n = 10, 15, and 27, respectively). In contrast, the NumbΔAB transgenic protein failed to segregate exclusively into the cortex of the future GMC in the telophase neuroblasts (Fig. 3M; 61%, N = xx). Thus, we conclude that the ACBD3 binding region is necessary for asymmetric segregation of Numb.
We next ectopically expressed this series of the UAS-numb transgenes in type I neuroblasts, where Notch signaling is dispensable for maintenance of their identity, to determine which domains mediate the function of Numb in suppressing reversion of progenitor cells in lgl mutant brains. While ectopic expression of Numb, NumbΔN and NumbΔC efficiently suppressed supernumerary neuroblasts in lgl mutant brains, expression of NumbΔPTB did not have any effects on the supernumerary neuroblast phenotype (Figs. 3O and Q–S). The PTB domain mediates Numb binding to the Notch receptor protein and is essential for Numb suppression of Notch signaling (Frise et al., 1996; Yaich et al., 1998). Additionally, ectopic expression of Numb in type I neuroblasts of wild-type brains using ase-GAL4 had no effect on neuroblast number (data not presented). These data strongly suggest that aberrant activation of Notch signaling leads to supernumerary type I neuroblasts in lgl mutant brains. Most importantly, the NumbΔAB transgenic protein also failed to suppress supernumerary neuroblasts in lgl mutant brains (Figs. 3P and S). We independently tested whether the ACBD3 binding region is indeed necessary for suppressing supernumerary neuroblasts induced by unrestrained cortical aPKC kinase activity. While ectopic expression of Numb or NumbΔC efficiently suppressed massive supernumerary neuroblasts induced by aPKCcaax, expression of NumbΔAB or NumbΔPTB did not have any effects on the supernumerary neuroblast phenotype (Figs. 3T–V; data not presented). Thus, Numb requires the ACBD3 binding region to suppress reversion of progenitor cells in lgl mutant brains. Together, we conclude that the ACBD3 binding region is necessary for the localization and the function of Numb during asymmetric division of brain neuroblasts.
Serines 48 and 52 are required for asymmetric localization of Numb but likely dispensable for regulating specification of progenitor cells
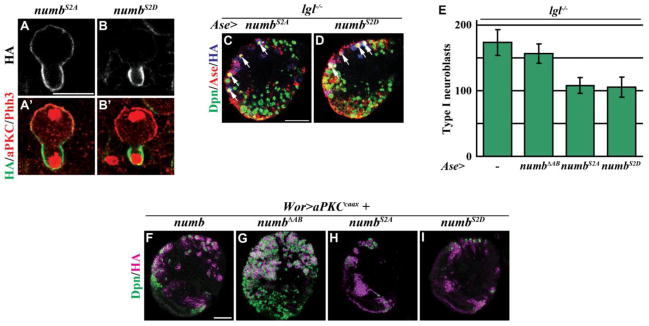
Our data showed that the ACBD3 binding region is indispensable for the localization and the function of Numb during neuroblast asymmetric division; therefore, we investigated if aPKC might regulate Numb through the phosphorylation sites in this domain. The ACBD3 binding region of Numb harbors two aPKC phosphorylation sites, serines 48 and 52 (Nishimura and Kaibuchi, 2007; Smith et al., 2007). If aPKC indeed regulates the localization of Numb through the ACBD3 binding region, the phosphorylation status at serines 48 and 52 should directly impinge on the distribution of the Numb protein in mitotic neuroblasts. Consistently, the non-phosphorylatable NumbS2A transgenic protein localized throughout the cortex of the telophase neuroblasts (Figs. 3I and 4A; 100%, n = 17). In contrast, the phosphomimetic NumbS2D transgenic protein became basally enriched in the telophase neuroblasts (Figs. 3I and 4B; 74%, n = 19). Together, these data strongly suggest that aPKC excludes Numb from the apical cortex of mitotic neuroblasts by phosphorylating serines 48 and 52.
Figure 4. Phosphorylation of Numb at serines 48 and 52 regulates its localization, but not function.
(A–B) NumbS2A localized uniformly throughout the entire cortex of the telophase neuroblast, while NumbS2D was enriched in the basal cortex. (n = 17 and 19, respectively) (C–D) Expression of NumbS2A or NumbS2D driven by Ase-GAL4 suppressed the supernumerary neuroblasts in lgl334/3644 mutant brains. (n = 8 and 9, respectively) (E) Quantification of the number of type I neuroblasts per brain lobe is shown for expression of the transgenic Numb proteins by Ase-GAL4 in lgl334/3644 mutant brains. (F–I) Similar to full-length Numb, expression of NumbS2A or NumbS2D suppressed the supernumerary neuroblasts induced by expression of aPKCcaax driven by Wor-GAL4, while expression of NumbΔAB did not have an effect on the supernumerary neuroblast phenotype. (n = 8 per genotype) Brains were stained with the indicated markers. White arrows indicate the type I neuroblasts. Scale bars are 10 μm (A–B) and 25 μm (C–I).
We next tested if increased cortical aPKC kinase activity inactivates the function of Numb by phosphorylating serines 48 and 52 in the ACBD3 binding region during neuroblast asymmetric division. We ectopically expressed NumbS2A or NumbS2D in type I neuroblasts in lgl mutant brains. Surprisingly, either NumbS2A or NumbS2D efficiently suppressed supernumerary type I neuroblasts in lgl mutant brains (Figs. 4C–E). In addition, while NumbΔAB failed to suppress supernumerary neuroblasts induced by aPKCcaax in larval brains, NumbS2A or NumbS2D completely suppressed the supernumerary neuroblast phenotype in the same genetic background (Figs. 4F–I). Thus, the phosphorylation status of serine 48 and 52 has no effects on the ability of the Numb transgenic protein to restore restricted potential in progenitor cells in lgl mutant brains. We propose that serine 48 and 52 play a critical role in asymmetric localization of Numb but are likely dispensable for regulation of progenitor cell potential.
The ACBD3 binding region mediates Numb-dependent suppression of Notch signaling specifically in brain neuroblasts
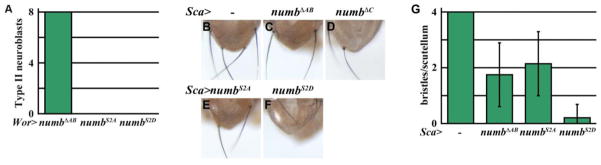
The ACBD3 binding region is necessary for the function of the mouse Numb protein, but how this domain mediates the function of the fly Numb protein has never been investigated (Zhou et al., 2007). The ACBD3 binding region is necessary for Numb to suppress supernumerary type I neuroblasts in larval brains lacking lgl function or ectopically expressing aPKCcaax, phenotypes that required activation of Notch signaling (Figs. 3 and S5). Thus, we hypothesize that the ACBD3 binding region mediates Numb suppression of Notch signaling. We tested this hypothesis by ectopically expressing the UAS-numbΔAB transgene under the control of a pan-neuroblast Wor-Gal4 driver in the larval brain. While increased function of numb or decreased function of Notch led to premature differentiation of type II neuroblasts, expression of NumbΔAB did not have any effects on maintenance of the type II neuroblast identity (Figs. S1C–E, G and 5A). Importantly, expression of NumbS2A or NumbS2D led to complete loss of type II neuroblasts prematurely in larval brains, indicating that the phosphorylation status at serines 48 and 52 does not affect the function of Numb to antagonize Notch signaling (Fig. 5A).
Figure 5. The ACBD3 binding region of Numb specifically mediates the inhibition of Notch signaling in larval neuroblasts.
(A) Quantification of type II neuroblasts per brain lobe is shown for Wor-GAL4 driving expression of NumbΔAB, NumbS2A, or NumbS2D. (n = 5 per genotype) (B–F) Compared to wild-type flies, overexpression of NumbΔAB, NumbS2A, NumbS2D, or NumbΔC driven by Sca-GAL4 leads to a loss of scutellar bristles. (n = 259, 187, 83, and 34, respectively) (G) Quantification of the number of bristles per scutellum is shown for Sca-GAL4 driving expression of NumbΔAB, NumbS2A, or NumbS2D.
Asymmetric divisions of sensory organ precursors give rise to the bristles on the scutellum of the adult fly and are highly sensitive to changes in Notch signaling (Frise et al., 1996; Knoblich et al., 1997; Rhyu et al., 1994; Yaich et al., 1998). Similar to over-expression of NumbΔC, unexpectedly, ectopic expression of NumbΔAB, NumbS2A or NumbS2D all led to cell fate transformation in the sensory organ precursor lineage and resulted in decreased bristles on the scutellum (Figs. 5B–G). This result indicates that the ACBD3 binding region is dispensable for Numb-mediated suppression of Notch signaling in sensory organ precursor cells in the peripheral nervous system. Together, we conclude that the ACBD3 binding region specifically mediates the function of Numb in suppressing Notch signaling in the brain regardless of the phosphorylation by aPKC.
Discussion
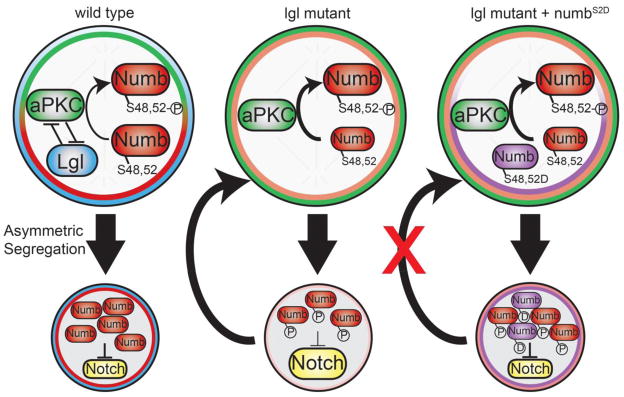
The antagonistic interaction between the polarity proteins Lgl and aPKC provides an evolutionarily conserved mechanism for regulating the cell fate determinants inherited into the daughter siblings during asymmetric cell division (Beatty et al., 2010; Betschinger et al., 2003; Hoege et al., 2010; Lee et al., 2006). However, regulation of the localization and the function of fate determinants by the polarity proteins can be uncoupled in a context-dependent manner. Our study led us to conclude that the antagonistic interaction between Lgl and aPKC maintains limited potential in progenitor cells at least in part by ensuring asymmetric partitioning of Numb into the future progenitor cells, where Numb acts irrespective of its phosphorylation by aPKC (Fig. 6). We showed that neuroblasts in lgl mutant brains undergo asymmetric division to self-renew and to generate progenitor cells, which can produce post-mitotic progeny but can also revert back into neuroblasts (Figs. 1 and 2). This indicates that although progenitor cells initially establish the proper identity these cells fail to maintain their limited potential. Additionally, Numb remained localized in the cortex of telophase neuroblasts lacking lgl function or ectopically expressing aPKCcaax (Fig. 3F and data not presented). The phosphomimetic Numb2D transgenic protein at serines 48 and 52, which are essential for asymmetric cortical localization of Numb, also remained localized in the cell cortex of telophase neuroblasts (Fig. 4B). Finally, increased function of Numb efficiently suppressed supernumerary neuroblasts in lgl mutant brains irrespective of the phosphorylation status at serines 48 and 52 (Fig. 4C–D). Thus, we propose that Lgl antagonizes aPKC to ensure a necessary threshold of Numb in the progenitor cells where Numb maintains limited potential regardless of phosphorylation by aPKC (Fig. 6).
Figure 6. Diagram of Proposed Model.
In wild-type neuroblasts, the mutual antagonism between Lgl and aPKC ensures asymmetric segregation of Numb into the progenitor cell, where Numb antagonizes Notch signaling via its PTB and ACDB3 protein binding domains. In the absence of lgl, increased cortical aPKC kinase activity redistributes Numb in the cortex of the dividing neuroblast, potentially leading to insufficient Numb to inhibit Notch in the progenitor cell. This increases the Notch activity in the progenitor cells and drives the progenitor cell to revert back into a neuroblast. Furthermore, expression of the phosphomimetic NumbS2D rescues the lgl mutant supernumerary neuroblast phenotype by restoring Numb and the inhibition of Notch activity in the progenitor cell.
Lgl maintains limited potential in progenitor cells
How Lgl suppresses formation of supernumerary neuroblasts in larval brains has remained a mystery largely due to the existence of a phenomenon called “telophase rescue” (Albertson and Doe, 2003; Cai et al., 2001). In lgl mutant brains, the basal proteins including Miranda and Numb fail to localize to the basal crescent in metaphase neuroblasts but by and large re-localize asymmetrically in telophase neuroblasts (Lee et al., 2006; Rolls et al., 2003; Wirtz-Peitz et al., 2008). Furthermore, Miranda and Numb appear to localize independently of each other in mitotic neuroblasts (Lu et al., 1998; Shen et al., 1997). Thus, the transcription factor Dpn whose expression and localization pattern is not impinged upon by defective cortical cell polarity provides an excellent cell identity marker to investigate the cellular origin of supernumerary neuroblasts in lgl mutant brains (Fig. S1A; Komori and Lee, unpublished). Surprisingly, only the 48-hour, but not the 24-hour, type I and II neuroblast lineage clones in lgl mutant brains contained supernumerary neuroblasts, which frequently localized basally from the parental neuroblasts (Fig. 1). The most recently born daughter always remains immediately adjacent to the parental neuroblasts while the earlier born progeny gradually becomes displaced away from the parental neuroblasts (Bayraktar et al., 2010; Bowman et al., 2008; Weng et al., 2010). Thus, supernumerary neuroblasts in lgl mutant brains most likely originated from the progenitor cells rather than symmetric neuroblast division. We propose that in the type I neuroblast lineage Lgl prevents aPKC kinase activity in the basal cortex to ensure that GMCs maintain limited potential and generate only post-mitotic progeny. In the type II neuroblast lineage, Lgl prevents aPKC kinase activity in the basal cortex to ensure that after maturation the INP can maintain limited potential and generate only GMCs during limited rounds of asymmetric divisions.
Although these results do not exclude the possibility that GMCs in the type II neuroblast lineages in lgl mutant brains can revert back into neuroblasts, we believe that reacquisition of the type II neuroblast fate by GMCs might be less likely. First, the basal protein Prospero plays a critical role in regulating the function of GMCs, and mosaic clones derived from prospero mutant INPs contained massive supernumerary INPs but never supernumerary type II neuroblasts (Hirata et al., 1995; Knoblich et al., 1995; Spana and Doe, 1995; Weng et al., 2010). Second, mosaic clones derived from numb mutant INPs contained supernumerary INPs but never supernumerary type II neuroblasts (Komori and Lee, unpublished). Thus, blocking differentiation in GMCs allows them to retain the identity of their immediate parental cell type, which is INP in the type II neuroblast lineage. Until the enhancer elements that exhibit GMC-specific expression become available, we cannot conclusively rule out the possibility that GMCs can re-acquire the type II neuroblast fate in lgl mutant brains.
aPKC regulates asymmetric localization but not the function of Numb in neuroblasts
A previous study strongly suggested that increased cortical aPKC kinase activity induces supernumerary neuroblasts in lgl mutant brains by phosphorylating Numb, therefore, displacing it from the neuroblast cortex and inactivating its function (Wirtz-Peitz et al., 2008). This proposed mechanism was in part based on studies in vertebrates showing that phosphorylation by aPKC perturbs cortical localization and the function of Numb (Nishimura and Kaibuchi, 2007; Smith et al., 2007). Inconsistent with this proposed mechanism, we reproducibly detected disruption in asymmetric distribution of Numb in the cortex of mitotic neuroblasts in lgl mutant brains (Fig. 3E–H). Failure to displace Numb from the cortex of mitotic neuroblasts in lgl mutant brains was unlikely due to insufficient aPKC kinase activity as Numb remained localized uniformly in the cortex of neuroblasts over-expressing aPKCcaax (Fig. S4). Additionally, the phosphomimetic Numb2D transgenic protein at serines 48 and 52, two residues required for asymmetric segregation of Numb in mitotic neuroblasts, remained localized in the neuroblast cortex (Fig. 4B). Thus, increased cortical aPKC kinase activity most likely disperses Numb in the cortex of mitotic neuroblasts and reduces accumulation of Numb in the cortex of the future progenitor cell in lgl mutant brains. Most importantly, over-expression of Numb2D suppressed supernumerary neuroblasts in lgl mutant brains as efficiently as Numb2A, strongly suggesting that phosphorylation by aPKC does not inactivate the function of Numb (Fig. 4). Together, these data led us to propose that increased cortical aPKC kinase activity induces supernumerary neuroblasts in lgl mutant brains by reducing accumulation of Numb rather than inactivation of Numb in the progenitor cells (Fig. 6).
Studies in vertebrates identified five conserved aPKC phosphorylation sites in the fly Numb, and the non-phosphorylatable Numb5A transgenic protein at these sites localized uniformly cortical in mitotic sensory organ precursors (Nishimura and Kaibuchi, 2007; Smith et al., 2007). Surprisingly, Numb5A localized asymmetrically in mitotic brain neuroblasts, indicating that these sites are dispensable for exclusion of Numb from the apical cortex by aPKC (Fig. 3J). Thus, many important questions regarding the significance of these five conserved aPKC phosphorylation sites on the localization and the function of Numb remain to be tested. For example, does the phosphomimetic Numb5D transgenic protein indeed fail to localize to the cell cortex of mitotic sensory organ precursors? Furthermore, is Numb5D indeed non-functional? Does over-expression of Numb5D have any effects on cell fate determination in the sensory organ precursor cell lineage? Are the non-conserved aPKC phosphorylation sites dispensable for the function of Numb in the sensory organ precursor cell lineage? Most importantly, the extent to which these conserved and non-conserved aPKC phosphorylation sites might impinge on other biological processes regulated by Numb requires additional direct and rigorous assessment.
The ACBD3 binding region mediates Numb suppression of Notch signaling specifically in the brain
Numb is a highly conserved protein and exerts its antagonistic effect on Notch signaling via the PTB domain, which mediates direct binding to the Notch receptor protein (Frise et al., 1996; Knoblich et al., 1997; Yaich et al., 1998; Zhong et al., 1997). A previous study identified that the ACBD3 binding region is required for asymmetric localization of the Numb protein and Numb-dependent suppression of Notch signaling (Zhou et al., 2007). Our study extended this result and showed that the ACBD3 binding region mediates asymmetric cortical localization of the fly Numb protein in mitotic neuroblasts through an aPKC-regulated mechanism (Fig. 3). Interestingly, the ACBD3 binding region regulates tissue-specific suppression of Notch signaling by Numb despite the presence of the PTB domain (Fig. 5). Since the ACBD3 binding region appears to mediate direct protein-protein interactions, we propose that this domain serves as a platform in which tissue-specific regulators can exert precise control of the Numb function in antagonizing Notch signaling. Identification and functional characterization of proteins that interact with Numb through the ACBD3 binding region will provide novel mechanistic insight into how the evolutionarily conserved Numb-dependent suppression of Notch signaling can be precisely regulated in a tissue-specific manner.
Supplementary Material
(A) A diagram of the type I and type II neuroblast lineages is shown. (B–E) Reductions in Notch signaling by aph-1 mutation, RNAi knock-down of the Notch receptor, or ectopic expression of Numb leads to a loss of Notch reporter (mγ-GFP) expression and premature loss of type II neuroblasts. (n = 5 per genotype) (F–G) Quantification of the number of type I and type II neuroblasts per brain lobe corresponding to the genotypes in B–E is shown.
(A–E) Various lgl mutant alleles show supernumerary type I and type II neuroblasts. (F) Illustrations of the isolated mutations in lgl are shown. (G–J) The isolated mutations in lgl show a loss of Lgl protein compared to wild-type brains.
(A) Quantification of the number of type II neuroblasts per lobe is shown for wild-type brains and brains ectopically expressing aPKCcaax by Erm-GAL4. (B) Quantification of the number of type II neuroblasts and INPs per brain lobe is shown for wild-type, lgl334/3644 mutant, and lgl334/3644, aPKC06403/+ mutant brains. (n = 13, 17, and 14 brains, respectively)
(A–B) Neuroblasts ectopically expressing aPKCcaax showed uniform cortical Numb localization at metaphase and telophase. (C–D) Ectopic expression of a kinase-dead aPKCcaax showed asymmetric localization of Numb in neuroblasts at both metaphase and telophase.
(A–C) Mutation of aph-1 reduced the number of type I and type II neuroblasts in the lgl334/3644 mutant brains. (n = 20 per genotype) (D–F) Knock-down of the Notch receptor by RNAi using Ase-GAL4 rescued the supernumerary type I neuroblast phenotype in lgl334/3644 mutant brains. (n = 20 per genotype) (G–I) Reductions in Notch signaling by RNAi knock-down of the Notch receptor or a critical component of the Notch signaling pathway, spdo, suppressed the supernumerary neuroblasts induced by Wor-GAL4 driven aPKCcaax expression. (n = 8 per genotype) (J–K) Both type I and type II clones expressing Notchintra showed supernumerary neuroblasts.
Highlights.
Progenitor cells revert back to form ectopic neuroblasts in lgl mutant brains.
Lgl and aPKC together ensure asymmetric segregation of Numb in mitotic neuroblasts.
aPKC regulates asymmetric localization of Numb via the ACBD3 protein binding domain.
Serines 48/52 are necessary for localization but dispensable for function of Numb.
The ACBD3 binding domain of Numb exerts neuroblast-specific suppression of Notch.
Acknowledgments
We thank Drs. C. Doe, S. Goode, J. Knoblich, F. Schweisguth, J. Skeath and G. Struhl for fly stocks and antibody reagents. We thank the Bloomington Drosophila Stock Center and Vienna Drosophila RNAi Center for fly stocks and the Developmental Studies Hybridoma Bank for antibodies. We thank the anonymous reviewer and members of the Lee lab for reading the manuscript and providing critical comments. J.M.H. was supported by the NIH Cellular and Molecular Biology Training Grant T32-GM007315. C.K. was also supported by the NIH MSTP Training Grant T32-GM07863. C.-Y.L was supported by University of Michigan start-up, the Burroughs Wellcome Fund Career Award in the Biomedical Sciences (1006160.01), a Sontag Foundation Distinguished Scientist Award and an NIH grant R01-GM092818.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–70. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- Bayraktar OA, Boone JQ, Drummond ML, Doe CQ. Drosophila type II neuroblast lineages keep Prospero levels low to generate large clones that contribute to the adult brain central complex. Neural Dev. 2010 Oct;1:26. doi: 10.1186/1749-8104-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty A, Morton D, Kemphues K. The C. elegans homolog of Drosophila Lethal giant larvae functions redundantly with PAR-2 to maintain polarity in the early embryo. Development. 2010;137:3995–4004. doi: 10.1242/dev.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello BC, Izergina N, Caussinus E, Reichert H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 2008;3 doi: 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bischof J, Basler K. Recombinases and their use in gene activation, gene inactivation, and transgenesis. Methods Mol Biol. 2008;420:175–95. doi: 10.1007/978-1-59745-583-1_10. [DOI] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–95. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila. Dev Cell. 2008;14:535–46. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Chia W, Yang X. A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J. 2001;20:1704–14. doi: 10.1093/emboj/20.7.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W, Somers WG, Wang H. Drosophila neuroblast asymmetric divisions: cell cycle regulators, asymmetric protein localization, and tumorigenesis. J Cell Biol. 2008;180:267–72. doi: 10.1083/jcb.200708159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HM, Struhl G. Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila. Nat Cell Biol. 2001;3:1129–32. doi: 10.1038/ncb1201-1129. [DOI] [PubMed] [Google Scholar]
- Dho SE, Trejo J, Siderovski DP, McGlade CJ. Dynamic regulation of mammalian numb by G protein-coupled receptors and protein kinase C activation: Structural determinants of numb association with the cortical membrane. Mol Biol Cell. 2006;17:4142–55. doi: 10.1091/mbc.E06-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–87. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Egger B, Chell JM, Brand AH. Insights into neural stem cell biology from flies. Philos Trans R Soc Lond B Biol Sci. 2008;363:39–56. doi: 10.1098/rstb.2006.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc Natl Acad Sci U S A. 1996;93:11925–32. doi: 10.1073/pnas.93.21.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Hirata J, Nakagoshi H, Nabeshima Y, Matsuzaki F. Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature. 1995;377:627–30. doi: 10.1038/377627a0. [DOI] [PubMed] [Google Scholar]
- Hoege C, Constantinescu AT, Schwager A, Goehring NW, Kumar P, Hyman AA. LGL can partition the cortex of one-cell Caenorhabditis elegans embryos into two domains. Curr Biol. 2010;20:1296–303. doi: 10.1016/j.cub.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–97. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–60. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–7. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc Natl Acad Sci U S A. 1997;94:13005–10. doi: 10.1073/pnas.94.24.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–8. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Lu B, Rothenberg M, Jan LY, Jan YN. Partner of Numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell. 1998;95:225–35. doi: 10.1016/s0092-8674(00)81753-5. [DOI] [PubMed] [Google Scholar]
- Neumüller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C, Svirskas R, Kadonaga JT, Doe CQ, Eisen MB, Celniker SE, Rubin GM. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–20. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehoda KE. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect Biol. 2009 Aug;1:a001388. doi: 10.1101/cshperspect.a001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–91. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–98. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Juán BP, Baonza A. The bHLH factor deadpan is a direct target of Notch signaling and regulates neuroblast self-renewal in Drosophila. Dev Biol. 2011;352:70–82. doi: 10.1016/j.ydbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Shen CP, Jan LY, Jan YN. Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell. 1997;90:449–58. doi: 10.1016/s0092-8674(00)80505-x. [DOI] [PubMed] [Google Scholar]
- Smith CA, Lau KM, Rahmani Z, Dho SE, Brothers G, She YM, Berry DM, Bonneil E, Thibault P, Schweisguth F, Le Borgne R, McGlade CJ. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 2007;26:468–80. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Lu B. Regulation of cell growth by Notch signaling and its differential requirement in normal vs. tumor-forming stem cells in Drosophila. Genes Dev. 2011;25:2644–58. doi: 10.1101/gad.171959.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S, Díaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166:549–57. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–95. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006;20:3453–63. doi: 10.1101/gad.1487506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Golden KL, Lee CY. dFezf/Earmuff maintains the restricted developmental potential of intermediate neural progenitors in Drosophila. Dev Cell. 2010;18:126–35. doi: 10.1016/j.devcel.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Haenfler JM, Lee CY. Changes in Notch signaling coordinates maintenance and differentiation of the Drosophila larval optic lobe neuroepithelia. Dev Neurobiol. 2011 doi: 10.1002/dneu.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Lee CY. Keeping neural progenitor cells on a short leash during Drosophila neurogenesis. Curr Opin Neurobiol. 2011;21:36–42. doi: 10.1016/j.conb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–73. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaich L, Ooi J, Park M, Borg JP, Landry C, Bodmer R, Margolis B. Functional analysis of the Numb phosphotyrosine-binding domain using site-directed mutagenesis. J Biol Chem. 1998;273:10381–8. doi: 10.1074/jbc.273.17.10381. [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997;124:1887–97. doi: 10.1242/dev.124.10.1887. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Atkins JB, Rompani SB, Bancescu DL, Petersen PH, Tang H, Zou K, Stewart SB, Zhong W. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasing ACBD3 during mitosis. Cell. 2007;129:163–78. doi: 10.1016/j.cell.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127(2):409–22. 409–22. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) A diagram of the type I and type II neuroblast lineages is shown. (B–E) Reductions in Notch signaling by aph-1 mutation, RNAi knock-down of the Notch receptor, or ectopic expression of Numb leads to a loss of Notch reporter (mγ-GFP) expression and premature loss of type II neuroblasts. (n = 5 per genotype) (F–G) Quantification of the number of type I and type II neuroblasts per brain lobe corresponding to the genotypes in B–E is shown.
(A–E) Various lgl mutant alleles show supernumerary type I and type II neuroblasts. (F) Illustrations of the isolated mutations in lgl are shown. (G–J) The isolated mutations in lgl show a loss of Lgl protein compared to wild-type brains.
(A) Quantification of the number of type II neuroblasts per lobe is shown for wild-type brains and brains ectopically expressing aPKCcaax by Erm-GAL4. (B) Quantification of the number of type II neuroblasts and INPs per brain lobe is shown for wild-type, lgl334/3644 mutant, and lgl334/3644, aPKC06403/+ mutant brains. (n = 13, 17, and 14 brains, respectively)
(A–B) Neuroblasts ectopically expressing aPKCcaax showed uniform cortical Numb localization at metaphase and telophase. (C–D) Ectopic expression of a kinase-dead aPKCcaax showed asymmetric localization of Numb in neuroblasts at both metaphase and telophase.
(A–C) Mutation of aph-1 reduced the number of type I and type II neuroblasts in the lgl334/3644 mutant brains. (n = 20 per genotype) (D–F) Knock-down of the Notch receptor by RNAi using Ase-GAL4 rescued the supernumerary type I neuroblast phenotype in lgl334/3644 mutant brains. (n = 20 per genotype) (G–I) Reductions in Notch signaling by RNAi knock-down of the Notch receptor or a critical component of the Notch signaling pathway, spdo, suppressed the supernumerary neuroblasts induced by Wor-GAL4 driven aPKCcaax expression. (n = 8 per genotype) (J–K) Both type I and type II clones expressing Notchintra showed supernumerary neuroblasts.