Abstract
Homozygous and compound heterozygous mutations in SLC34A3, the gene encoding the sodium-dependent co-transporter NaPi-IIc, cause hereditary hypophosphatemic rickets with hypercalciuria (HHRH), a disorder characterized by renal phosphate-wasting resulting in hypophosphatemia, elevated 1,25(OH)2 vitamin D levels, hypercalciuria, rickets/osteomalacia, and frequently kidney stones or nephrocalcinosis. Similar albeit less severe biochemical changes are also observed in heterozygous carriers, which are furthermore indistinguishable from those encountered in idiopathic hypercalciuria (IH). We now searched for SLC34A3 mutations (exons and introns) in two previously not reported HHRH kindreds, which resulted in the identification of three novel mutations. The affected members of kindred A were compound heterozygous for two different mutations, c.1046_47del and the intronic mutation c.560+23_561-42del, while the index case in kindred B was homozygous for the nonsense SLC34A3 mutation c.1764C>G (p.Y588X). The patient in kindred C was diagnosed with IH because of bilateral medullary nephrocalcinosis, suppressed PTH levels, and hypercalciuria; she was found to have a novel heterozygous c.1571_1880del mutation. The HHRH patients in kindred A were treated for up to 7 years with oral phosphate, which led to reversal of hypophosphatemia, hypercalciuria, and prevention or healing of the mild bone abnormalities. PTH levels were normal throughout the observation period, while 1,25(OH)2 vitamin D levels remained elevated and may thus be helpful for assessing treatment efficacy and patient compliance in HHRH.
1. INTRODUCTION
Phosphate is essential for numerous cellular processes, DNA synthesis, and it is part of structural hydroxyapatite, thereby providing strength to the skeleton, which furthermore serves as a reservoir for this mineral [1]. Intestinal phosphate absorption occurs through paracellular, as well as active transcellular mechanisms involving the sodium-dependent phosphate co-transporter NaPi-IIb (SLC34A2) [2,3]. In the proximal tubules of the kidney, two closely related transporters, namely NaPi-IIa (SLC34A1) [4] and NaPi-IIc (SLC34A3) [5] reabsorb more than 70% of filtered phosphate. Expression of both transporters at the brush border membrane is directly or indirectly influenced by two phosphaturic hormones, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23), and possibly by the biologically active form of vitamin D, 1,25(OH)2 vitamin D [6].
NaPi-IIa and NaPi-IIc functions have been extensively studied through different in vitro and in vivo approaches. For example, studies in rodents have shown that injection of PTH rapidly reduces expression of NaPi-IIa and with some delay NaPi-IIc [7], while FGF23 requires several hours before a significant decrease in serum phosphate levels can be observed [8,9]. Furthermore, 1,25(OH)2 vitamin D was previously shown to regulate expression of NaPi-IIa in juxtamedullary kidney cortex, but not in the superficial cortex [6]; the role of 1,25(OH)2 vitamin D in the regulation of NaPi-IIc has not yet been investigated. Although the ablation of NaPi-IIc provided no clear evidence for a role of this transporter in renal phosphate handling [5], the combined ablation of NaPi-IIa and NaPi-IIc revealed that both transporters have an important role in maintaining rodent serum phosphorus levels within normal limits [10].
However, most significant insights into the importance of NaPi-IIc in human phosphate homeostasis were obtained by revealing that inactivating mutations on both parental alleles are the cause of hereditary hypophosphatemic rickets with hypercalciuria (HHRH; OMIM: 241530) [11-13]. HHRH was first defined as a distinct disorder when Tieder et al. reported a Bedouin kindred, in which consanguinity suggested an autosomal recessive mode of inheritance [14,15]. Individuals affected by HHRH, who carry homozygous or compound heterozygous NaPi-IIc mutations, show increased urinary phosphate excretion leading to hypophosphatemic rickets, bowing, and short stature, as well as elevated 1,25(OH)2 vitamin D levels leading to hypercalciuria because of enhanced intestinal absorption of calcium and reduced PTH-dependent calcium-reabsorption in the distal renal tubules. Heterozygous NaPi-IIc mutations are frequently associated with hypercalciuria, but none of the heterozygous carriers of the originally described HHRH patients had renal calcifications and kidney stones [14,15]; subsequent investigations, however, revealed renal complications in numerous patients with homozygous or compound heterozygous NaPi-IIc mutations [13,16-21]. The presence of kidney stones or nephrocalcinosis in HHRH kindreds is different from the findings in FGF23-dependent hypophosphatemic disorders such as X-linked hypophosphatemia (XLH; mutant PHEX) [22], autosomal dominant hypophosphatemia (ADHR; mutant FGF23) [23], or autosomal recessive hypophosphatemia (ARHR; mutant DMP1 or ENPP1) [24-26], in which affected individuals show inappropriately normal or suppressed 1,25(OH)2 vitamin D levels despite significant hypophosphatemia and thus no increase in urinary calcium excretion (at least prior to treatment with oral phosphate) [12,27].
Based on the underlying molecular defect that results in an FGF23-independent increase in urinary phosphate excretion, HHRH is thought to require a different therapeutic approach than FGF23-dependent hypophosphatemic disorders such as XLH. While oral phosphate supplements in combination with active vitamin D analogs are generally required for the treatment in the latter disorder [28], treatment with phosphate supplements alone is deemed sufficient for individuals with HHRH [14,15]; particularly since endogenously elevated 1,25(OH)2 vitamin D levels are predicted to prevent an increase in PTH secretion triggered by intermittent elevations in serum phosphate. In fact, therapy of HHRH patients with oral phosphate alone raises blood phosphate levels and is generally successful in resolving acute symptoms and abnormal bone mineralization, but no long-term follow-up studies exist evaluating the efficacy of this therapeutic approach. Despite persistent hypophosphatemia, phosphate treatment usually also leads to an improvement of the rachitic bone changes in XLH, yet it is well established that these patients often develop secondary or tertiary hyperparathyroidism when treated with oral phosphate alone [29]. Similar data evaluating outcomes in HHRH are lacking and it is conceivable that long-term oral phosphate supplementation of HHRH patients may affect parathyroid function and/or increase the risk for the development of nephrocalcinosis and kidney stones by increasing the urinary phosphate load, even if treatment with activated vitamin, which may worsen the degree of hypercalciuria, is avoided [16,18,30].
We here describe two previously not reported HHRH kindreds, in whom we discovered novel homozygous and compounded heterozygous NaPi-IIc mutations. Occurrence of renal calcifications as the initial finding in a third unrelated patient with a heterozygous NaPi-IIc mutation strengthens previous observations suggesting an increased risk for nephrocalcinosis and renal stones in this population. Follow-up of the three affected members in one of these families allowed us to assess the efficacy of standard oral phosphate supplementation with regard to skeletal and laboratory findings.
2. METHODS
2.1. Laboratory Assays
With the exception of genetic analyses, all laboratory studies were performed at local laboratories (normal ranges are provided in parenthesis after each value). Initial laboratory tests were done for each patient before oral phosphate supplements were administered. The 25(OH) vitamin D levels were measured by liquid chromatography with tandem mass spectroscopy or chemiluminescence immunoassay, 1,25(OH)2 vitamin D levels by radioimmunoassay or enzyme-linked immunoassay. Serum intact PTH levels were determined by electrochemiluminescence immunoassay and FGF23 level were measured by c- terminal FGF23 ELISA (Immutopics, San Clemente, CA). The renal tubular reabsorption of phosphorus was calculated using the following formula: %TRP = 100 × (1 - (urine phosphorus × serum creatinine)/(serum phosphorus × urine creatinine); when serum phosphorus is below the reference range for age, %TRP should be above 90. TmP/GFR was estimated using the Walton and Bijvoet nomogram [31,32].
2.2. SLC34A3 genetic analysis
Mutational and haplotype analysis of SLC34A3 was performed after informed written consent was obtained using forms approved by the institutional review board of Massachusetts General Hospital. The entire SLC34A3 gene, including approximately 800 bp 5′ of the transcriptional start site, all intervening sequences and approximately 200 bp of the 3′ UTR, was amplified by PCR from genomic DNA of the index cases (A/II-3, B/II-2, C/II-3), followed by nucleotide sequence analysis at the Massachusetts General Hospital DNA Sequencing Core Facility or at Genewiz Inc. (Cambridge, MA). PCR assays to confirm the findings in index cases and to analyze family members and controls were designed as described [11,12] using Qiagen reagents (Valencia, CA) at standard PCR cycling conditions. The following primers were used for c.1046_47del: 5′-CTCTGACCTCTGTCTGCC-3′ and 5′-GGAAGGGGAAGTCTATGG-3′, followed by nucleotide sequence analysis; for c.560+23_561-42del: 5′-AGCATGGTGGCTGCTAAGC-3′ and 5′-GGGTGTCAGGCTGGCGGC-3′, and for c.1571_1880del: 5′-CATCCACTTCTTCTTCAACCTG-3′ and 5′-CCATTCCTTGGGAGCTTC-3′, both latter amplicons were separated by 3% agarose TAE gel electrophoresis to detect mutant alleles that are shortened by 30 and 310 bp, respectively. The primers for c.1764C>G were 5′-GCTCCTTCTGTAGGGTGGAG-3′ and 5′-AAGCAGGTGACCGGAGG-3′, followed by restriction enzymatic digestion with Bfa1, resulted in the generation of two fragments (520 bp and 142 bp, respectively), whereas the PCR product derived from the wild-type allele revealed a single band of 662 bp.
Searches of NCBI-dbSNP [33] and the 1000 genomes project [34] were negative for the identified mutations. GenBank accession numbers for SLC34A3 are as follows: genomic contig NT_024000.15; cDNA, NM_080877.1; protein, NP_543153.1.
3. RESULTS
3.1. Kindred A
The 8 year old index case A/II-3 was referred to S.R.S. for evaluation of bowed legs. With the exception of an uncomplicated clavicular fracture as a toddler, childhood development was within normal limits. Physical examination confirmed genu valgum without evidence for pain or swelling, but revealed no additional skeletal findings.
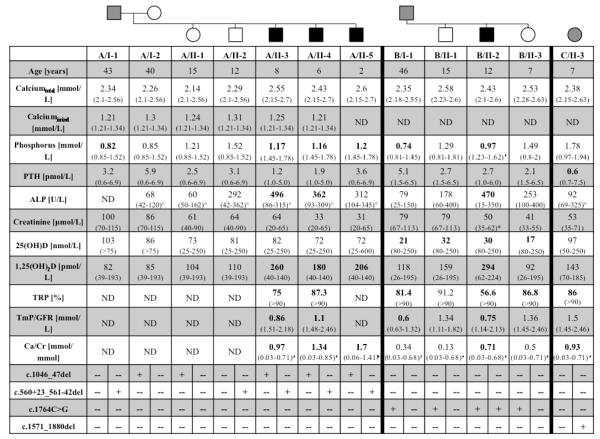
The initial laboratory testing (Fig. 1) showed a low serum phosphorus level of 1.17 mmol/L (1.45-1.78 mmol/L) and an elevated alkaline phosphatase of 496 U/L (86-315 U/L) with normal serum calcium, creatinine, PTH and 25(OH) vitamin D levels. Despite hypophosphatemia, tubular reabsorption of phosphate (TRP) was decreased at 75% (>90%) and the maximal renal phosphate reabsorption per glomerular filtration rate (TmP/GFR) was low at 0.86 mmol/L (1.51-2.18 mmol/L), which was consistent with renal phosphate wasting. Inappropriately low tubular reabsorption of phosphate along with an appropriately elevated 1,25(OH)2 vitamin D level of 260 pmol/L (40-140 pmol/L) raised the suspicion for HHRH. A subsequently obtained 24-hour-urine collection showed an elevated level of urinary calcium excretion (0.13 mmol/kg/day; normal: 0.05-0.1 mmol/kg/day); the urinary calcium/creatinine ratio was elevated at 0.97 mmol/mmol (0.03-0.71 mmol/mmol). Urine analysis showed no evidence for glucose, protein, or amino acids; serum bicarbonate level was within normal limits. Radiographs of both knees revealed valgus deformity and generalized osteopenia with numerous transversely oriented reinforcement lines in the medullary portions of the distal femora and proximal tibiae, as evidence for previous intermittent mineralization defects (Suppl. Fig. 1).
Fig. 1. Laboratory findings in patients with hereditary hypophosphatemic rickets with hypercalciuria (HHRH) or idiopathic hypercalciuria (IH) caused by novel SLC34A3 utations.
Biochemical parameters for the index cases in kindreds A, B, and C were measured at the indicated ages before therapy was initiated. Age-related reference ranges in parentheses (*http://www.mayomedicallaboratories.com/, °http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index/1D336E0232533D3285256B9C0059ECEA, and respective clinical laboratories); age-related reference ranges for TmP/GFR and Ca/Cr are from [43] and [44], respectively. All abnormal values are shown in bold. Circles denote females, squares denote men. Black symbols indicate affected individuals, who presented with hypophosphatemia, hypercalciuria, elevated 1,25(OH)2 vitamin D levels, and skeletal findings consistent with rickets, while grey symbols indicate affected individuals, who had one or more of the above biochemical abnormalities. Open symbols indicate healthy individuals.
Abbreviations are as follows:
ALP, alkaline phosphatase; Ca/Cr= urinary calcium/creatinine ratio; n.d., not determined; PTH; parathyroid hormone; TmP/GFR, maximum tubular phosphate reabsorption per glomerular filtrate; TRP, tubular phosphate reabsorption; 1,25(OH)2D, 1,25(OH)2 vitamin D; 25(OH)D, 25(OH) vitamin D
Laboratory investigations of the patient’s four siblings were conducted, which revealed no abnormalities for his sister (A/II-1) and one of his brothers (A/II-2). The affected brothers, A/II-4 and A/II-5, were asymptomatic and showed no evidence for bowing. Radiographic evaluation, however, revealed multiple growth arrest lines and demineralization consistent with hypophosphatemic rickets. Both children showed biochemical abnormalities including hypophosphatemia, hypercalciuria, and increased 1,25(OH)2 vitamin D levels (Fig.1). The non-consanguineous parents were found to be healthy and their laboratory results were within normal limits, with the exception of a slightly reduced serum phosphorus level of the father (0.82 mmol/L; normal: 0.85-1.52 mmol/L).
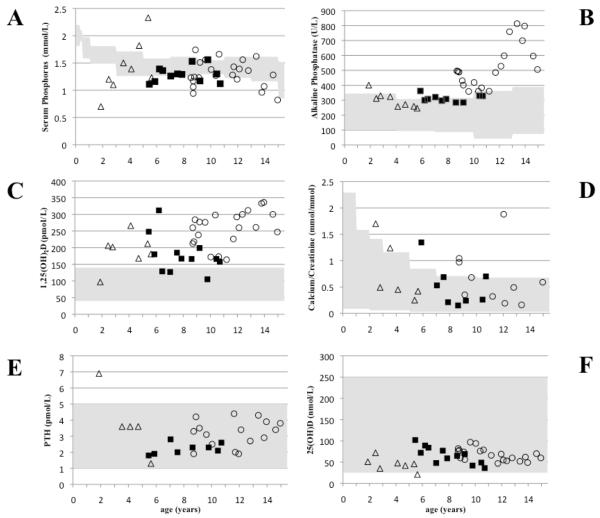
Treatment of A/II-3 with oral phosphate supplements (250 mg bid; 20 mg/kg/day) was started at the age of 91/12 years; the dose was slowly increased to 40 mg/kg/day (divided into three doses). Similar treatment was initiated for A/II-4 and A/II-5, which led to normalization of serum phosphorus levels and urinary calcium excretion in all three children; PTH and 25(OH) vitamin D remained within normal limits. Conversely, 1,25(OH)2 vitamin D levels remained elevated in all three individuals (Fig. 2A-F), while alkaline phosphatase activity normalized in A/II-4 and A/II-5, but increased during puberty in A/II-3. After improving serum phosphorus levels, A/II-3 showed some catch-up growth from the 3rd to the 25th percentile; if growth continues along this percentile he would reach the mid-parental height. There was no change in growth velocity for A/II-5, who continued growth along the 3rd percentile after oral phosphate supplements had been started. A/II-4 showed only mild improvement in growth velocity; 5th to the 10th percentile (data not shown). No episodes of hematuria were reported for the three affected members and screening by renal ultrasound showed no evidence for nephrolithiasis.
Fig. 2. Long-term follow-up of several laboratory parameters for the affected members in kindred A (II-3, II-4, and II-5).
Serum phosphate (panel A); alkaline phosphatase (panel B), 1,25(OH)2 vitamin D (panel C), urinary calcium/creatinine ratio (panel D), parathyroid hormone (panel E), and 25(OH) vitamin D (panel F).
Grey areas indicate normal range, age-matched where indicated. Open circles, A/II-3; closed squares, A/II-4; open triangles, A/II-5.
3.2. Kindred B
The index case (B/II-2) was evaluated for non-specific bone pain in her native country as a toddler. After immigrating at the age of 5 years with her family to the United States of America, she again came to medical attention for bilateral genu valga. At the age of 7 years, laboratory evaluation revealed hypophosphatemia, decreased 25(OH) vitamin D levels, and elevated alkaline phosphatase levels. At the age of 8 years, an eight-plate was placed to correct alignment without compressing the growth plate, and was removed one year later. She was initially treated with ergocalciferol and calcium carbonate for suspected vitamin D deficiency, which resulted in normalization of her 25(OH) vitamin D levels, but did not correct her hypophosphatemia. When she was referred at the age of 11 years to N.D., hypercalciuria was noted leading to the presumptive diagnosis of HHRH and she was started on 1000 mg K-Phos tid.
The patient’s maternal great-grandmother and paternal grandfather were siblings. Her father’s height is 172.7 cm, he has no evidence for bowed legs and no history of bone pain, but had previously passed a calcium oxalate stone. Laboratory investigations showed a low serum phosphorus level (0.74 mmol/L; normal: 0.81-145 mmol/L), low 25(OH) vitamin D (21 nmol/L; normal: 80-250 nmol/L) and decreased TRP (81.4%, normal: >90%) as well as a low TmP/GFR of 0.6 mmol/L (0.63-1.32mmol/L). The patient’s mother is healthy, her height is 165.1 cm and she has no history of bone or kidney disease; the two siblings of B/II-2 showed some biochemical abnormalities (Fig. 1).
At presentation to our hospital (A.S.) she was 124/12 years old, her height was 152.4 cm (54th percentile) and her weight 74 kilogram (>97th percentile). Physical examination revealed no bony abnormalities for the upper extremities with normal range of motion and strength, but the lower extremities showed scars from prior surgery and marked genu valgum with intramedullary distance of 12 cm. A renal ultrasound was significant for nephrocalcinosis (data not shown).
Laboratory evaluation revealed a low serum phosphorus level of 0.97 mmol/L (1.23-1.62 mmol/L) and an elevated alkaline phosphatase level of 470 U/L (15-350 U/L). Her 1,25(OH)2 vitamin D level was elevated to 294 pmol/L (62-224 pmol/L), while the 25(OH) vitamin D level was at 30 nmol/L (82-250 nmol/L). Serum calcium, PTH, and creatinine were within normal limits, as was c-terminal FGF23 (68 RU/ml; normal: <230 RU/ml).
Urine analysis revealed a TRP of only 56.6% (>90%) and a low TmP/GFR of 0.75 mmol/L (1.14-2.13 mmol/L). The urinary calcium/creatinine ratio was slightly elevated at 0.71 mmol/mmol (0.03-0.68 mmol/mmol), but there was no evidence for generalized tubular dysfunction as indicated by the lack of proteinuria, glucosuria, or aminoaciduria, and a normal serum bicarbonate level.
3.3. Kindred C
The index case (C/II-3) was referred to T.S. at the age of 710/12 years, because of abdominal pain over the flank area for the past year, which prompted ultrasonographic studies revealing bilateral medullary nephrocalcinosis (Suppl. Fig. 2). Her history was otherwise unremarkable except for increased urinary frequency and mild asthma. There was no history of macroscopic hematuria, passage of stones/gravel or urinary tract infection. The family history was significant for the maternal grandfather being diagnosed with kidney stones and Crohn’s disease; other members of the father’s family had been diagnosed with what was referred to as “nephritis”. The older brother was found to have hypercalciuria when he was evaluated for discomfort with urination two years prior to the presentation of the index case; subsequent evaluations showed normal urinary calcium excretion. Both parents and the older sister are healthy without urinary symptoms, stones, osteopenia and/or bone fractures.
The patient’s height at presentation was 123.9 cm (30th percentile), weight 23.6 kg (34th percentile), and BMI 15.4 kg/m2. Laboratory studies revealed normal levels for calcium, phosphorus, alkaline phosphatase, creatinine, 1,25(OH)2 vitamin D and 25(OH) vitamin D. PTH was below the normal range (0.6 pmol/L; normal: 0.7-7.5 pmol/L) and c-terminal FGF23 was within the normal range (109 RU/ml; normal: <230 RU/ml). The urinary calcium/creatinine ratio was elevated at 0.93 mmol/mmol (0.03-0.71 mmol/mmol), TRP was 86% (>90% in the setting of hypophosphatemia) and TmP/GFR was at the lower end of the normal range at 1.5 mmol/L (1.45-2.46 mmol/L). A 24-hour urine collection with simultaneous blood evaluation for nephrocalcinosis made primary hyperoxaluria, Dent’s disease, renal tubular acidosis, hypophophatasia, and an activating mutation in calcium-sensing receptor unlikely; familial hypomagnesemia with hypercalciuria as the cause of nephrocalcinosis was equally unlikely. The patient’s hypercalciuria was treated symptomatically with Amiloride and potassium citrate. On this therapy her laboratory studies were: calcium, 2.55 mmol/L; phosphorus, 1.71 mmol/L; magnesium, 0.9 mmol/L; bicarbonate, 29 mmol/L; alkaline phosphatase, 241 U/L; creatinine, 61.9 μmol/L; 1,25(OH)2 vitamin D, 120 pmol/L; and 25(OH) vitamin D, 130 nmol/L. The serum PTH was at the lower end of the normal range with 1.5 pmol/L (1.5-6.5 pmol/L). The repeat TRP measurement was 76%, a fasting TmP/GFR was low at 1.2 mmol/L (1.45-2.46 mmol/L). The urinary calcium/creatinine ratio was elevated at 0.76 mmol/mmol (0.03-0.71 mmol/mmol) with an excretion of 0.12 mmol/kg/day. The renal ultrasound revealed no change in the medullary nephrocalcinosis. The increase in urine phosphorus excretion despite a low serum PTH level, associated with an 1,25(OH)2 vitamin D at the upper end of the normal range and increased urine calcium excretion led us to search for a defect in one of the sodium-phosphate co-transporters.
3.4. Mutational Analysis
Mutational analysis of the SLC34A3 gene for individuals A/II-3, A/II-4 and A/II-5 revealed compound heterozygosity for two novel mutations: c.1046_47del and c.560+23_561-42del (Fig. 1). c.1046_47del leads to a frame-shift in exon 10 of SLC34A3, while the consequences of c.560+23_561-42del in intron 6 are uncertain, but may involve alternative splicing as has been reported for other intronic SLC34A3 deletions [13]. c.1046_47del was inherited from the healthy mother A/I-2, while c.560+23_561-42del was inherited from the father A/I-1. The unaffected siblings A/II-1 and A/II-2 are each carriers of either one of the two mutations.
Consistent with consanguinity of her parents, patient B/II-2 was found to be homozygous for a novel missense mutation in exon 13 (c.1764C>G; p.Y588X) (Fig. 1), which prematurely terminates the open reading-frame 12 amino acids before the natural termination codon. The c.1764C>G p.Y588X nucleotide change introduces a novel Bfa1 endonuclease restriction site, which permitted confirmation of the results obtained by nucleotide sequence analysis and showed that her parents and her two siblings are heterozygous carrier of the mutation. B/II-2 was furthermore homozygous for several known single nucleotide polymorphisms throughout SLC34A3 (Suppl. Fig. 3).
Patient C/II-3 revealed a novel heterozygous deletion of 310 bp in exon 13 (c.1571_1880del), but no evidence for a mutation on the second allele. c.1571_1880del is predicted to change the NaPi-IIc co-transporter protein after amino acid residue 523, followed by 13 unrelated amino acids and a termination codon. The deletion was also identified in her father C/I-2. Several known heterozygous and homozygous polymorphisms were found throughout the gene (Suppl. Fig. 3). One of the heterozygous polymorphisms (rs34372115; c.200G/A), which changes arginine 67 to histidine (R67H), has a minor allele frequency of only 8.6-15% [34] and thus could have represented the second mutation; however, it was inherited paternally on the same allele as c.1571_1880del.
4. DISCUSSION
The index cases in kindreds A and B showed hypophosphatemia due to increased urinary phosphate excretion, normal calcium, and normal or suppressed PTH levels, thus excluding hyperparathyroidism. In response to the hypophosphatemia, 1,25(OH)2 vitamin D levels were appropriately increased and normal FGF23 levels made FGF23-dependent causes of increased urinary phosphate excretion unlikely. Furthermore, urinary calcium excretion was increased, presumably because of 1,25(OH)2 vitamin D-mediated enhanced intestinal calcium absorption, but there was no evidence for generalized renal tubular defects, i.e. metabolic acidosis, aminoaciduria, glucosuria, or hematuria. Based on the laboratory and radiographic findings, the diagnosis of HHRH was considered and confirmed through the identification of previously not reported mutations in SLC34A3/NaPi-IIc that are predicted to affect co-transporter function and thus likely lead to impaired phosphate reabsorption in the renal proximal tubules. The identified SLC34A3/NaPi-IIc mutations co-segregate with biochemical and clinical abnormalities in the respective kindreds and were not found in publically available SNP databases, including the 1000 genomes project, making it likely that these are the causes of HHRH.
In family A, we discovered two novel compound heterozygous mutations: c.1046_47del and c.560+23_561-42del, which are predicted to severely alter or abolish NaPi-IIc function. One of the two mutations, c.560+23_561-42del, deletes 30 bp in intron 6, which may result in abnormal spliceosome assembly [35], and may lead to non-sense mediated decay of the mRNA, as has been described previously for deletions in intron 9 and 10 [13]. The change in open reading frame caused by the second mutation, c.1046_47del, introduces 242 novel amino acid residues in the C-terminal portion, which have no homology with proteins in the RefSeq database and likely impair function of the NaPi-IIc co-transporter, or lead to non-sense mediated decay of the mRNA. Likewise, the homozygous mutation c.1764C>G (p.Y588X) in exon 13 found in the index case of kindred B, most likely provides a molecular explanation for her HHRH. This mutation changes tyrosine at position 588 at the C-terminal end to a termination codon thus deleting the terminal 12 amino acids of the co-transporter protein. The equivalent C-terminal portion of the closely related NaPi-IIa contains a class 1 PDZ binding domain (TRL), which interacts with several sodium hydrogen exchange regulatory factors (NHERF) [36-39]. Although NaPi-IIc lacks a PDZ binding domain, interaction with NHERF1 and NHERF3 has been reported, raising the possibility that the identified mutation impairs this interaction [40,41]. Functional evaluation of c.1764C>G (p.Y588X) in Xenopus oocytes has not been performed, but a mutation (p.V446Stop) that we recently reported in an unrelated kindred leads to a complete loss of [P32]-transport in support of this conclusion [19].
The deletion of 310 bp in exon 13, c1571_1880del detected in C/II-3, is predicted to cause a frame-shift and thus likely results in a truncated and presumably nonfunctional protein or in non-sense mediated decay of the mRNA. This deletion was identified on the paternal allele, while there was no evidence for a second mutation on the maternal allele. Consistent with the lack of a second mutation, patient C/II-3 showed only hypercalciuria and biochemical findings suggestive of a PTH- and FGF23-independent renal phosphate leak.
Kindred A further supports the notion that HHRH is frequently caused by compound heterozygous NaPi-IIc mutations. This suggests either that SLC34A3/NaPi-IIc has a high mutation frequency or that heterozygous carrier status may be relatively frequent in the population, as also illustrated in C/II-3 and other previously described patients [13,16,18-21,30]. The subtle proximal tubular abnormalities associated with heterozygous carrier status in the original Bedouin kindred were initially not thought to increase the risk for kidney stones [13]. However, several heterozygous carriers of SLC34A3 mutations in subsequently investigated HHRH kindreds revealed kidney stones, nephrolithiasis, and/or increased urinary calcium excretion, similar to the findings in C/II-3 [16,18,30]. At least certain heterozygous SLC34A3 mutations may thus increase the relative risk for developing nephrocalcinosis and/or kidney stones, which frequently occur in the general population. Alternatively, it is possible that a SLC34A3 mutation was missed for C/II-3 on the second allele, or that other genetic modifiers of renal phosphate handling, for example NHERF-1 or SLC34A1/NaPi-IIa, carry additional nucleotide sequence variations in this individual.
Improvement of rachitic changes in XLH are often observed despite persistent hypophosphatemia, while oversupplementation or treatment with oral phosphate alone may cause secondary or tertiary hyperparathyroidism [29]. Likewise in HHRH patients therapy with oral phosphate supplements alone is generally effective in resolving bone pain and abnormal mineralization, and it was shown to reverse most biochemical abnormalities, except the decrease in TmP/GFR [14]. However, there are no long-term studies documenting that treatment of HHRH patients with oral phosphate alone is efficacious and safe. In fact, it is unknown whether secondary hyperparathyroidism can develop as reported in FGF23-dependent renal phosphate-wasting disorders such as XLH [42], whether 1,25(OH)2 vitamin D levels or other parameters remain abnormal in this group of patients, or whether an increased urinary phosphate load can lead to renal calcifications.
Based on biochemical follow-up data that were available for the affected members in kindred A (A/II-3, A/II-4 and A/II-5), oral phosphate supplement resulted in normalization of serum phosphorus and urine calcium levels, while there was no evidence for the development of secondary hyperparathyroidism over the course of up to 7 years. Alkaline phosphatase levels normalized in A/II-4 and A/II-5 over the course of therapy, but improved only partially in A/II-3 and increased during pubertal development, which could be indicative of skeletal growth, since catch-up growth and follow-up X-rays suggest that his rickets had healed. Despite improvement of most abnormalities with oral phosphate treatment, all three affected siblings had persistently elevated 1,25(OH)2 vitamin D levels. It is therefore conceivable that higher daily doses and/or more frequent administration of phosphate are needed to suppress the 1-alpha-hydroxylase and/or that our patients were not fully compliant with their supplements.
To monitor compliance with oral phosphate supplements, measurement of 24 hour urinary phosphate excretion is usually performed; this requires, however, considerable cooperation of the often pediatric patients. In HHRH patients, 1,25(OH)2 vitamin D levels are typically elevated because hypophosphatemia increases renal 1-alpha-hydroxylase activity without abatement by elevated FGF23 levels. In fact, FGF23 levels should be suppressed due to the primary renal defect in SLC34A3/NaPi-IIc, which is responsible for increased urinary phosphate excretion in HHRH; however, current FGF23 assays are not sufficiently sensitive to distinguish normal from suppressed levels. Our observations in kindred A thus suggest that measurement of 1,25(OH)2 vitamin D levels, a widely available test, can be helpful for monitoring therapy and compliance. Since 1,25(OH)2 vitamin D stimulates FGF23 production, which could lead to a further increase in urinary phosphate excretion, normalization of this parameter may be an important goal and further improve outcomes.
In summary, we have identified four novel mutations in SLC34A3/NaPi-IIc in two unrelated HHRH kindreds and a case of IH with bilateral nephrocalcinosis. Follow-up of the three affected cases in kindred A suggests that sole therapy with oral phosphate supplements resolved or improved hypophosphatemia, hypercalciuria, and prevented progression or development of skeletal abnormalities without increasing PTH levels. In the absence of other biomarkers, 1,25(OH)2 vitamin D levels may be a helpful laboratory parameter for assessing the treatment efficacy and compliance.
Supplementary Material
HIGHLIGHTS.
-
➢
Three novel NaPi-IIc/SLC34A3 mutations causing HHRH and one novel mutation in this gene causing IH
-
➢
Long-term follow up during treatment with oral phosphate
-
➢
Persistent elevation of 1,25(OH)2 vitamin D levels
Acknowledgments
Grants and Fellowships
This work was supported by the Studienstiftung des deutschen Volkes (The German National Merit Foundation) (Y.Y), Biomedical Sciences Exchange Program (Y.Y), and NIH/NIDDK 5K08DK078361 (C.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WEB RESOURCES
1000 Genomes Project, http://www.1000genomes.org/
dbSNP, http://www.ncbi.nlm.nih.gov/projects/SNP/
Mayo Clinic, Mayo Medical Laboratories, http://www.mayomedicallaboratories.com/
NIH Clinical Center, http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index/1D336E0232533D3285256B9C0059ECEA
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/omim
NCBI Reference Sequence, http://www.ncbi.nlm.nih.gov/RefSeq/
The Sequence Manipulation Suite, http://www.bioinformatics.org/sms/
University of California-Santa Cruz (UCSC) Human Genome Browser, http://genome.ucsc.edu/
REFERENCES
- [1].Bergwitz C, Jüppner H. Disorders of phosphate homeostasis and tissue mineralisation. Endocr Dev. 2009;16:133–156. doi: 10.1159/000223693. doi:10.1159/000223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM. Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem. Biophys. Res. Commun. 1999;258:578–582. doi: 10.1006/bbrc.1999.0666. doi:10.1006/bbrc.1999.0666. [DOI] [PubMed] [Google Scholar]
- [4].Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, et al. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, et al. Growth-related renal type II Na/Pi cotransporter. J. Biol. Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. doi:10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- [6].Taketani Y, Segawa H, Chikamori M, Morita K, Tanaka K, Kido S, et al. Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J. Biol. Chem. 1998;273:14575–14581. doi: 10.1074/jbc.273.23.14575. [DOI] [PubMed] [Google Scholar]
- [7].Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, et al. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch. 2010;460:677–687. doi: 10.1007/s00424-010-0841-1. doi:10.1007/s00424-010-0841-1. [DOI] [PubMed] [Google Scholar]
- [8].Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal Physiol. 2009;297:F282–291. doi: 10.1152/ajprenal.90742.2008. doi:10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tomoe Y, Segawa H, Shiozawa K, Kaneko I, Tominaga R, Hanabusa E, et al. Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am. J. Physiol. Renal Physiol. 2010;298:F1341–1350. doi: 10.1152/ajprenal.00375.2009. doi:10.1152/ajprenal.00375.2009. [DOI] [PubMed] [Google Scholar]
- [10].Segawa H, Onitsuka A, Furutani J, Kaneko I, Aranami F, Matsumoto N, et al. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am. J. Physiol. Renal Physiol. 2009;297:F671–678. doi: 10.1152/ajprenal.00156.2009. doi:10.1152/ajprenal.00156.2009. [DOI] [PubMed] [Google Scholar]
- [11].Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006;78:179–192. doi: 10.1086/499409. doi:10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 2006;78:193–201. doi: 10.1086/499410. doi:10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J. Clin. Endocrinol. Metab. 2006;91:4022–4027. doi: 10.1210/jc.2005-2840. doi:10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- [14].Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, et al. Hereditary hypophosphatemic rickets with hypercalciuria. N. Engl. J. Med. 1985;312:611–617. doi: 10.1056/NEJM198503073121003. doi:10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- [15].Tieder M, Modai D, Shaked U, Samuel R, Arie R, Halabe A, et al. “Idiopathic” hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N. Engl. J. Med. 1987;316:125–129. doi: 10.1056/NEJM198701153160302. doi:10.1056/NEJM198701153160302. [DOI] [PubMed] [Google Scholar]
- [16].Page K, Bergwitz C, Jaureguiberry G, Harinarayan CV, Insogna K. A patient with hypophosphatemia, a femoral fracture, and recurrent kidney stones: report of a novel mutation in SLC34A3. Endocr Pract. 2008;14:869–874. doi: 10.4158/EP.14.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kremke B, Bergwitz C, Ahrens W, Schütt S, Schumacher M, Wagner V, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp. Clin. Endocrinol. Diabetes. 2009;117:49–56. doi: 10.1055/s-2008-1076716. doi:10.1055/s-2008-1076716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Phulwani P, Bergwitz C, Jaureguiberry G, Rasoulpour M, Estrada E. Hereditary hypophosphatemic rickets with hypercalciuria and nephrolithiasis-Identification of a novel SLC34A3/NaPi-IIc mutation. Am. J. Med. Genet. A. 2011;155A:626–633. doi: 10.1002/ajmg.a.33832. doi:10.1002/ajmg.a.33832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jaureguiberry G, Carpenter TO, Forman S, Jüppner H, Bergwitz C. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am. J. Physiol. Renal Physiol. 2008;295:F371–379. doi: 10.1152/ajprenal.00090.2008. doi:10.1152/ajprenal.00090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mejia-Gaviria N, Gil-Peña H, Coto E, Pérez-Menéndez TM, Santos F. Genetic and clinical peculiarities in a new family with hereditary hypophosphatemic rickets with hypercalciuria: a case report. Orphanet J Rare Dis. 2010;5:1. doi: 10.1186/1750-1172-5-1. doi:10.1186/1750-1172-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tencza AL, Ichikawa S, Dang A, Kenagy D, McCarthy E, Econs MJ, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/type IIc sodium-phosphate cotransporter: presentation as hypercalciuria and nephrolithiasis. J. Clin. Endocrinol. Metab. 2009;94:4433–4438. doi: 10.1210/jc.2009-1535. doi:10.1210/jc.2009-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat. Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. doi:10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- [23].Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. doi:10.1038/81664. [DOI] [PubMed] [Google Scholar]
- [24].Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. doi:10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. doi:10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. doi:10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamamoto T, Michigami T, Aranami F, Segawa H, Yoh K, Nakajima S, et al. Hereditary hypophosphatemic rickets with hypercalciuria: a study for the phosphate transporter gene type IIc and osteoblastic function. J. Bone Miner. Metab. 2007;25:407–413. doi: 10.1007/s00774-007-0776-6. doi:10.1007/s00774-007-0776-6. [DOI] [PubMed] [Google Scholar]
- [28].Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. doi:10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carpenter TO, Keller M, Schwartz D, Mitnick M, Smith C, Ellison A, et al. 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets--a clinical research center study. J. Clin. Endocrinol. Metab. 1996;81:2381–2388. doi: 10.1210/jcem.81.6.8964881. [DOI] [PubMed] [Google Scholar]
- [30].Kremke B, Bergwitz C, Ahrens W, Schütt S, Schumacher M, Wagner V, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp. Clin. Endocrinol. Diabetes. 2009;117:49–56. doi: 10.1055/s-2008-1076716. doi:10.1055/s-2008-1076716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr. Nephrol. 1994;8:250–251. doi: 10.1007/BF00865491. [DOI] [PubMed] [Google Scholar]
- [32].Brodehl J, Krause A, Hoyer PF. Assessment of maximal tubular phosphate reabsorption: comparison of direct measurement with the nomogram of Bijvoet. Pediatr. Nephrol. 1988;2:183–189. doi: 10.1007/BF00862587. [DOI] [PubMed] [Google Scholar]
- [33].Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2007;35:D5–12. doi: 10.1093/nar/gkl1031. doi:10.1093/nar/gkl1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. doi:10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deutsch M, Long M. Intron-exon structures of eukaryotic model organisms. Nucleic Acids Res. 1999;27:3219–3228. doi: 10.1093/nar/27.15.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J. Biol. Chem. 2001;276:9206–9213. doi: 10.1074/jbc.M008745200. doi:10.1074/jbc.M008745200. [DOI] [PubMed] [Google Scholar]
- [37].Biber J, Gisler SM, Hernando N, Murer H. Protein/protein interactions (PDZ) in proximal tubules. J. Membr. Biol. 2005;203:111–118. doi: 10.1007/s00232-005-0738-7. doi:10.1007/s00232-005-0738-7. [DOI] [PubMed] [Google Scholar]
- [38].Gisler SM, Kittanakom S, Fuster D, Wong V, Bertic M, Radanovic T, et al. Monitoring protein-protein interactions between the mammalian integral membrane transporters and PDZ-interacting partners using a modified split-ubiquitin membrane yeast two-hybrid system. Mol. Cell Proteomics. 2008;7:1362–1377. doi: 10.1074/mcp.M800079-MCP200. doi:10.1074/mcp.M800079-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, et al. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int. 2003;64:1733–1745. doi: 10.1046/j.1523-1755.2003.00266.x. doi:10.1046/j.1523-1755.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- [40].Giral H, Lanzano L, Caldas Y, Blaine J, Verlander JW, Lei T, et al. Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J. Biol. Chem. 2011;286:15032–15042. doi: 10.1074/jbc.M110.199752. doi:10.1074/jbc.M110.199752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blaine J, Weinman EJ, Cunningham R. The regulation of renal phosphate transport. Adv Chronic Kidney Dis. 2011;18:77–84. doi: 10.1053/j.ackd.2011.01.005. doi:10.1053/j.ackd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [42].Whyte MP, Schranck FW, Armamento-Villareal R. X-linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J. Clin. Endocrinol. Metab. 1996;81:4075–4080. doi: 10.1210/jcem.81.11.8923863. [DOI] [PubMed] [Google Scholar]
- [43].Kruse K, Kracht U, Göpfert G. Renal threshold phosphate concentration (TmPO4/GFR) Arch. Dis. Child. 1982;57:217–223. doi: 10.1136/adc.57.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mitchell DM, Jüppner H. Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr Opin Endocrinol Diabetes Obes. 2010;17:25–30. doi: 10.1097/MED.0b013e328334f041. doi:10.1097/MED.0b013e328334f041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.