Abstract
Alterations in cerebrovascular function are evident acutely in moderate to severe traumatic brain injury (TBI), although less is known about their chronic effects. Adolescent and adult patients with moderate to severe TBI have been reported to demonstrate diffuse activation throughout the brain during functional magnetic resonance imaging (fMRI). Because fMRI is a measure related to blood flow, it is possible that any deficits in blood flow may alter activation. An arterial spin labeling (ASL) perfusion sequence was performed on seven adolescents with chronic moderate to severe TBI and seven typically developing (TD) adolescents during the same session in which they had performed a social cognition task during fMRI. In the TD group, prefrontal CBF was positively related to prefrontal activation and negatively related to non-prefrontal, posterior, brain activation. This relationship was not seen in the TBI group, who demonstrated a greater relationship between prefrontal CBF and non-prefrontal activation than the TD group. An analysis of CBF data independent of fMRI showed reduced CBF in the right non-prefrontal region (p<.055) in the TBI group. To understand any role reduced CBF may play in diffuse, extra-activation, we then related the right non-prefrontal CBF to activation. CBF in the right non-prefrontal region in the TD group was positively associated with prefrontal activation, suggesting an interactive role of non-prefrontal and prefrontal blood flow throughout the right hemisphere in healthy brains. However, the TBI group demonstrated a positive association with activation constrained to the right non-prefrontal region. These data suggest a relationship between impaired non-prefrontal CBF and the presence of non-prefrontal extra-activation, where the region with more limited blood flow is associated with activation limited to that region. In a secondary analysis, pathology associated with hyperintensities on T2-weighted FLAIR imaging over the whole brain was related to whole brain activation, revealing a negative relationship between lesion volume and frontal activation, and a positive relationship between lesion volume and posterior activation. These preliminary data, albeit collected with small sample sizes, suggest that reduced non-prefrontal CBF, and possibly pathological tissue associated with T2-hyperintensities, may provide contributions to the diffuse, primarily posterior extra-activation observed in adolescents following moderate to severe TBI.
Keywords: traumatic brain injury, cerebral blood flow, fMRI, social cognition, adolescents
1. INTRODUCTION
In addition to gray and white matter damage, acute moderate to severe traumatic brain injury (TBI) impairs cerebrovascular function (Maxwell et al., 1988; Rodriguez-Baeza et al., 2003). In children, increase in cerebral blood flow (CBF), i.e., hyperemia, has long been thought to occur acutely (Bruce et al., 1981), although more recently fewer cases with hyperemia and more cases with hypoperfusion have been observed when using comparison groups similar in age and gender (Adelson et al., 1997; Vavilala et al., 2004; Zwienenberg & Muizelaar, 1999). Other cerebrovascular changes that have been found acutely after moderate to severe pediatric TBI include impaired cerebral autoregulation, i.e., impaired ability to maintain constant CBF despite alteration in blood pressure, and hypotension, which can affect outcome (Chaiwat et al., 2009 ; Vavilala et al., 2004; Vavilala et al., 2006). However, little is known about how alterations in CBF after TBI may present chronically or whether they have implications for other brain functions during development (Giza et al., 2007; Udomphorn et al., 2008). In a study of 8 to 12-year old children three years following severe TBI, Chiu Wong et al. (2006) reported hypoperfusion in the cerebellum and, contrary to expectation, hyperperfusion in several cortical and subcortical areas, including the frontal lobes, suggested to be potentially the result of increased recruitment of uninjured regions, disrupted inhibitory mechanisms, or developmental delay.
In blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI), an increase in local CBF, blood volume, and oxygen extraction, i.e., hemodynamic response (HDR), is measured to infer neural activity during the performance of a task (Ogawa et al., 1992). This neurovascular coupling may be altered after TBI as a result of increased astrocytic growth, i.e., gliosis (D’Esposito et al., 2003), as astrocytes mediate neuronal firing and blood flow (D’Esposito et al., 2003; Schummers et al., 2008). Adolescents and adults with moderate to severe TBI often exhibit increased HDR in more regions of the brain compared to control subjects during fMRI (Christodoulou et al., 2001; Newsome et al., 2010; Scheibel et al., 2007; Turner & Levine, 2008). Because HDR during the performance of a task is associated with local CBF, it is possible that alterations in fMRI may be related to different patterns of CBF in TBI and control groups. However, results in adult TBI patients are unclear, and to the authors’ knowledge, there is no information on the relation of CBF to activation in the developing brain. Although reduced CBF has been reported in three adults with severe TBI (Hillary & Biswal, 2007), Palmer et al. (2010) reported intact HDR and extra-activation in primary visual cortex in adult patients with severe TBI despite the presence of impaired white matter integrity. Thus, CBF at rest in adolescents with chronic moderate to severe TBI may provide useful information regarding the nature of the increased activation often observed during cognitive and other fMRI tasks.
Many forms of cognition are altered after moderate to severe TBI in children, adolescents, and adults, including those required for social interaction. Following TBI, disrupted cognitive processes can include executive function, memory, and attention, which have been linked to injury severity (Anderson et al., 2005; Anderson, et al. 1998; Yeates et al., 2005). Each of these processes contribute to social cognition (Beer & Ochsner, 2006), the encoding and decoding of information about the self and others, with Muscara et al. (2008) and Yeates et al. (2004) specifically linking executive function deficits to impaired social problem-solving in children with chronic TBI. Impaired social information processing or social problem-solving skills are common sequelae (Levin & Eisenberg, 1991; Turkstra et al., 1996; Turkstra et al., 2001) and have been linked to reduced social competence, including heightened aggression, and diminished abilities to generate alternate solutions to problems in social situations and to incorporate age-appropriate resolutions (Ganesalingam et al., 2007; Janusz et al., 2002; Walz, et al. 2009; Warschausky et al. 1997). Patients with TBI can have diminished self awareness (Henry et al., 2006) and experience difficulty understanding the intentions and taking the perspectives of others (Dennis & Barnes, 1990; Milders et al., 2008; Muller et al., 2010; Yeates et al., 2007). Effects of TBI on social cognition are long-lasting; Janusz et al. (2002) reported diminished social reasoning an average of four years after TBI in children. Bibby & McDonald (2005) reported impairments in inferring mental the mental states of others in adult severe TBI patients an average of seven years after injury.
In an fMRI study that manipulated self-awareness and perspective-taking to investigate the ability to evaluate characteristics of one’s self from another person’s perspective, adolescents with moderate to severe TBI, who had lower scores in measures of subtle cognitive-communication (Functional Assessment of Verbal Reasoning and Executive Strategies (FAVRES); MacDonald & Johnson, 2005) and social dilemma-solving (Virtual Reality Social Problem-Solving Task (VR-SPS; Hanten et al., 2011) relative to typically developing (TD) control adolescents, had greater activation in posterior brain regions (posterior cingulate, lingual gyrus, parahippocampal gyrus, cuneus, and thalamus), primarily within the left hemisphere, than the TD adolescents (Newsome et al., 2010). In healthy adults the task has produced activation in medial prefrontal cortex (MPFC) (D’Argembeau et al., 2007), while bilateral and right-sided posterior brain regions such as the posterior cingulate and precuneus are also implicated in thinking about others and perspective taking in healthy children and adults (D’Argembeau et al., 2007; Pfeifer et al. 2007). Thus, the extra-activation occurred in posterior brain regions known to be involved in social cognition and in other regions of the brain not previously reported to be involved in social cognition.
To investigate the potential causes of this increased brain activation during a social cognition task, we measured CBF at rest during the same scanner session and related it to the task activation. In this preliminary study, CBF was measured in right and left prefrontal and remaining (non-prefrontal) regions of the brain to investigate 1) whether adolescents had CBF decrements several years after a moderate to severe TBI, and 2) whether CBF at rest related to activation during the fMRI task. Because of the high proportion of children and adults with frontal lesions after moderate to severe TBI (Graham et al. 2002; Levin et al., 1997), we studied the relation of prefrontal CBF to brain activation. As a secondary goal, to investigate extra-activation in relation to brain lesions, the volume of lesions containing gliosis with or without associated pathology was regressed onto brain activation.
2. METHODS
2.1 Participants
Resting CBF data were available from seven TBI (4 females; mean age = 16.3 years, SD = 2.5; range 12.8 – 19.1) and seven TD adolescents (4 females; mean age = 16.7 years, SD = 2.0; range 13.9 – 19.3) from the Newsome et al. (2010) study (Table 1). The TBI subjects had sustained moderate to severe TBI as defined by a post-resuscitation score of 3–12 on the Glasgow Coma Scale (GCS) (Teasdale & Jennett, 1974), or a higher score associated with brain pathology on computed tomography. Additionally, all TBI subjects evidenced focal pathology in the frontal lobes on imaging, with temporal pathology in five of the seven subjects as detailed in Table 1. The TBI group was 2.4 years post-injury and did not differ from the TD subjects in age, gender, ethnicity, or mother’s education (all p’s > .20). All subjects were right-handed (Oldfield, 1971). The TBI group scored lower than the TD group on a measure of subtle cognitive communication, the Functional Assessment of Verbal Reasoning and Executive Strategies (FAVRES, MacDonald & Johnson, 2005), Z=2.19, p<.03), and on a measure of social problem-solving (the Virtual Reality Problem-Solving Task (VR-SPS, Hanten et al., 2011), Z=2.27, p<.03). Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) verbal knowledge (i.e., vocabulary subtest) was also lower in the TBI, group, Z= −2.36, p < .02, but not visuospatial knowledge (i.e., block design subtest), p > .28.
Table 1.
Demographic and injury characteristics of patients.
| Age at Test (years) | Age at Injury (years) | Post-injury Interval (years) | Mother’s Education (years) | Gender | Mechanism of Injury | GCS score | Focal Pathology Sites^ | |
|---|---|---|---|---|---|---|---|---|
| TBI1 | 18.33 | 16.01 | 2.32 | 14 | F | Fall | 15 (+complications) | R OrbG, B IFG, R MFG, R MedFG, B SFG, R Temp Pole |
| TBI2 | 16.03 | 13.07 | 2.96 | 12 | M | Fall | 3 | L GyrRect, B OrbG, B IFG, L MFG, B Temp Pole, L Thal |
| TBI3 | 12.83 | 9.36 | 3.47 | 6 | F | Fall | 9 | B OrbG, R IFG, R MFG, L Cblm |
| TBI4 | 18.66 | 16.67 | 1.99 | 16 | M | MVC | 10 | R MFG, L MedFG, B SFG, B MTG, B Temp Pole, L Operculum |
| TBI5* | 19.13 | 17.03 | 2.10 | 12 | F | MVC | 7 | L OrbG, B ITG |
| TBI6 | 14.42 | 12.37 | 2.06 | 9 | M | MVC | 8 | L MFG, L SFG |
| TBI7 | 14.62 | 12.71 | 1.92 | 16 | F | Fall | 7 | B OrbG, L MFG, B SFG, L STG, R MTG, R ITG, R Temp Pole, B Ant Temp Pole, Midline Ant CC |
GCS=Glasgow Coma Scale, F=female, M=Male, Ant=Anterior, B=Bilateral, L=Left, R=Right
Cblm=Cerebellum, GyrRect=Gyrus Rectus, IFG=Inferior Frontal Gyrus, ITG=Inferior Temporal Gyrus, MedFG=Medial Frontal Gyrus, CC=Corpus Callosum, MFG=Middle Frontal Gyrus, OccLobe=Occipital Lobe, OrbG=Orbital Gyrus, SFG=Superior Frontal Gyrus, Temp Pole= Temporal Pole, Thal=Thalamus
MVA = Motor Vehicle Collision
Lesions were identified as those involving blood (hematoma or hemorrhage), contusion or hemosiderin deposit, gliosis, shear injury, and encephalomalacia. Other abnormalities such as atrophy, siderosis, herniation and edema were also coded.
Results based on partial MRI report.
2.2 fMRI Task
The fMRI paradigm is described in Newsome et al., (2010) and was a 2×2 (Perspective x Target) design that manipulated whether subjects took their own perspective or that of a well-known other (self vs. other) to evaluate a personality trait in either themselves or the other person (self vs. other) (D’Argembeau et al., 2007). Four conditions (self-self, self-other, other-self, and other-other) were presented. Prior to scan, subjects were asked to provide the name of a person whom they knew well and who knew them well, and that person’s name was incorporated into the stimuli. Across the top of a computer screen, the participant viewed one of four incomplete statements from one of the conditions, respectively: “You think you are…”, “You think (Veronica) is…”, “(Veronica) thinks you are…”, and “(Veronica) thinks she is…” After 3 s, adjectives, e.g., “happy”, “interesting”, “lazy” were presented below each statement for 4.5 s, during which time the participant pressed a right response button if the adjective described the target some or all of the time, or a left response button if the adjective did not describe the target or did so to a limited degree. Each block presented 5 trials in 22.5 s, and was followed by an interblock interval in which a fixation cross was presented for 9 to 15 s. After all 4 block conditions were presented, they were repeated during each run for a total of 8 blocks, or 40 trials, per run. The duration of each run was 5 minutes and 3 seconds. There were 4 runs with 1 minute of rest between runs. Order of conditions was counterbalanced between runs and between subjects. Forty adjectives were selected from age of acquisition and familiarity normative data (Wilson, 1988) to be on a third to fourth grade reading level and to be high in familiarity, with a mean familiarity rating of 552 (normative data mean = 488, range 100–700). E-Prime (www.pstnet.com/eprime) was used to present stimuli and collect responses. Participants were familiarized with the adjectives and task requirements immediately prior to the scan.
2.3 Image Data Acquisition
Imaging data were acquired with 3.0 T Philips Achieva scanners with multi-channel SENSE headcoils using the same parameters and software releases at Texas Children’s Hospital in Houston, Texas and the Advanced Imaging Center at University of Texas, Southwestern, in Dallas, Texas. Similar ranges of values for Weisskoff stability measurements (Weisskoff, 1996) (minimum 1/SNR index, peak-to-peak and RMS stability) taken on the day of scan indicated stability of both scanners over time. Additionally, we examined whether there appeared to be any systematic site differences in either the TBI or TD groups, and none were detected.
Arterial spin tag labeling (ASL) with the Transfer Insensitive Labeling Technique (TILT; Golay et al,. 1999) was used with a single-shot echoplanar imaging (EPI) sequence to acquire 13 axial slices starting at the most inferior boundary of the thalamus and continuing superiorly (thickness = 5.0 mm thick, 1.0 mm gap; FOV = 220 mm × 220 mm; acquisition matrix = 64 × 64; reconstruction matrix = 256 × 256; TR = 3000 ms; TE = 8.49 ms; flip angle 90 degrees; SENSE factor 2.0; TD (label delay time) = 1500 ms; label thickness = 120 mm; label offset = −117 mm; 50 pairs of control and label images; 5.25 minutes duration). The TILT sequence is a pulsed ASL technique and minimizes artifacts from venous inflow and magnetization transfer (Golay et al., 1999).
Blood oxygen level dependent (BOLD) T2* weighted single-shot gradient-echo echoplanar images (EPI) were acquired in 32 axial slices (thickness = 3.75 mm, 1.0 mm gap; FOV = 240 mm × 240 mm; matrix = 64 × 64; TR = 1700 ms; TE = 30 ms; flip angle 73 degrees; SENSE factor 2.0), which were followed by high- resolution T1-weighted 3D-Turbo Field Echo (TFE) anatomical images (132 axial slices of 1.0 mm thickness (no gap); FOV = 240 mm × 240 mm; matrix = 256 × 256; TR = 9.9 ms; TE = 4.6 ms; 8.0 degree flip angle, and SENSE factor 1.2).
Additional sequences were acquired to review pathology (T2-weighted gradient echo imaging, T2- weighted FLAIR, and T2-weighted GRASE), with the T2-weighted FLAIR primarily used for detection of gliosis, which was defined as a persistent hyperintensity on T2 FLAIR in the absence of blooming, i.e., evidence of hemorrhage, on T2-weighted gradient echo imaging. Lesion volume and nature of the pathology on imaging was determined by a boardcertified neuroradiologist (JVH).
2.4 ASL Image Processing and Analysis
ASL images were processed with in-house scripts written in Interactive Data Language (IDL, ITT Visual Information Solutions, Boulder, CO). The raw images were realigned using Statistical Parameter Mapping (SPM, University College London, UK). The control and labeled images were averaged across the 50 time points, respectively, to improve the signal-to-noise ratio. Image subtraction was performed for the mean control and labeled images. The quantification of CBF value (in milliliters of blood per 100 grams tissue per minute) from the ASL difference signal was based on a kinetic model (Buxton, et al. 1998; Donahue, et al. 2006):
| (1) |
Where
and .
M0 is the equilibrium magnetization of the tissue and was measured from the control signal in thalamus after accounting for saturation recovery during the delay time. The other parameter values in the model were based on imaging parameters and literature reports at the field of 3T: TI <τd ; λ = 0.9(ml blood)/(g tissue) (Donahue et al., 2006); τa = 0.45 s (Hendrikse et al., 2003) TI = 1.5 s; T1a = 1.627 s (Lu et al., 2004); T1 = 1.122s (Lu et al., 2005). A perfusion value represents mean CBF of each individual region (left and right prefrontal, left and right non-prefrontal).
Boundaries between the prefrontal and non-prefrontal regions were drawn on each slice for each subject. On each slice, four regions were drawn: in the axial plane, the posterior boundary of the right and left prefrontal region was placed just anterior to the genu of the corpus callosum; parenchyma posterior to that boundary was designated as non-prefrontal. The prefrontal and non-prefrontal regions were also divided into right and left sides, using the interhemispheric fissure as the boundary. Regions included both gray and white matter. Consistency in the placement of the boundary between pre-frontal and non-frontal boundaries from slice to slice was verified through comparison with other slices when the genu of the corpus callosum was not visible. Thirteen axial slices starting at the most inferior boundary of the thalamus and continuing superiorly were used. As image realignment tends to result in partial slice coverage in the top and bottom slices, those slices were omitted from analyses.
2.5 fMRI Image Processing and Analysis
Preprocessing of the fMRI data was performed in Statistical Parametric Mapping software (Friston et al., 1995; SPM2, Wellcome Department of Cognitive Neurology, London, UK) with the MarsBaR toolbox (Brett et al., 2002) implemented in Matlab (Mathworks Inc. Sherborn MA, USA). Spikes greater than 2.5 standard deviations above the mean voxel intensity across time series were replaced with the median voxel intensity for that time series using Analysis of Functional Neuroimages software (AFNI) (Cox, 1996). Thereafter all processing were completed in SPM2. After slice-timing correction, the fMRI time series was realigned and corrected for head motion and susceptibility-by-movement interactions. Series with head motion component on any axis greater than 2.0 mm translational or 2.0 degrees rotational were eliminated from analysis. The high-resolution anatomical scan was co-registered to the fMRI images and was transformed to the stereotactic coordinates of the Montreal Neurologic Institute using the SPM2 Normalise procedure. The same transformation was applied to the functional images, which were then resliced to 2 mm isotropic voxels, and spatially smoothed with a 6 mm isotropic full width at half maximum Gaussian filter. SPM8 random effects regression analyses were used to relate resting CBF values to activation during the task, and to compare the strength of the relation of CBF to activation in the two groups. In all analyses, perfusion values were regressed onto activation produced during the condition when participants were asked to indicate whether or not the person they knew well would think they had particular traits. The total volume of T2 hyperintensities that were coded by the neuroradiologist as containing gliosis for each subject was entered in the regression of lesion volume with activation. All reported clusters were statistically significant (p<0.05) at the Family-Wise-Error (FWE) corrected cluster level of inference, corrected for multiple comparisons over the whole brain volume. To facilitate the comparison of the anatomical regions associated with activation in the original paper to those in the present investigation, methods from the original paper were adopted; significant coordinates were converted to the coordinates of the Talairach Atlas (Talairach & Tournoux, 1988) using the mni2tal script (Brett, 1999), and the Talairach Daemon (Lancaster et al., 2000) and the Talairach Atlas were then used to determine the anatomical locations and approximate Brodmann’s Areas (BA) of the Talairach coordinates. Betas of the regressions with group are reported at 90%, rather than 95%, confidence rather intervals due to the features of MarsBar.
3. RESULTS
3.1 ASL Results
Please see Tables 2a and 2b. To investigate potential differences in CBF asymmetry between the right and the left hemispheres within the groups, we calculated a difference score (right hemisphere minus left hemisphere) for both prefrontal and non-prefrontal regions and used the Signed Rank Test to examine laterality within the groups. Differences in perfusion measures (milliliters of blood per 100 grams tissue per minute) of right versus left prefrontal regions, and in right versus left non-prefrontal regions, did not differ within groups and had small effect sizes (all p’s > 0.30; all Cohen’s d’s ≤ 0.18).
Table 2a.
Laterality of Perfusion. Mean, median, and standard deviation of difference scores (right vs. left) for frontal and nonfrontal regions in milliliters of blood per 100 grams tissue per minute, p (Signed Rank Test for within group analyses; Wilcoxon rank sum two sample tests for between group analyses), and Cohen’s d values in within and between TBI and TD groups.
| Frontal | Frontal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TBI Right vs Left |
TD Right vs Left |
TBI vs TD | ||||||||
| Perfusion | Difference | p | Cohen’s d | Perfusion | Difference | p | Cohen’s d | p | Cohen’s d | |
| Right | 27.41 (3.27) | −0.67 −1.46 (3.67) |
0.47 | 0.15 | 30.67 (3.72) | 1.00 1.17 (2.41) |
0.30 | 0.15 | 0.25 | 0.54 |
| Left | 28.08 (5.61) | 29.64 (9.13) | ||||||||
| Nonfrontal | Nonfrontal | |||||||||
| Right | 24.94 (2.78) | −0.75 −0.66 (2.84) |
0.58 | 0.18 | 28.62 (6.36) | 0.98 1.40 (2.75) |
0.58 | 0.15 | 0.37 | 0.62 |
| Left | 25.69 (5.36) | 27.64 (6.76) | ||||||||
Wilcoxon rank sum two sample tests were then performed to investigate any group difference in asymmetry. The results indicated that the groups did not significantly differ in the degree or direction of asymmetry (Prefrontal: z=1.15, p=0.25; Non-prefrontal: z= 0.89, p=0.37, although effect sizes were moderate (Prefrontal: Cohen’s d = 0.54; Non-prefrontal: Cohen’s d=0.62), with the TBI group tending to have higher CBF in the left hemisphere in both regions and the TD group tending to have a higher CBF on the right side.
When investigating group differences by region, a Wilcoxon rank sum two sample test revealed no group differences in the right prefrontal, left prefrontal, and left non-prefrontal regions (all p’s > 0.20) with small to moderate effect sizes, while group differences in the right non-prefrontal region approached significance with a large effect size, (TBI M = 24.94 ml/100g/min, SD = 2.78 vs. TD M = 28.62 ml/100g/min, SD = 6.39), z=−1.92, p =0.055, Cohen’s d = 0.75.
3.2 Relating Cerebral Blood Flow to fMRI Activation
3.2.1 Prefrontal regions
Please see Figure 1 and Table 3. Because there were no significant differences and small effect sizes in the right versus left prefrontal regions in each group, perfusion values for both prefrontal regions were combined to examine their relation with activation.
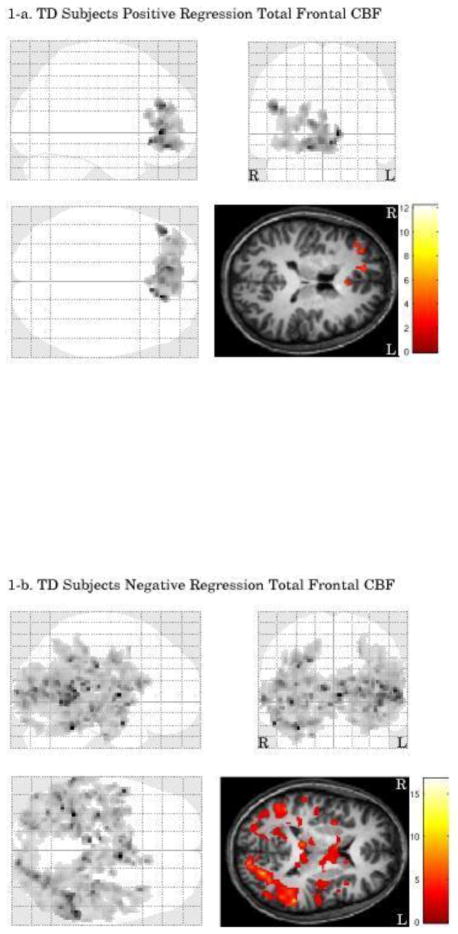
Figure 1.
Table 2b.
Group Comparisons of ROI Perfusion. Mean, median, and standard deviation of perfusion in milliliters of blood per 100 grams tissue per minute, p (Wilcoxon rank sum two sample tests) and Cohen’s d values for between group comparisons of left frontal, right frontal, left nonfrontal, and right nonfrontal regions.
| Frontal | |||||||
|---|---|---|---|---|---|---|---|
| Left | Right | ||||||
| Perfusion | p< | Cohen’s d | Perfusion | p< | Cohen’s d | ||
| TBI | Mean | 28.08 | 0.70 | 0.23 | 27.41 | 0.20 | 0.58 |
| Median | 26.27 | 26.53 | |||||
| St dev | (5.61) | (3.27) | |||||
| TD | Mean | 29.85 | 30.85 | ||||
| Median | 31.60 | 32.65 | |||||
| St dev | (9.09) | (7.67) | |||||
| Nonfrontal | |||||||
| TBI | Mean | 25.69 | 0.52 | 0.32 | 24.94 | 0.055 | 0.75 |
| Median | 26.97 | 25.66 | |||||
| St dev | (5.36) | (2.78) | |||||
| TD | Mean | 27.64 | 28.62 | ||||
| Median | 27.58 | 30.10 | |||||
| St dev | (6.76) | (6.39) | |||||
3.2.1.1 TD Group
TD adolescents had a significant positive correlation between prefrontal CBF and activation in one cluster within the prefrontal cortex. This cluster included areas of prefrontal cortex previously reported to be involved in social cognition e.g., (D’Argembeau et al., 2007), including bilateral anterior cingulate (L BA 32, R BA 24), left medial frontal gyrus (BA10, 11), right middle frontal gyrus (BA 10), and superior frontal gyrus (BA 10). A significant negative correlation was found within posterior areas that included bilateral thalamus, middle temporal gyrus (BA 22), left insula (BA 13), and superior temporal gyrus (BA 22).
3.2.1.2 TBI Group
Positive and negative regressions were nonsignificant in the TBI group.
3.2.1.3 TBI vs. TD
Relative to the TD group, the TBI patients demonstrated a stronger regression between prefrontal CBF and activation in two posterior clusters. The first cluster included left posterior cingulate gyrus (BA 23, 30), middle temporal gyrus (BA 39), superior temporal gyrus (BA 39) hippocampus, precuneus, (BA 18), and cuneus (BA 7, 18) (group difference in mean beta = 0.24, 90% CI = 0.03 – 0.44). The second cluster included right superior parietal lobule (BA7), precuneus (BA39), middle temporal gyrus (BA 39), hippocampus, fusiform gyrus (BA 37) (group difference in mean beta = 0.29, 90% CI = 0.12 – 0.47). There were no brain areas where the TD group exhibited a stronger regression between prefrontal CBF and fMRI activation than the TBI group.
The group difference in regression may indicate a greater positive relationship between prefrontal CBF and activation in the TBI group than in the TD group, or simply reflect the greater negative relation found in the TD group. In order to investigate the voxels unique to the TBI > TD regression analysis, the clusters from both analyses (TD negative regression and TBI > TD regression strength comparison) were examined to determine their overlap (i.e., the voxels in common to both analyses). However, the significant cluster from the TD regression was very large (number of voxels = 17,929) and may exaggerate any overlap between the two analyses. As a result, we used a more conservative cluster-defining (height) threshold for the TD negative regression (p<.01, number of voxels in cluster = 2148) to examine more carefully any overlap with each of the two clusters that were significant in the TBI>TD regression analysis. After eliminating overlapping voxels, areas unique to the TBI>TD regression comparison within the first cluster included right middle temporal gyrus (BA 39), posterior cingulate gyrus (BA 23, 30), parahippocampal gyrus, hippocampus, cuneus (BA 18), and lingual gyrus, with the voxel closest to the center of mass (Talairach coordinates: [29, 59, 17] mm) in sub-gyral temporal white matter. Within the second cluster, areas unique to the TBI>TD regression comparison included left superior parietal lobule (BA 7), precuneus (BA 19), posterior cingulate gyrus (BA 30), middle temporal gyrus white matter, parahippocampal gyrus (BA 28, 36), hippocampus, amygdala, and cerebellum, with the voxel closest to the center of mass (−32, 48, −8) in sub-gyral occipital white matter.
3.2.2 Right non-prefrontal region
Please see Table 4. Right non-prefrontal perfusion was related to activation to investigate the implications of the significant group difference for right non-prefrontal perfusion values.
Table 3.
Statistical parametric mapping summary tables for regressions of activation on total frontal cerebral blood flow (CBF)a
|
TD Group Positive Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.018 | 2,252 | 44, 42, 20 | Right middle frontal gyrus (BA 10) |
| −12, 33, −8 | Left medial frontal gyrus (BA 10) | ||
| 6, 35, 4 | Right anterior cingulate (BA 24) | ||
| −4, 50, −11 | Left medial frontal gyrus (BA 11) | ||
| −2, 43, 3 | Left anterior cingulate (BA 32) | ||
| 34, 50, −11 | Right middle frontal gyrus (white matter) | ||
| 18, 49, 18 | Right superior frontal gyrus (white matter) | ||
| 10, 54, −11 | Right medial frontal gyrus (white matter) | ||
| 34, 56, −1 | Right superior frontal gyrus (BA 10) | ||
| −10, 40, −12 | Left medial frontal gyrus (white matter) | ||
|
TD Group Negative Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.0001 | 17,929 | −6, −2, 6 | Left thalamus (anterior nucleus) |
| −55, −40, 11 | Left superior temporal gyrus (white matter) | ||
| 10, −19, 14 | Right thalamus (lateral dorsal nucleus) | ||
| −12, −91, 12 | Left cuneus (white matter) | ||
| −55, −44, 4 | Left middle temporal gyrus (BA 22) | ||
| 63, −43, 4 | Right middle temporal gyrus (BA 22) | ||
| −51, −54, 10 | Left superior temporal gyrus (BA 39) | ||
| 4, 6, −2 | Right caudate head | ||
| 34, −65, 18 | Right middle temporal gyrus (white matter) | ||
| TBI group > TD group | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.001 | 3,931 | −18, −56, 6 | Left posterior cingulate (BA 30) |
| −2, −88, 19 | Left cuneus (BA 18) | ||
| −57, −55, 25 | Left superior temporal gyrus (BA 39) | ||
| 2, −56, 16 | Right posterior cingulate (BA 23) | ||
| −34, −37, −12 | Left fusiform gyrus (BA 37) | ||
| −48, −75, 11 | Left middle temporal gyrus (BA 39) | ||
| −16, −78, 30 | Left cuneus (BA 7) | ||
| −30, −36, −15 | Left fusiform gyrus (BA 20) | ||
| −30, −28, −7 | Left hippocampus | ||
| −42, −53, −19 | Left cerebellum (culmen) | ||
| 0.001 | 3,802 | 63, −43, 4 | Right middle temporal gyrus (BA 22) |
| 48, −47, −14 | Right fusiform gyrus (BA 37) | ||
| 48, −43, −13 | Right fusiform gyrus (BA 37) | ||
| 38, −62, 34 | Right precuneus (BA 39) | ||
| 57, −56, 10 | Right middle temporal gyrus (BA 39) | ||
| 30, −37, −2 | Right hippocampus | ||
| 28, −84, 28 | Right cuneus (white matter) | ||
| 14, −65, 55 | Right superior parietal lobule (BA 7) | ||
| 28, −10, −1 | Right putamen | ||
| 26, −12, −6 | Right lateral globus pallidus | ||
Cluster-defining voxel height threshold: T = 2.19, degrees of freedom = 5.0
Smoothness: FWHM = 6.2 6.4 6.5 (voxels)
Search Volume = 189110 voxels = 678.8 resolution elements (resels)
Cluster-defining voxel height threshold: T = 1.93, degrees of freedom = 11.0
Smoothness: FWHM = 5.8 6.0 6.1 (voxels)
Search Volume = 173291 voxels = 763.4 resolution elements (resels)
BA = Brodmann area; TBI = traumatic brain injury; TD = typically developing
Relative maxima shown are greater than 4 mm apart.
Probability at the cluster level of significance after random field theory family-wise error correction over the whole brain search volume.
Number of voxels within a cluster.
Negative values along the x-axis are defined to be in the subject’s left hemisphere.
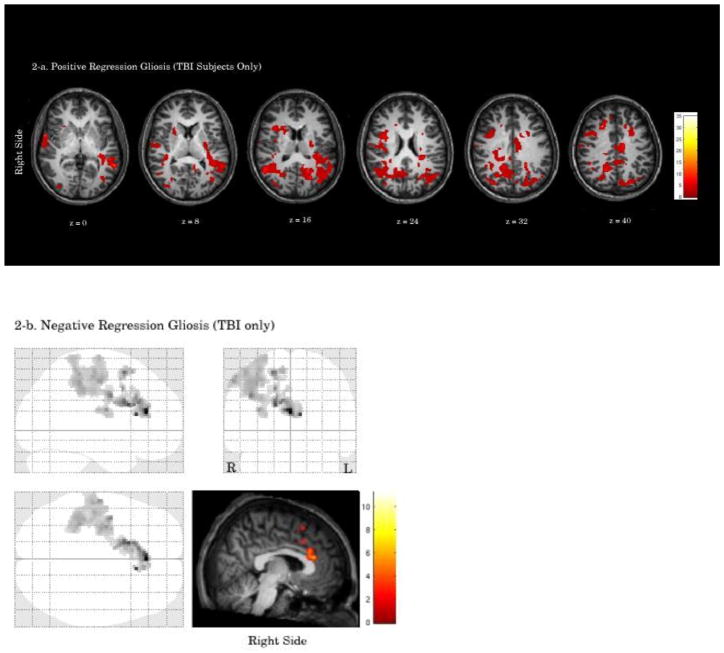
3.2.2.1 TD Group
TD adolescents had a significant positive correlation between right non-prefrontal CBF and activation in one cluster within the prefrontal cortex, similar to that found in the regression with prefrontal CBF in the TD group. The activation included bilateral anterior cingulate gyrus (L BA 32, R BA 24), left medial frontal gyrus (BA 10, 11), right middle frontal gyrus (BA 10), and right superior frontal gyrus (BA 10). Negative regression for the TD group was nonsignificant.
3.2.2.2 TBI Group
The TBI group had a significant positive correlation between right non-prefrontal CBF and activation in two right hemisphere non-prefrontal clusters which included right precuneus (BA 7, BA 19), inferior and superior parietal lobules (BA 40, BA 7, respectively) and subcortical, midbrain, and cerebellar structures. Negative regression for the TBI group was nonsignificant.
3.2.2.3 TBI vs. TD
Compared to the TD group, the TBI group demonstrated a stronger relation between right non-prefrontal CBF and activation in one cluster in the right non-prefrontal region, including superior parietal lobule (BA 7) and precuneus (BA 19) (group difference in mean beta = 0.28, 90% CI = −0.05 – 0.63). There were no brain areas where the TD group exhibited a stronger relation between right non-prefrontal CBF and fMRI activation than the TBI group.
3.3 Relating Gliotic Lesions to fMRI Activation
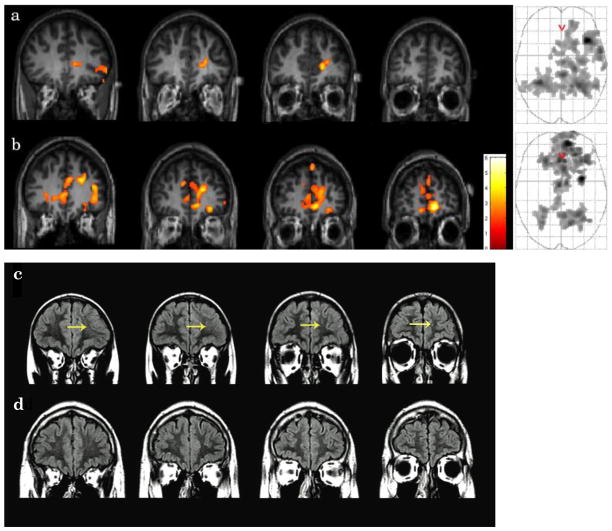
Please see Figure 2 and Table 5. Complete lesion information was available for six of the TBI patients; therefore, data from these patients were used in the following regression analyses. Partial data (T1-weighted imaging) was available for one patient; however, because gliosis is difficult to detect on T1-weighted imaging, this patient was not included in these analyses. A positive regression of activation on volume of gliotic lesions was significant in one cluster that included bilateral superior temporal gyrus (left BA 41), right superior frontal gyrus (BA 8), precentral gyrus, and left middle occipital gyrus. A negative regression of activation on volume of gliotic lesions was significant in one cluster that included bilateral anterior cingulate (BA 32), right medial frontal gyrus (BA 32), superior frontal gyrus (BA 6), precentral gyrus (BA 6), inferior parietal lobule (BA 40)1.
Figure 2.
Table 4.
Statistical parametric mapping summary tables for regressions of activation with right nonfrontal cerebral blood flow (CBF)a
|
TD Group Positive Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.007 | 2,864 | 6, 33, 6 | Right anterior cingulate (BA 24) |
| 6, 56, −11 | Right medial frontal gyrus (BA 11) | ||
| 4, 36, −9 | Right anterior cingulate (BA 32) | ||
| −2, 45, 3 | Left anterior cingulate (BA 32) | ||
| −8, 54, −14 | Left medial frontal gyrus (white matter) | ||
| 18, 38, 13 | Right anterior cingulate (BA 32) | ||
| 10, 42, −12 | Right medial frontal gyrus (white matter) | ||
| 46, 33, 33 | Right middle frontal gyrus (BA 9) | ||
| 24, 24, −16 | Right middle frontal gyrus (BA 11) | ||
|
TBI Group Positive Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.024 | 1,760 | 24, −3, −12 | Right amygdala |
| 26, −51, −16 | Right cerebellum (culmen) | ||
| 12, −33, 2 | Right parahippocampal gyrus | ||
| 30, −14, 1 | Right putamen | ||
| 12, −18, −16 | Right midbrain | ||
| 36, −38, −15 | Right fusiform gyrus (BA 20) | ||
| 16, −1, −17 | Right parahippocampal gyrus (BA 34) | ||
| 46, −37, −12 | Right fusiform gyrus (white matter) | ||
| 24, −14, −18 | Right parahippocampal gyrus (white matter) | ||
| 22, 3, −19 | Right uncus (BA 34) | ||
| 0.024 | 1,761 | 38, −48, 52 | Right inferior parietal lobule (BA 40) |
| 57, −61, 23 | Right superior temporal gyrus (BA 39) | ||
| 18, −68, 33 | Right precuneus (white matter) | ||
| 53, −65, 27 | Right middle temporal gyrus (BA 39) | ||
| 16, −73, 52 | Right precuneus | ||
| 16, −69, 57 | Right superior parietal lobule (BA 7) | ||
| 24, −80, 35 | Right precuneus | ||
| 26, −76, 39 | Right precuneus (BA 19) | ||
| 18, −78, 43 | Right precuneus (BA 7) | ||
| 36, −74, 39 | Right precuneus (BA 19) | ||
| TBI group > TD group | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.038 | 2,123 | 38, −62, 36 | Right precuneus (BA 39) |
| 34, −54, 51 | Right superior parietal lobule (BA 7) | ||
| 34, −58, 53 | Right superior parietal lobule (BA 7) | ||
| 42, −18, 21 | Right insula (white matter) | ||
| 34, −82, 26 | Right superior occipital gyrus (BA 19) | ||
| 50, −59, 25 | Right middle temporal gyrus (BA 39) | ||
| 26, −76, 39 | Right precuneus (BA 19) | ||
| 20, −56, 43 | Right precuneus (white matter) | ||
| 48, −54, 17 | Right superior temporal gyrus (white matter) | ||
| 50, −48, 13 | Right superior temporal gyrus (white matter) | ||
Cluster-defining voxel height threshold: T = 2.19, degrees of freedom = 5.0
Smoothness: FWHM = 6.4 6.6 6.8 (voxels)
Search Volume = 189110 voxels = 614.1 resolution elements (resels)
Cluster-defining voxel height threshold: T = 2.19, degrees of freedom = 5.0
Smoothness: FWHM = 5.8 5.9 6.1 (voxels)
Search Volume = 179553 voxels = 797.9 resolution elements (resels)
Cluster-defining voxel height threshold: T = 1.93, degrees of freedom = 11.0
Smoothness: FWHM = 5.8 6.1 6.2 (voxels)
Search Volume = 173291 voxels = 732.9 resolution elements (resels)
BA = Brodmann area; TBI = traumatic brain injury; TD = typically developing
Relative maxima shown are greater than 4 mm apart.
Probability at the cluster level of significance after random field theory family-wise error correction over the whole brain search volume.
Number of voxels within a cluster.
Negative values along the x-axis are defined to be in the subject’s left hemisphere.
4. DISCUSSION
To investigate factors contributing to alterations in activation following moderate to severe traumatic brain injury (TBI) in adolescents, we sought to relate cerebral blood flow (CBF) to brain activation during an fMRI task in which adolescents were asked to take the perspective of another person to think of themselves. Because MPFC has been implicated in this task (D’Argembeau et al., 2007) and because frontal lobes are the most affected after TBI (Graham et al., 2002; Levin et al., 1997), we focused on the relationship of frontal CBF to task activation. In the typically developing (TD) adolescents, CBF in the prefrontal regions was positively related to activation in prefrontal regions, including bilateral MPFC. In addition, CBF of the prefrontal regions was negatively related to activation in regions posterior to the prefrontal area. This pattern demonstrates that relating resting CBF to activation provides reasonable results and may be a reasonable method to further investigate effects of CBF on activation in TBI patients. The pattern was disrupted in adolescents who had sustained moderate to severe TBI, who demonstrated no significant relationships between prefrontal CBF and activation. TBI results in heterogeneous damage to the brain, and the lack of an effect may in part be due to increased variability in the regions recruited. In a group comparison, a positive relation of prefrontal CBF and whole brain activation was stronger in bilateral non-prefrontal regions in the TBI group, suggesting that prefrontal CBF may be associated with the re-routing of neural resources available to complete the task, including but not limited to recruitment of task-appropriate non-prefrontal region, and may contribute to extra-activation seen in posterior alternate regions.
Regions where the TBI group had a stronger relationship between prefrontal CBF and activation than the healthy adolescents included posterior cingulate and parahippocampal gyri, which have been found to be more activated during this social cognition task in the TBI group (Newsome et al., 2010). Potentially, prefrontal CBF may have played a role in the greater HDR originally observed in non-prefrontal regions. The relationship between task activation and prefrontal CBF in the TBI group was also stronger in precuneus, amygdala, which have been implicated in social cognition (Pfeifer et al., 2007; Schultz et al., 2003). Bilateral hippocampi were also activated, potentially suggesting a role between prefrontal CBF and the access of long-term representations of when traits had been evident to the person whose perspective was adopted, and have been linked to the amygdala, which has been implicated in processing social information in episodic autobiographical memory (Markowitsch & Staniloiu, 2011).
TBI appeared to disrupt the relation between prefrontal perfusion and neural activation since the relation of prefrontal CBF and activation was stronger in non-prefrontal regions in the TBI group compared with the TD group. When considered independently of the fMRI data, CBF in the TBI group tended to be lower in the right prefrontal region, although this trend was nonsignificant, suggesting that extra-activation in posterior brain regions may result from sources in addition to or other than altered CBF in the frontal lobes, e.g., lesions and diffuse axonal injury to frontal brain areas. Research consistent with this interpretation exists in adults with chronic moderate to severe TBI, where an association between diffuse injury and reduced CBF in posterior cingulate cortices was observed (Kim et al., 2010). Further, we conducted a secondary investigation to relate the volume of gliotic lesions in the TBI patients to activation with the hypothesis that lesions associated with gliosis would be associated with non-prefrontal activation. Widespread non-prefrontal activation, including temporal, occipital, and parietal regions, was positively related to gliotic lesion volume, while anterior cingulate and medial prefrontal cortex activation (regions associated with social cognition) were negatively related to gliotic lesion volume. Given the variability in age range, we also performed regressions with age to investigate the specificity of the pattern, and the results were nonsignificant with rather high p-levels (p=0.99). While the sample size is small, and other mediating factors are not ruled out, it may be that overall lesion volume associated with gliosis and possibly abnormalities in astrocytes may play a role in extra-activation. If confirmed in future studies, this finding may be consistent with the linkage between neuronal response, astrocytes, and blood flow that can be disturbed after TBI (D’Esposito et al., 2003; Schummers et al., 2008). Indeed, Golding (2002) had suggested that after TBI astrocytes may play a role in constricting artioles and thereby decreasing blood flow (Golding, 2002). Although the hyperintensities on the T2-FLAIR were coded by the neuroradiologist as being or containing gliosis, presence of T2 hyperintensities on MRI may indicate pathology other than astrocytosis, and the analysis with gliotic lesions should be interpreted with caution.
In a region where the TBI group did have significantly lower CBF relative to the TD group, the right non-prefrontal region, relation of CBF to activation was suggestive. In TD adolescents, CBF in the right non-prefrontal region was significantly related to activation in the right prefrontal region, suggesting an interactive role of non-prefrontal and prefrontal blood flow throughout the right hemisphere in activating MPFC to perform the task. In the group comparison, however, the TBI group demonstrated a stronger association between right non-prefrontal CBF and activation in the right non-prefrontal region, relative to the TD adolescents, suggesting a disruption to the continuity of right prefrontal and non-prefrontal CBF.
Do the non-prefrontal CBF findings have any implications for the greater left-sided activation seen during the social cognition task in the original study? It may simply be that the CBF in the left non-prefrontal region was sufficient to provide the resources needed for the extra-activation to occur. Under this interpretation, neurons in the underperfused right non-prefrontal area may not have fired efficiently because of decreased oxygen and glucose delivery caused by the diminished blood supply or needed as much blood flow, and the neurons in the left non-prefrontal region compensated by firing more.
The TD group did not demonstrate any relationships between CBF and activation that were stronger than in the TBI group, which may be due to subthreshold activation in the TBI group observed in the medial and right lateral prefrontal cortex, similar areas to those observed in the TD group.
Chiu Wong et al. (2006) reported mainly hyperperfusion in their chronic pediatric TBI sample. However, the children in their sample were much younger (approximate mean 10.3 years vs. 16.3 years) and had their injuries at younger ages (approximate mean 7 years vs 13.9 years). These two factors may contribute to the difference in our findings. Palmer et al., (2010) reported extra-activation and normal HDR in primary visual cortex in adults with moderate to severe TBI. The present study did not examine the HDR curve per se, only the relation of its amplitude to CBF in general regions. Since altered blood flow can affect HDR, larger follow-up studies fully examining HDR parameters in relation to multiple regions of interest may further elucidate the role of HDR in activation after TBI.
It is not clear why CBF was lower in the right hemisphere, particularly in posterior portions. Effects of CBF laterality have been found nine days after admission to ICU and suggested to be due to ipsilateral lesions (Vavilala et al., 2008). Many of the adolescents with TBI in the current study had bilateral lesions, and so focal lesion alone may not account for the laterality in CBF seen in the more chronic sample here. Two of the TBI adolescents had an intracranial pressure monitor inserted in the right frontal region at the time of treatment, potentially contributing to the trend in the lower value seen there, and possibly to the right non-prefrontal region, as well, because a connection between right prefrontal activation and right non-prefrontal CBF was observed in the TD adolescents. Barclay et al., (1985) found greater decreases in ipsilateral CBF in patients with focal injury, relative to patients with diffuse injury 13 months after injury, and global decreases in patients regardless of focal or diffuse injury. Differences in other regions may not have been detected in the current sample due to small sample size, and future studies with larger sample sizes and more specific brain regions would be helpful in identifying the degree of contribution of multiple bilateral focal lesions to CBF. In addition, resting CBF may have important implications for understanding alterations in resting state functional connectivity after TBI (Mayer et al., 2011).
There are several limitations of this study, including a small sample size with a range of severity (mild with complications to severe), and we were unable to control for injury severity. The reduction in CBF may result mainly from the more severely injured patients, and may not be universal to all moderate-to-severe TBI cases. However, a range in values permitted regression analyses that revealed associations with activation patterns, and in that sense a range of values may be considered informative in this preliminary investigation. Given the moderate effect size in the group comparison for the right prefrontal CBF, it is possible that with more subjects, diminished perfusion might be found in that prefrontal region. As is common in moderate-to-severe TBI samples, ours evidenced significant heterogeneity in the specific location, nature and degree of focal pathology, and this likely contributed to the pattern of findings in this study. However, the pattern of significant pathology in frontal and temporal areas characteristic of many TBI cohorts (Graham et al., 2002; Levin et al., 1997) was reflected in our sample, rendering it fairly representative despite the small sample size. While the TILT ASL sequence limited acquisition to 13 slices, which excluded signal in any non-prefrontal region inferior to the thalamus, its complete coverage of the frontal lobes and increased signal to noise ratio relative to other sequences measuring CBF (Golay et al., 1999) provided sensitivity in detecting differences between groups.
Individual-level analyses were completed with a general linear model, although some reports suggest that nonlinear, or linear in combination with nonlinear models may be more accurate (Magri et al. 2011; Sheth et al., 2004) and it is possible that the relationship between hemodynamic and neural responses becomes even more complex after TBI. Furthermore, problematic aspects of fMRI that have been suggested in uninjured populations, e.g., imprecise neural transmission (volume transmission) (Agnati et al., 2010; Logothetis, 2008), may have a greater influence after moderate to severe TBI. Impairments in cerebrovascular function following TBI (Golding, 2002; Maxwell et al., 1988, Rodriguez-Baeza et al., 2003) may alter fMRI signal. Rodriguez-Baeza et al., 2003 found alterations in microvessels including arterioles, a source of fMRI signal, that were related to changes in the endothelium, i.e., the lining of blood vessels, which has been implicated in affecting CBF associated with neural activity (Peppiatt et al., 2006). Oxygenation changes in the capillary bed may also alter the spatial specificity of the BOLD signal (Logothetis 2008 Supplementary Information), and damage to the capillary bed or other vasculature in a particular brain region may be associated with a reduced BOLD fMRI response there, even though the regional neuronal activity has not changed or possibly even increased. Thus, it is possible that the frontal pathology in these patients contributed to the reduced frontal activation. A large-scale study that finely details and relates coordinates of lesion location to the absence or presence of activation in regions near the lesion and in regions structurally and functionally connected would shed more light on this possibility. Finally, further investigation of the many vascular and supportive mechanisms, e.g., oxygen metabolism, blood oxygenation, endothelium morphology, cerebrovascular smooth muscle and glial functioning, and presence of drugs such as COX-2 inhibitors, will facilitate better understanding of the complex underpinnings of decreased blood flow in relation to neural activity (Golding, 2002; Lindauer et al., 2010)
Figure 3.
Table 5.
Statistical parametric mapping summary tables for regressions of activation with volume of lesions containing gliosisa
|
TBI Group Positive Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.0001 | 12,092 | −6, −14, 67 | Left medial frontal gyrus (white matter) |
| 65, −10, 26 | Right precentral gyrus | ||
| 59, 2, 2 | Right superior temporal gyrus | ||
| 22, 24, 47 | Right superior frontal gyrus (BA8) | ||
| −42, −72, 7 | Left middle occipital gyrus | ||
| −24, −69, 18 | Left precuneus (white matter) | ||
| −38, −30, 16 | Left superior temporal gyrus (BA41) | ||
| −51, −61, 31 | Left angular gyrus (white matter) | ||
| 18, −53, 38 | Right precuneus (white matter) | ||
| 55, −54, 17 | Right superior temporal gyrus (white matter) | ||
|
TBI Group Negative Regression | |||
|---|---|---|---|
| Cluster-Level P Value (corrected)b | Cluster Size (k)c | Coordinatesd (x, y, z mm) | Location |
| 0.001 | 2,831 | 18, 14, 53 | Right superior frontal gyrus (BA 6) |
| 10, 26, 24 | Right anterior cingulate (BA 32) | ||
| 57, −11, 47 | Right postcentral gyrus (BA 3) | ||
| −8, 32, 13 | Left anterior cingulate (white matter) | ||
| 30, −9, 47 | Right middle frontal gyrus (BA 6) | ||
| 55, −17, 51 | Right postcentral gyrus (BA 1) | ||
| 40, −29, 40 | Right inferior parietal lobule (BA 40) | ||
| 14, 10, 47 | Right medial frontal gyrus (BA 32) | ||
| 26, −10, 67 | Right precentral gyrus (BA 6) | ||
| −6, 37, 11 | Left anterior cingulate (BA 32) | ||
| 40, −18, 38 | Right precentral gyrus (BA 4) | ||
| 32, −13, 19 | Right insula (white matter) | ||
Cluster-defining voxel height threshold: T = 2.33, degrees of freedom = 4.0
Smoothness: FWHM = 6.0 6.2 6.2 (voxels)
Search Volume = 180947 voxels = 718.5 resolution elements (resels)
BA = Brodmann area; TBI = traumatic brain injury; TD = typically developing
Relative maxima shown are greater than 4 mm apart.
Probability at the cluster level of significance after random field theory family-wise error correction over the whole brain search volume.
Number of voxels within a cluster.
Negative values along the x-axis are defined to be in the subject’s left hemisphere.
Highlights.
Cerebral blood flow (CBF) may be altered in chronic traumatic brain injury (TBI) in adolescents.
Adolescents with chronic TBI often demonstrate extra-activation in fMRI tasks.
CBF may be implicated in the extra-activation.
Typically developing adolescents served as a comparison group.
Adolescents with TBI showed reduced CBF in right nonfrontal regions and altered relationships between CBF and activation.
Activation in TBI adolescents was also related to their volume of gliotic lesions.
Reduced nonfrontal CBF and gliotic lesion volume may contribute to the diffuse, primarily nonfrontal, extra-activation
Acknowledgments
This work was supported by the National Institute Neurological Disorders and Stroke grant R01-NS21889 (“Neurobehavioral outcome of head injury in children,” Levin, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to the adolescents and their families for taking part in the research, to Jason Crowther, Ragini Yallampalli Sanyal, Zahra Kadivar, Joshua Cooper, and Vanessa Abadia for assistance in the preparation of the manuscript, and to the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education, and Clinical Center (MIRECC) for providing facilities that allowed the analyses of the fMRI data.
Footnotes
As a test of the specificity of patterns found with the regressions with gliotic volumes, we also regressed age onto activation. Positive and negative regressions of age and activation were nonsignificant with all corrected cluster thresholds p=0.99.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26(4):200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64(1):137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Injury. 2005;19:699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- Anderson V, Fenwick T, Manly T, Robertson I. Attentional skills following traumatic brain injury in childhood: a componential analysis. Brain Injury. 1998;12:937–949. doi: 10.1080/026990598121990. [DOI] [PubMed] [Google Scholar]
- Barclay L, Zemcov A, Reichert W, Blass JP. Cerebral blood flow decrements in chronic head injury syndrome. Biol Psychiatry. 1985;20(2):146–157. doi: 10.1016/0006-3223(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Bibby H, McDonald S. Theory of mind after traumatic brain injury. Neuropsychologia. 2005;43(1):99–114. doi: 10.1016/j.neuropsychologia.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Brett M. 2002 The MNI brain and the Talairach atlas. 1999 February 14; from http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
- Bruce DA, Alavi A, Bilaniuk L, Dolinskas C, Obrist W, Uzzell B. Diffuse cerebral swelling following head injuries in children: the syndrome of "malignant brain edema". J Neurosurg. 1981;54(2):170–178. doi: 10.3171/jns.1981.54.2.0170. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Chaiwat O, Sharma D, Udomphorn Y, Armstead WM, Vavilala MS. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J Neurotrauma. 2009;26(5):657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Wong SB, Chapman SB, Cook LG, Anand R, Gamino JF, Devous MD., Sr A SPECT study of language and brain reorganization three years after pediatric brain injury. Prog Brain Res. 2006;157:173–185. doi: 10.1016/s0079-6123(06)57011-6. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;71(2):161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA. Knowing the meaning, getting the point, bridging the gap, and carrying the message: aspects of discourse following closed head injury in childhood and adolescence. Brain Lang. 1990;39(3):428–446. doi: 10.1016/0093-934x(90)90149-b. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, Pekar JJ, van Zijl PC. An account of the discrepancy between MRI and PET cerebral blood flow measures. A high-field MRI investigation. NMR Biomed. 2006;19(8):1043–1054. doi: 10.1002/nbm.1075. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Ganesalingam K, Yeates KO, Sanson A, Anderson V. Social problem-solving skills following childhood traumatic brain injury and its association with self-regulation and social and behavioural functioning. Journal of neuropsychology. 2007;1(Pt 2):149–170. doi: 10.1348/174866407x185300. [DOI] [PubMed] [Google Scholar]
- Giza CC, Mink RB, Madikians A. Pediatric traumatic brain injury: not just little adults. Curr Opin Crit Care. 2007;13(2):143–152. doi: 10.1097/MCC.0b013e32808255dc. [DOI] [PubMed] [Google Scholar]
- Golay X, Stuber M, Pruessmann KP, Meier D, Boesiger P. Transfer insensitive labeling technique (TILT): application to multislice functional perfusion imaging. J Magn Reson Imaging. 1999;9(3):454–461. doi: 10.1002/(sici)1522-2586(199903)9:3<454::aid-jmri14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Golding EM. Sequelae following traumatic brain injury. The cerebrovascular perspective. Brain Res Brain Res Rev. 2002;38(3):377–388. doi: 10.1016/s0165-0173(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Graham DI, Gennarelli TA, McIntosh TR. Trauma. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7. New York: Oxford University Press; 2002. pp. 823–897. [Google Scholar]
- Hanten G, Cook L, Orsten K, Chapman SB, Li X, Wilde EA, et al. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia. 2011;49(3):486–497. doi: 10.1016/j.neuropsychologia.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikse J, Lu H, van der Grond J, Van Zijl PC, Golay X. Measurements of cerebral perfusion and arterial hemodynamics during visual stimulation using TURBO-TILT. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;50(2):429–433. doi: 10.1002/mrm.10525. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Ietswaart M, Summers F. Theory of mind following traumatic brain injury: the role of emotion recognition and executive dysfunction. Neuropsychologia. 2006;44(10):1623–1628. doi: 10.1016/j.neuropsychologia.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Biswal B. The influence of neuropathology on the FMRI signal: a measurement of brain or vein? Clin Neuropsychol. 2007;21(1):58–72. doi: 10.1080/13854040601064542. [DOI] [PubMed] [Google Scholar]
- Janusz JA, Kirkwood MW, Yeates KO, Taylor HG. Social problem-solving skills in children with traumatic brain injury: long-term outcomes and prediction of social competence. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2002;8(3):179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Avants B, Europa E, Wang J, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. 27(8):1399–1411. doi: 10.1089/neu.2009.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Yeakley J, Song J, Scheibel RS, et al. Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: a test of the Ommaya-Gennarelli model. Neurosurgery. 1997;40(3):432–440. doi: 10.1097/00006123-199703000-00002. discussion 440–431. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Dirnagl U, Fuchtemeier M, Bottiger C, Offenhauser N, Leithner C, et al. Pathophysiological interference with neurovascular coupling - when imaging based on hemoglobin might go blind. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC. Routine clinical brain MRI sequences for use at 3.0 Tesla. Journal of magnetic resonance imaging : JMRI. 2005;22(1):13–22. doi: 10.1002/jmri.20356. [DOI] [PubMed] [Google Scholar]
- MacDonald S, Johnson CJ. Assessment of subtle cognitive-communication deficits following acquired brain injury: A normative study of the Functional Assessment of Verbal Reasoning and Executive Strategies (FAVRES) Brain injury : [BI] 2005;19(11):895–902. doi: 10.1080/02699050400004294. [DOI] [PubMed] [Google Scholar]
- Magri C, Logothetis NK, Panzeri S. Investigating static nonlinearities in neurovascular coupling. Magn Reson Imaging. 2011 doi: 10.1016/j.mri.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Staniloiu A. Amygdala in action: relaying biological and social significance to autobiographical memory. Neuropsychologia. 2011;49(4):718–733. doi: 10.1016/j.neuropsychologia.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Irvine A, Adams JH, Graham DI, Gennarelli TA. Response of cerebral microvasculature to brain injury. J Pathol. 1988;155(4):327–335. doi: 10.1002/path.1711550408. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milders M, Ietswaart M, Crawford JR, Currie D. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. Journal of the International Neuropsychological Society : JINS. 2008;14(2):318–326. doi: 10.1017/S1355617708080351. [DOI] [PubMed] [Google Scholar]
- Muller F, Simion A, Reviriego E, Galera C, Mazaux JM, Barat M, et al. Exploring theory of mind after severe traumatic brain injury. Cortex; a journal devoted to the study of the nervous system and behavior. 2010;46(9):1088–1099. doi: 10.1016/j.cortex.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Muscara F, Catroppa C, Anderson V. Social problem-solving skills as a mediator between executive function and long-term social outcome following paediatric traumatic brain injury. Journal of neuropsychology. 2008;2(Pt 2):445–461. doi: 10.1348/174866407x250820. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Hanten G, Chu Z, Steinberg JL, Hunter JV, et al. Brain activation while thinking about the self from another person’s perspective after traumatic brain injury in adolescents. Neuropsychology. 2010;24(2):139–147. doi: 10.1037/a0017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palmer HS, Garzon B, Xu J, Berntsen EM, Skandsen T, Haberg AK. Reduced fractional anisotropy does not change the shape of the hemodynamic response in survivors of severe traumatic brain injury. J Neurotrauma. 27(5):853–862. doi: 10.1089/neu.2009.1225. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. "I know you are but what am I?!": neural bases of self- and social knowledge retrieval in children and adults. J Cogn Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Baeza A, Reina-de la Torre F, Poca A, Marti M, Garnacho A. Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat Rec A Discov Mol Cell Evol Biol. 2003;273(1):583–593. doi: 10.1002/ar.a.10069. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, et al. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil Neural Repair. 2007;21(1):36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320(5883):1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42(2):347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 1. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, Kaufmann PM. Assessment of pragmatic communication skills in adolescents after traumatic brain injury. Brain injury : [BI] 1996;10(5):329–345. doi: 10.1080/026990596124359. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, DePompei R. Social information processing in adolescents: data from normally developing adolescents and preliminary data from their peers with traumatic brain injury. The Journal of head trauma rehabilitation. 2001;16(5):469–483. doi: 10.1097/00001199-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology. 2008;71(11):812–818. doi: 10.1212/01.wnl.0000325640.18235.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38(4):225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilala MS, Lee LA, Boddu K, Visco E, Newell DW, Zimmerman JJ, et al. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med. 2004;5(3):257–263. doi: 10.1097/01.pcc.0000123545.69133.c3. [DOI] [PubMed] [Google Scholar]
- Vavilala MS, Muangman S, Tontisirin N, Fisk D, Roscigno C, Mitchell P, et al. Impaired cerebral autoregulation and 6-month outcome in children with severe traumatic brain injury: preliminary findings. Dev Neurosci. 2006;28(4–5):348–353. doi: 10.1159/000094161. [DOI] [PubMed] [Google Scholar]
- Vavilala MS, Tontisirin N, Udomphorn Y, Armstead W, Zimmerman JJ, Chesnut R, et al. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9(1):45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]
- Walz NC, Yeates KO, Wade SL, Mark E. Social information processing skills in adolescents with traumatic brain injury: Relationship with social competence and behavior problems. J Pediatr Rehabil Med. 2009;2(4):285–295. doi: 10.3233/PRM-2009-0094. [DOI] [PubMed] [Google Scholar]
- Warschausky S, Cohen EH, Parker JG, Levendosky AA, Okun A. Social problem-solving skills of children with traumatic brain injury. Pediatr Rehabil. 1997;1(2):77–81. doi: 10.3109/17518429709025850. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Weisskoff RM. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magn Reson Med. 1996;36(4):643–645. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- Wilson MD. MRC Psycholinguistics Database: Machine Readable Dictionary, Version 2. Behavioural Research Methods, Instruments and Computers. 1988;20:6–11. [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 2004;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, Drotar D. Long-term attention problems in children with traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwienenberg M, Muizelaar JP. Severe pediatric head injury: the role of hyperemia revisited. J Neurotrauma. 1999;16(10):937–943. doi: 10.1089/neu.1999.16.937. [DOI] [PubMed] [Google Scholar]