Abstract
Background
An important function of the fat body in adult female mosquitoes is the conversion of blood meal derived amino acids (AA) into massive amounts of yolk protein precursors. A highly efficient transport mechanism for AAs across the plasma membrane of the fat body trophocytes is essential in order to deliver building blocks for the rapid synthesis of large amounts of these proteins. This mechanism consists in part of AA transporter proteins from the solute carrier family. These transporters have dual function; they function as transporters and participate in the nutrient signal transduction pathway that is activated in the fat body after a blood meal. In this study we focused on the solute carrier 7 family (SLC7), a family of AA transporters present in all metazoans that includes members with strong substrate specificity for cationic AAs.
Methodology/Principal Findings
We identified eleven putative SLC7 transporters in the genome sequence of Aedes aegypti. Phylogenetic analysis puts five of these in the cationic AA transporter subfamily (CAT) and six in the heterodimeric AA transporter (HAT) subfamily. All eleven Aedes aegypti SLC7 genes are expressed in adult females. Expression profiles are dynamic after a blood meal. We knocked down six fat body-expressed SLC7 transporters using RNAi and found that these ‘knockdowns’ reduced AA-induced TOR signaling. We also determined the effect these knockdowns had on the number of eggs deposited following a blood meal.
Conclusions/Significance
Our analysis stresses the importance of SLC7 transporters in TOR signaling pathway and mosquito reproduction.
Keywords: SLC7 transporter, Mosquito, Target of rapamycin, Amino acid, Aedes aegypti, RNA interference
1. Introduction
Obligatory anautogenous mosquitoes such as the malaria mosquito Anopheles gambiae and the yellow fever mosquito Aedes aegypti, need vertebrate blood in order to complete their life cycle. This “need for blood” is the underlying reason this category of mosquitoes are efficient vectors for parasitic protozoans, viruses, and nematodes (Attardo et al., 2005). Blood meal-derived amino acids (AA) are used for vitellogenesis, a process that involves the synthesis of large amounts of yolk proteins and their deposition in the developing oocytes. These maternal proteins are later used by the developing embryo.
In mosquitoes, yolk proteins are synthesized exclusively in the fat body; the primary metabolic organ of insects. During mosquito vitellogenesis the fat body tissue takes up AA from the hemolymph and converts it into yolk proteins that are secreted back into the hemolymph and then taken up by the developing oocytes via receptor-mediated endocytosis. However, prior to blood meal consumption yolk protein expression is tightly repressed. Following a blood meal, fat body yolk protein biosynthesis is de-repressed by the rise in hemolymph AA concentration. The AA signal is relayed to and causes the expression of yolk protein genes via the target of rapamycin (TOR) signaling pathway. Interruption of TOR signaling has been shown to negatively affect vitellogenesis and egg development (Attardo et al., 2006; Hansen et al., 2004; Hansen et al., 2005; Park et al., 2006).
Uptake of massive amounts of AA by the fat body requires an effective battery of AA transporters that facilitate transport of these building blocks over the plasma membrane of the fat body trophocytes. Despite their importance for reproduction only few AA transporters have been functionally characterized in mosquitoes so far. A LAT type AA transporter with specificity for large neutral and basic AAs is expressed in the alimentary system of A. aegypti larvae (Jin et al., 2003). A glutamate/aspartate transporter with expression in the thoracic ganglia of adult A. aegypti has been described [7]. Phylogenetic analysis of the nutrient AA transporter (NAT) family has been performed and several NATs of the mosquito larval alimentary system have been characterized in detail. A NAT with high specificity for phenylalanine has been identified in the larval midgut of A. Aegypti (Boudko et al., 2005). This transporter is a member of the sodium-neurotransmitter symporter family. A second NAT has been cloned and characterized from Anopheles gambiae (Meleshkevitch et al., 2006). Electrophysiological analysis showed that this B0 type transporter is sodium-dependent and highly selective for aromatic AAs or other precursors of catecholamine synthesis pathways. Two other members of the NAT family with similar specificities for aromatic AAs and expression in the larval alimentary system have been characterized in A. gambiae (Okech et al., 2008). A proton-dependent AA transporter with low affinity and low substrate specificity was described in epithelial cell membranes of larval caecae and the adult female midgut (Evans et al., 2009).
The SLC7 family of AA transporters consists of two closely related subfamilies that are well characterized in vertebrates (Verrey et al., 2004). The cationic AA transporters (CAT) have a strong specificity for positively charged AAs: histidine, lysine, arginine and may be involved in NO synthesis via regulation of arginine uptake. The heterodimeric AA transporters (HAT) have a more diverse spectrum of substrates. HATs are only functional when associated with a membrane glycoprotein from the SLC3 family. Two members of the CAT subfamily have been cloned and characterized in A. aegypti (Attardo et al., 2006). AaCAT1 and AaCAT2 are both expressed in the adult fat body. RNAi-mediated knockdown of these AA transporters resulted in a strong inhibition of AA-induced yolk protein expression in the female fat body. Electrophysiological characterization of AaCAT1 expressed in Xenopus oocytes has shown that this transporter has narrow substrate specificity – at neutral pH it transports only L-histidine (Hansen et al., 2011).
Considering the important functions of SLC7 AA transporters in vertebrate cell physiology, this study focuses on the identification and expression analysis of AA transporters of the SLC7 family in mosquitoes. We also addressed the role of SLC7 transporters in AA-induced TOR signaling in the fat body of adult A. aegypti females and the effect of RNAi-mediated knockdown on egg production after a blood meal.
2. Materials and methods
2.1 Ethics Statement
The research plan used for this work involving animals was specifically approved by the Institutional Animal Care and Use Committee (IACUC) at New Mexico State University under approval ID #2008-034. All procedures and care are described in the New Mexico State University Animal Care Facility Standard Operating Procedure and on file in the IACUC office there. All persons involved in animal work successfully completed Animal Welfare Training at New Mexico State University and were specifically trained in protocols used in the research plan. All New Mexico State University IACUC care and protocols follow the NIH guidelines described in Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15400-6.
2.2 Sequence Identification & Phylogenetic Analysis
Predicted cDNA and deduced AA sequences of Ae. aegypti SLC7 transporters were identified using BLAST tools at two databases: Genbank (http://www.ncbi.nlm.nih.gov/genbank/), and VectorBase, http://www.vectorbase.org. Thirteen Homo sapiens SLC7 sequences were chosen according to http://www.membranetransport.org/ (Paulsen et al., 2007) and their sequences retrieved via Genbank.
ClustalX was used to perform phylogenetic analysis of the data set (Higgins et al., 2007). A putative membrane protein of Grosmannia clavigera was used as outgroup. Bootstrap values (1000 replicates are indicated on the nodes of the Bootstrap N-J tree).
2.3 Animals
The A. aegypti mosquito strain UGAL was maintained in laboratory culture as has been previously described by Hays and Raikhel (Hays and Raikhel, 1990). The strain was reared at a temperature of 28°C with 80% humidity and a photoperiod of 14h light and 10h dark. Larvae were fed on a diet of dry cat food pellets. Mosquitoes were blood fed by placing a live chicken (Gallus gallus domesticus) on top of their cage for approximately 30 minutes.
2.4 RNA Isolation
For expression profiling, whole mosquito RNAs were isolated from A. aegypti at each larval stage from triplicate samples. We used a sample size of 125 individuals from the 1st and 2nd larval stages, 75 from the 3rd larval stage, 30 from the 4th larval stage, 25 from the pupal stage, 8 adult females, and 8 adult males in order to produce the developmental expression profiles. In addition, 8 females were taken 72 hours post-eclosion, and time points 3, 9, 12, 24, 48, 72, and 96 hours post-blood meal (PBM) to produce the PBM expression profiles. For each time point we isolated RNA from three biological replicates. Total RNAs were isolated from 30 individual mosquitoes including larval stages, pupae, adult males, previtellogenic females, and females at various time points post blood meal. Total RNA was isolated by means of a commercially available procedure using TRIzol® solution (Invitrogen) (Chomczynski and Sacchi, 1987).
2.5 qPCR Expression Analysis
SLC7-specific primers were developed using Primer BLAST (Remm and Koressaar, 2007). Relative transcript levels were quantified on an Eppendorf Mastercycler ep realplex® (Eppendorf, Hamburg, Germany) using iQ Supermix (Biorad, Hercules, CA). Primers were as follows: AaCAT1F: CGC GTG TCA ACC CAC CGA AGA; AaCAT1R: CCG GAG CCA AGA ACC GAA TGT; AaCAT2F: TCG GCC TGC TGC TGC TCA TC; AaCAT2R: ACG CGT ACA GGG CCA AAC CA; AaCAT3F: AAC GTG GGT CCG ATT CGC CG; AaCAT3R: TCT CGG CCG GGA CTG TCG AT; AaCAT4F: GAC ATG GTG GGC GCT GGG AC; AaCAT4R: CCG GAG CCA CGT CAG GAA GC; AaCAT5F: ATG GCC ACC AAC GAC GAC GG; AaCAT5R: TTT CGC TTC GGG CCG CTG TT; AaHAT1F: TCC AAC TGT GGG TCC CAA CGC; AaHAT1R: TGG CAG AAC TGT GTC GCC CG; AaHAT2F: TCC TCC TGA CGG CAG GGT TCC; AaHAT2R: GGC ATG CAC AGG AAG AGT TTC GC; AaHAT3F: TGG CTT TCG GTG GCA GCT TG; AaHAT3R: TAC GGG CAC TCC GGA CAG GG; AaHAT4F: TCG GTG GGC CCA ACC AAA TCT; AaHAT4R: TGG GCT TGT TTC GCC ACG CA; AaHAT5F: CGG CGC GAT GGT CCT CTA CG; AaHAT5R: ATG GGC GCC GCC ACT AGG TA; AaHAT6F: AGC GTT CAT CGC ATT GCT GAC CT AaHAT6R: ACG GCG TGT AGA CAG CCA CG.
2.6 RNA interference
Generation of double-stranded RNAs (dsRNA) was accomplished by PCR amplification and cloning of 750 to 1,000 bp long AaCAT2, AaCAT3, AaHAT1, AaHAT2, AaHAT4, AaHAT5, and AaHAT6 partial cDNAs with T7 sequences attached at both ends into the pCRII-TOPO vector. eGFP cDNA was utilized as a negative control. dsRNA was produced by in vitro transcription with T7-RNA polymerase using the Megascript T7 Kit (Ambion, Austin, TX, USA). Approximately 0.5–1·μg of dsRNA in 0.3–0.5·μl of H2O was injected into the thorax of CO2-anesthetized 3-day-old female mosquitoes. The mosquitoes were allowed to recover for 3 days before further processing. Knockdown effectiveness was confirmed with qPCR. Primers were as follows (The T7 sequence is included): eGFPF- TAA TAC GAC TCA CTA TAG GGG ACG GCG ACG TAA ACG GCC A; eGFPR- TAA TAC GAC TC CTA CTA TAG GGC GTC GCC GAT GGG GGT GTT C; AaCAT2F -TAA TAC GAC TCA CTA TAG GGG CCA CGA CCG GAG AGG AGG T; AaCAT2R - TAA TAC GAC TCA CTA TAG GGC CAG GGT CAA GGC CCA CGG T; AaCAT3F - TAA TAC GAC TCA CTA TAG GGG TTC CTG GTG GCG GCC GTT G; AaCAT3R - TAA TAC GAC TCA CTA TAG GGT GCA CAG CGC GAA GAT GGC T; AaHAT1F - TAA TAC GAC TCA CTA TAG GGG GAC GAT TGA TGG CGG CGG A; AaHAT1R - TAA TAC GAC TCA CTA TAG GGC TGG GCG AAT GTC ACG GCG A; AaHAT2F - TAA TAC GAC TCA CTA TAG GGT GGC CAA CGT GTG AAC CAC CG; AaHAT2R - TAA TAC GAC TCA CTA TAG GGG CGT CCC GAT GGC AAC CTC T; AaHAT3F - TAA TAC GAC TCA CTA TAG GGA CCA TGG CCA AGC TGA CGG C; AaHAT3R - TAA TAC GAC TCA CTA TAG GGG TCG GAG AGC TCC CGG TCG T; AaHAT4F - TAA TAC GAC TCA CTA TAG GGT GGC GGT GGG GAT GGG GAA A; AaHAT4R - TAA TAC GAC TCA CTA TAG GGC GTC GGC GTT AGG CGT TGG A; AaHAT5F - TAA TAC GAC TCA CTA TAG GGT GGG TGT CCA CGC TGG TCC T; AaHAT5R - TAA TAC GAC TCA CTA TAG GGG CCG GCA ATC AGC GAG TGG A; AaHAT6F - TAA TAC GAC TCA CTA TAG GGC AGC CCT GGG CAC ATC GCA T; AaHAT6R - TAA TAC GAC TCA CTA TAG GGG CGT GTA GAC AGC CAC GCC A.
2.7 Fat body culture
Fat body tissue culture was performed in media replicating the AA conditions in post blood meal mosquito hemolymph as described previously (Deitsch et al., 1995; Raikhel et al., 1997). Fat bodies were incubated for time intervals of 5, 10, 15, 30, and 45 minutes, 1 and 2 hours at 27°C before collection and processing. In the knockdown experiments for AaCAT2, AaCAT3, AaHAT2, AaHAT4, AaHAT5, AaHAT6 fat bodies were incubated for 30 minutes prior to processing.
2.8 Western Blot Analysis
We determined TOR kinase activity via Western blot analysis. An antibody specific for mammalian S6 kinase when phosphorylated at the residue Thr-389 and Aedes S6 kinase when phosphorylated at the residue Thr-388 (number 07-018, Upstate, Lake Placid, NY)(Hansen et al., 2005) was used for this purpose. Fat bodies were isolated and subjected to experimental treatments in fat body culture. At the end of the culture period groups of 8 fat bodies were homogenized in 120 μl of breaking buffer (50 mM Tris, pH 7.4; 1% IGEPAL; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM protease inhibitor mixture; 1 mM phosphatase inhibitor mixture 1, (all purchased from Sigma) using a pellet pestle. 30 μl aliquots of the protein extract were resolved on 10% gradient sodium dodecyl sulfate-polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes by the use of Trans-Blot SD semi-dry electrophoretic transfer cell (Bio-Rad). The membranes were blocked overnight in StartingBlockTMT20 (TBS) blocking buffer (Pierce) at 4 °C. After washing with Tris-buffered saline-Tween 20 buffer (TBST), membranes were incubated with the primary antibody in blocking buffer solution (BLOTTO, Thermo Scientific) for one hour on a platform shaker at room temperature. After extensive washing in TBST, the membranes were incubated with an alkaline phosphatase-labeled secondary antibody in BLOTTO for one hour at room temperature. Bands were visualized using blue tetrazolium chloride/5-Bromo-4-chloro-3-indolyl phosphate, toluidine salt (NBT/BCIP).
We used an anti p70-S6K antibody (Santa Cruz, #sc-230, dilution 1:250) and an anti Drosophila actin antibody (Millipore, #MAB1501, dilution 1:5000) for controls.
2.9 Egg counts
Female mosquitoes 72 hrs after emergence were injected with dsRNA against AaCAT2, AaCAT3, AaHAT1, AaHAT2, AaHAT4, AaHAT5, AaHAT6, or with the control dsRNA (eGFP). After a 3-day recovery, mosquitoes were given a blood meal. Only fully engorged mosquitoes were used in this experiment. After 3 days, egg deposition was triggered by allowing the mosquitoes access to a cotton ball soaked in water. Egg numbers were determined by a stereo microscope 24 hrs later.
3. Results
3.1 Phylogenetic Analysis of SLC7 AA Transporters
We identified eleven putative SLC7-encoding genes in the genome on Ae. aegypti (Table 1). Five are members of the CAT subfamily characterized by fourteen transmembrane domains. We named these putative proteins AaCAT1-5. Six are members of the HAT subfamily with twelve transmembrane domains and we named these putative proteins AaHAT1-6.
Table 1. Aedes aegypti SLC7 transporters.
The identifiers and accession numbers refer to public bioinformation libraries at http://vectorbase.org/ and http://www.ncbi.nlm.nih.gov/genbank/.
| Name | VectorBase Identifier | Genbank Accession Number |
|---|---|---|
| AaCAT1 | AAEL012128 | XP_001662273.1 |
| AaCAT2 | AAEL009362 | XP_001659995 |
| AaCAT3 | AAEL012131 | XP_001662274.1 |
| AaCAT4 | AAEL009358 | XP_001659990.1 |
| AaCAT5 | AAEL011654 | XP_001661832.1 |
| AaHAT1 | AAEL002525 | XP_001655456.1 |
| AaHAT2 | AAEL014161 | XP_001648140.1 |
| AaHAT3 | AAEL003919 | XP_001647992.1 |
| AaHAT4 | AAEL002557 | XP_001655584.1 |
| AaHAT5 | AAEL003387 | XP_001656755.1 |
| AaHAT6 | AAEL008406 | XP_001653251.1 |
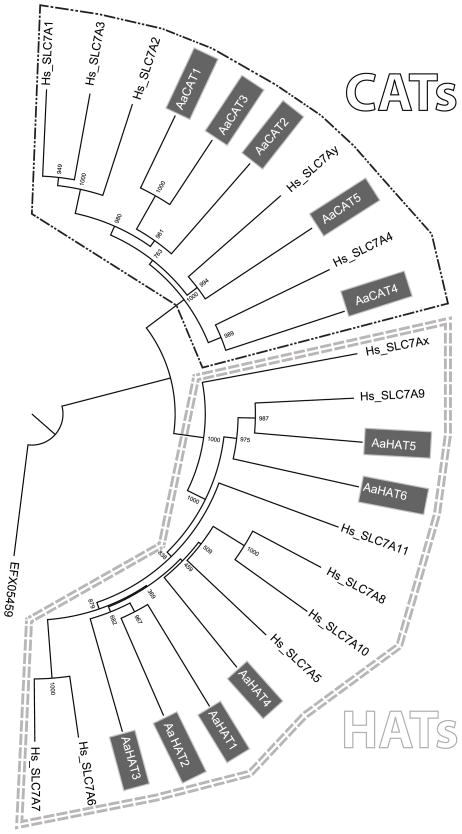
A phylogenetic analysis involving human and Ae. aegypti SLC7 sequences placed AaCAT1-3 in a separate group closely related to human SLCA1-3 while AaCAT1 form a group with human SLC7A4 and AaCAT5 with another human SLC7 transporter (Figure 1). AaHAT1-3 form a well supported group distinct from human SLC7s while the other AaHATs group together with human SLC7 proteins.
Fig. 1. Phylogenetic tree of SLC7 transporters.
The evolutionary history was inferred using the neighbor-joining method. The percentage of bootstrap test (1,000 replicates) is shown next to the nodes. The tree was drawn using Fig Tree software. The analysis involved 13 human (black letters) and 11 mosquito AA sequences (white letters in grey boxes). Predicted members of the CAT subfamily are surrounded by a black line, members of the HAT subfamily are surrounded by two grey lines.
3.2 Expression Profiles of SLC7 AA Transporters
We used quantitative RT PCR to determine SLC7 expression throughout larval development, in adult males and in female mosquitoes 72hrs post eclosion, and at several time points during the process of vitellogenesis. We found that each transporter had a varied expression profile throughout the different developmental stages (Figures 2A & 2B). The observed patterns of SLC7 expression were as follows:
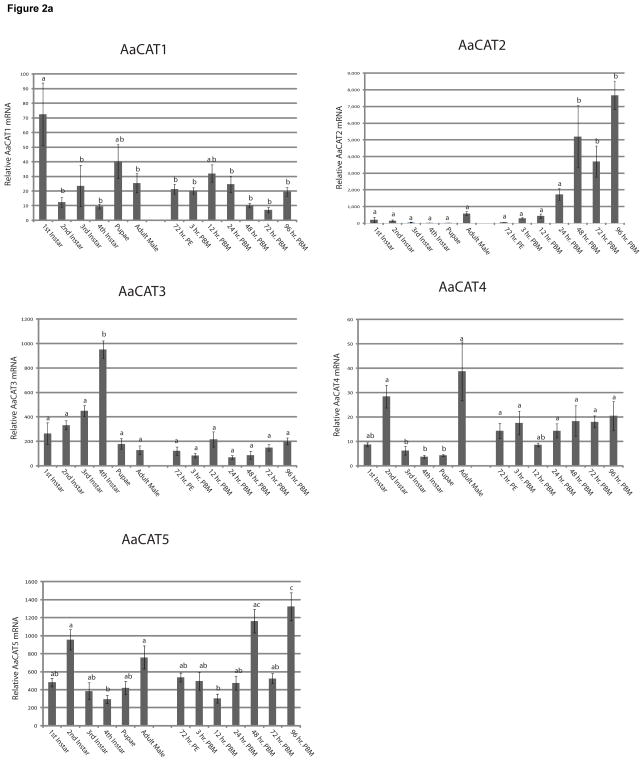
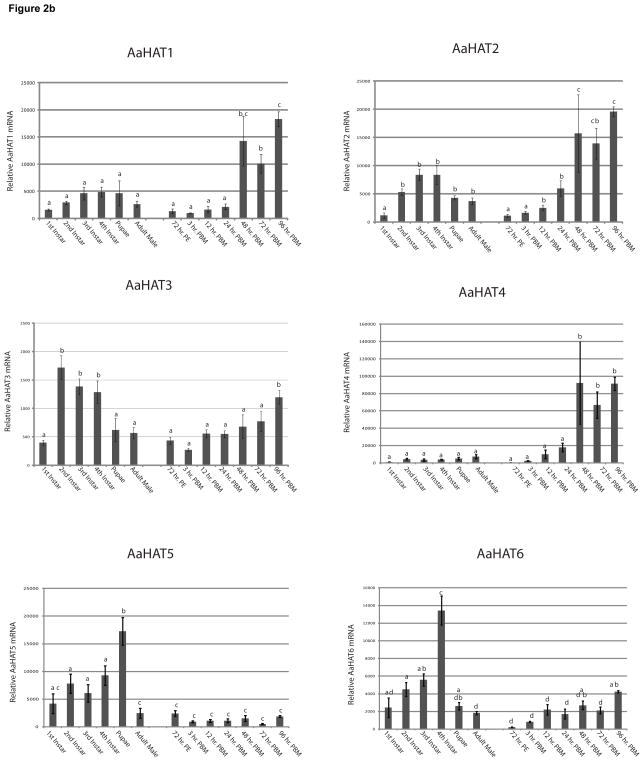
Fig. 2. Expression levels of SLC7 transporters in whole mosquitoes during development and vitellogenesis.
A. Overall expression of AaCATs during larval stages, adult male, and adult female mosquitoes during vitellogenesis. Expression was assayed with q-RT-PCR. RNA was isolated from three groups of 150 1st instar larvae, 130 2nd instar larvae, 75 3rd instar larvae, 30 4th instar larvae, 15 pupae, and 3 adult mosquitoes. PE- Post Eclosion, PBM- Post Blood Meal. Five replicates were analyzed, and their means separated by Tukey-Kramer HSD (p,0.05). Means which share the same letter are not significantly different. B. Expression of HATs during larval stages and in adult mosquitoes. Overall expression of AaHATs in during larval stages, adult male, and adult female mosquitoes during vitellogenesis. Expression was assayed as described above.
AaCAT1
AaCAT1, originally described as a homologue of Drosophila Slimfast (Attardo et al., 2006), has been previously characterized and found to have a strong substrate specificity to L- histidine (Hansen et al., 2011). This transporter had highest expression in the 1st instar larval stage with lower expression in the other developmental stages. We did not observe any statistically significant change of AaCAT1 expression in adult females after a blood meal.
AaCAT2
AaCAT2 had low expression levels throughout all developmental stages. Expression levels of AaCAT2 were significantly up-regulated in adult females at 48, 72, and 96 hrs PBM.
AaCAT3
This transporter shows rather unique expression that peaked in the 4th instar. We did not detect any significant changes in expression during the vitellogenic period.
AaCAT4
AaCAT4 exhibited peak expression during the second instar compared to the other larval instars. We also observed high expression in adult males. We did not observe significant changes in AaCAT4 expression during vitellogenesis.
AaCAT5
We found that this transporter is expressed at relatively low levels during development and at the onset of vitellogenesis. We found it up regulated at 48 and 96h PBM in adult females.
AaHAT1
This transporter displayed a non-significant trend of increasing expression levels from 1st through 4th instar during larval development. Additionally, it had fairly constant expression levels between adult males, adult females, up to and including 24 hrs PBM. Expression levels were significantly increased at 48, 72, and 96 hrs PBM.
AaHAT2
AaHAT2, a homologue to Drosophila minidisks (Shearn et al., 2000) showed expression profiles similar to AaHAT1, with the exception that PBM up-regulation began at 12 hrs instead of 48 hrs (Figure 3).
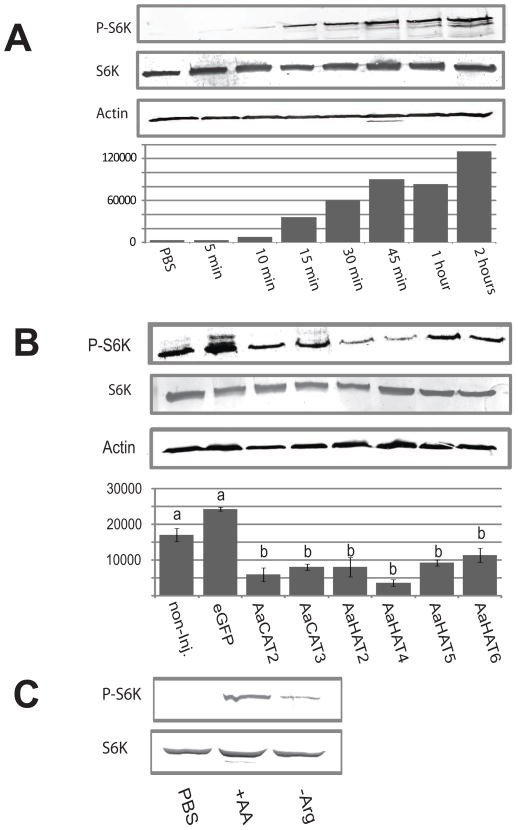
Fig. 3. SLC7 knockodown reduces TOR signaling in mosquito fat bodies.
A: AA induced S6K phosphorylation in fat body culture. Upper panel: Western Blot transfer membranes showing the increase of P-S6K in fat bodies of mosquitoes at different time points after placement in FBCM. Second panel: A second blot was probed with anti p70-S6K antibodies as control. Third panel: A third blot was probed with anti actin antibodies as loading control. Lower panel: Densitometric analysis of expression levels. P-S6K levels were normalized against actin. B: Effect of RNAi-mediated SLC7 knockdowns on P-S6K levels. Upper panel: Western Blot showing the levels of P-S6K after knockdown of the transporter shown in the column. Second panel: A second blot was probed with anti p70-S6K antibodies as control. Third panel: A third blot was probed with anti actin antibodies as loading control. Lower panel: Densitometric analysis of P-S6K expression levels. P-S6K levels were normalized against actin. Three biological replicates were analyzed, and their means separated by Tukey-Kramer HSD (p,0.05). Means which share the same letter are not significantly different. C: The effect of L-arginine withdrawal on S6K phosphorylation. Upper panel: Western Blot showing phospho-S6K levels of fat bodies that were treated with PBS, balanced AA media, and AA media without L-arginine. Lower panel: A second blot was probed with anti p70-S6K antibodies as control.
AaHAT3
For this transporter, we observed a significant amount of up-regulation in the 2nd, 3rd, and 4th larval stages compared to the first larval instar. During vitellogenesis we did not notice any significant changes in gene expression until 96 hrs PBM.
AaHAT4
AaHAT4 was found to have very low expression levels throughout the larval developmental stages, into the adult mosquitoes, and into the beginning of vitellogenesis. There was a slight non-significant elevation in expression at 12 hrs PBM and 24 hrs PBM, and a greater significant elevation in expression at 48, 72, and 96 hrs PBM.
AaHAT5
This transporter exhibited high expression levels during postembryonic development with peak expression during the pupal stage. We detected no changes in expression levels PBM.
AaHAT6
AaHAT6 expression closely resembled the AaHAT5 expression with the exception that in adult females expression is up regulated 96h PBM.
3.5 The Effect of SLC7-Knockdown on Fat Body TOR Signaling
In order to demonstrate the TOR signaling pathway activation kinetics in isolated fat bodies after AA stimulation we dissected fat bodies and transferred them onto fat body culture media (FBCM). We then examined the phosphorylation level of S6K, a direct target of the TOR kinase, at specific time points after application on the FBCM via Western blotting. Figure 4A shows the result of a representative experiment. We found a significant increase in the protein level of P- S6K starting at 10 minutes post application and observed a continuous increase in the phosphorylation of S6K corresponding to the duration of time on the FBCM.
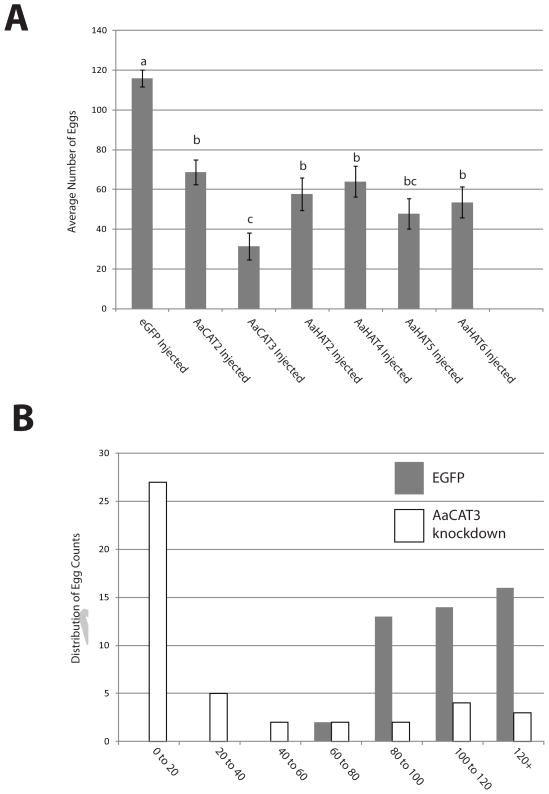
Fig. 4. RNAi-mediate knockdown causes a decrease in average clutch size per female.
A: Average number of eggs laid by each female. The total number of eggs was take from 45 females and measured by egg counting though a stereo microscope. B: Comparison of the egg distributions of AaCAT3 injected mosquitoes (white bars) and eGFP injected mosquitoes (grey bars).
To assess the effect of RNAi-mediated knockdown on fat body TOR signaling after AA stimulation we analyzed S6K phosphorylation levels within the fat bodies of SLC7 knockdown mosquitoes. We used RT-PCR to confirm expression of putative SLC7 transporters in the fat body of adult females (data not shown) and picked transporters with confirmed fat body expression for subsequent knockdown experiments. AaCAT2, AaCAT3, AaHAT2, AaHAT4, AaHAT5, AaHAT6 were knocked down by injection of specific dsRNA. Three days after dsRNA injection fat bodies were dissected combined in groups of eight and then incubated for 30 minutes on FBCM. P-S6K levels were determined via Western blotting. We found a significant decrease in the protein levels of P-S6K in each of the knockdowns compared to EGFP-injected control mosquitoes (Figure 4B).
To test if withdrawal of the cationic AA L-arginine has an effect on fat body TOR signaling in the context of AA stimulation we incubated fat bodies in three different media for 1h in fat body culture. We found strongly reduced P-S6K levels in fat bodies that were incubated in the media lacking L-arginine (see Figure 3C).
3.6 Lowered fecundity in RNAi mediated SLC7 knockdown females
To test whether individual SLC7 AA transporter proteins are important for mosquito egg development, we performed RNAi-mediated knockdowns of all transporters excluding AaCAT1 which has been previously characterized (Hansen et al., 2011). Global knockdown efficiency was determined via qPCR in three biological replicates of groups of three knockdown mosquitoes with efficiencies ranging from 76 to 98% reduction in relative mRNA expression levels. These values are comparable to knockdown efficiencies achieved in the Malpighian tubules of Ae. aegypti using the same technique (Drake et al., 2010). After knockdown of individual SLC7 transporters we observed a significant decrease in the overall number of eggs deposited when compared to the control (Figure 4A). Figure 4B shows the distribution of egg numbers deposited by AaCAT3-knockdown females.
4. Discussion
The publication of an annotated genome sequence for Ae. aegypti (Nene et al., 2007) greatly increased the feasibility of reverse genetic studies in this important disease vector such as the one presented here. While the first attempts to identify SLC7 genes in Ae. aegypti resulted in the identification and partial characterization of two transporters of the CAT subfamily (Attardo et al., 2006; Hansen et al., 2011) we were now able to identify a total of eleven putative SLC7 transporters, five of the CAT subfamily and six of the HAT subfamily (Table 1). Our phylogenetic study (Figure 1) confirmed and extended our previous analysis showing that the three closely related CAT1-3 form a separate group apart from the human CAT1-3 (SLC7A1-3) and two additional mosquito CATs (CAT4 & 5) that form two separate groups with human counterparts. The fact that CAT1-3 and HAT1-3 form separate insect-specific clades is promising when considering these proteins and their function as potential targets for insecticide development, since a potential drug targeting these proteins should be highly specific and target only insect SLC7 transporters.
Analysis of the expression profiles of all eleven SLC7 transporters and comparison of our qPCR data with published microarray data (Dissanayake et al., 2010) revealed an astonishingly diverse set of expression patterns and levels during both development and vitellogenesis (Figure 2). With the exception of AaCAT2 and AaHAT4 all SLC7 transporters are expressed at relatively high levels during the different larval stages and in pupae suggesting that these transporters might play an important role in the postembryonic development of mosquitoes. This is supported by a study in Drosophila that focused on the gene Slimfast, homologous to AaCAT3. Slimfast knockdown fruit flies showed growth pattern deficiencies similar to those of wild type Drosophila with AA restrictions (Colombani et al., 2003). Another interesting point is that we were not able to identify any female-specific SLC7-transporters. Since male mosquitoes do not take blood meals we expected generally lower expression levels of these transporters compared to females. However, expression levels between males and unfed females were in no case significantly different. Expression of CAT2 and CAT5 as well as HAT1-4 was however significantly up regulated in females at the later stages of vitellogenesis. This could be due to enhanced gene expression in female tissues or to synthesis and deposition of maternal mRNAs for these proteins in the developing oocytes. Further research, for example using in situ hybridization experiments, is necessary to clarify this point.
The TOR signaling pathway is highly conserved in eukaryotic organisms. It is nutrient sensitive and can be activated by multiple signals including essential AAs and growth factors (Goberdhan, 2010; Hay and Sonenberg, 2004). The cellular uptake of AAs via AA transporters is an important part of the regulation of the TOR kinase, however how cells measure cytoplasmic AA concentration and react accordingly is not known (Murphy et al., 2009). Previous research conducted in Ae. aegypti has shown that the SLC7 transporter proteins AaCAT1 and AaCAT2 are both components of the TOR signaling cascade in the mosquito fat body upstream of TOR and S6K (Attardo et al., 2006). Apparently these two AA transporters function as upstream components of the AA sensor system that allows the mosquito fat body to detect the rise in AA levels after the female takes a blood meal and subsequently to activate vitellogenic gene expression (Hansen et al., 2004; Hansen et al., 2005; Park et al., 2006). In the fat body culture system used here, dissected fat bodies were transferred onto FBCM that contains a balanced mixture of all 20 AAs at concentrations that resemble mosquito hemolymph after a blood meal. Our results show a strong activation of TOR signaling at fifteen minutes after transfer of the fat bodies onto the FBCM with increased signal intensity at the later times (Figure 3A). This is in concurrence with earlier results showing a strong activation of TOR signaling one hour after transfer (Hansen et al., 2005). We hypothesize that it takes the fat body approximately 15 minutes to transport sufficient amounts of AAs over the plasma membrane into the cytoplasm to reach the concentrations necessary to trigger the fat body nutrient sensor that leads of TOR activation, S6K phosphorylation and subsequent activation of yolk protein gene expression and synthesis. In addition it seems likely that a balanced increase of a group of essential AAs is necessary to activate the nutrient sensor since knockdown of any fat body SLC7 transporter leads to decreased TOR signaling (Figure 3B). This effect was astonishingly robust and reproducible despite the technical challenges associated with this experiment.
The transport specificity of all Ae. aegypti SLC7 transporters are unknown with the exception of AaCAT1 which turned out to have an uncommonly narrow substrate specificity since it transports only a single AA at neutral pH, L-histidine (Hansen et al., 2011). It is possible that the other SLC7 transporters have also narrow but overlapping substrate specificity so that knockdown of single transporters results in deprivation of the fat body of specific essential AAs resulting in the inability to fully activate the TOR signaling pathway.
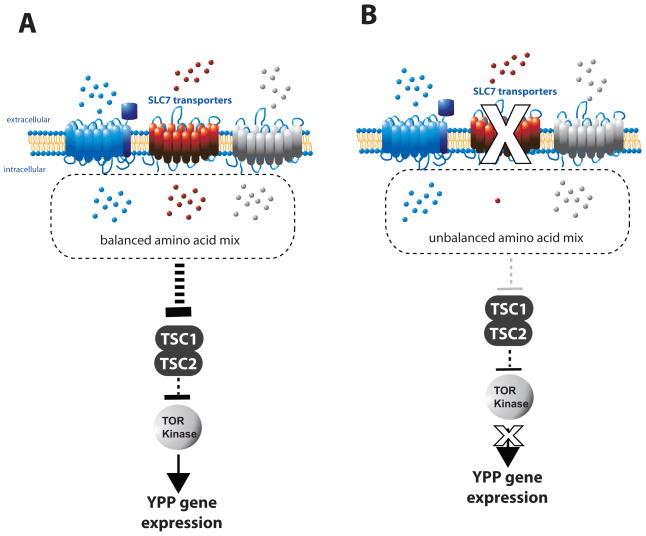
We have integrated earlier results and our recent findings in a working model for fat body AA/TOR signaling which is shown in Figure 5. In this model the knockdown of a single SLC7 AA transporter results in an unbalance of intracellular AAs within the fat body trophocytes after AA stimulation which results in decreased TOR signaling and subsequent yolk protein expression. This model is supported by the finding that withdrawal of single cationic AAs from the balanced mixture results in a strong reduction of yolk protein precursor mRNA expression in the Ae. agypti fat body culture system (Attardo et al., 2006). Also, in vertebrate cells AA availability is known to be required for TORC1 activity (Hay and Sonenberg, 2004) and it has been shown that vertebrate Jurkat cells require a balanced mixture of AAs in order to activate TOR signaling. Withdrawal of the cationic AAs L-arginine or L-lysine resulted in strongly reduced p70-S6K activity in this cell line (Hidayat et al., 2003). Accordingly, we found that withdrawal of L-arginine from the balanced amino acid media resulted in reduced TOR activity in fat bodies when stimulated with AA media for one hour in fat body culture (Figure 3C).
Fig. 5. Working Model: SLC7 regulation of TOR signaling in the Mosquito Fat Body.
A. Transfer of fat bodies onto FBCM results in a balanced rise of intracellular essential AAs and subsequent de-repression of TOR via the tuberous sclerosis complex (TSC1 & 2) which activates yolk protein precursor gene expression. B. RNAi-mediated knockdown of individual SLC7 transporters resulted in unbalanced intracellular AA concentrations. Under these conditions TOR signaling is reduced and yolk protein precursor gene expression is repressed.
Future electrochemical analysis of the other Ae. aegypti SLC7 transporters in combination with metabolomic profiling of the fat bodies of knockdown mosquitoes will enable us to test our working model and better understand the effect that RNAi-mediated knockdown has on the intracellular AA pools in the fat body.
It has been shown in several model organisms that knockdown or inhibition of TOR signaling leads to reduced fertility (Avruch et al., 2002; Hansen et al., 2004; Neufeld et al., 2000). In the hemimetabolous insect Blatella germanica TOR knockdown resulted in reduced Juvenile hormone production and stunted ovary development (Maestro et al., 2009). Taking this in account plus the fact that the fat body SLC7 transporters are upstream components of the TOR signaling cascade it is not surprising that knockdown of fat body SLC7 transporters resulted in decreased fertility in mosquito females (Figure 4A). An interesting phenotype we observed when studying AaCAT3 knockdown females was a high percentage of blood meal-induced mortality (data not shown) that resulted in strongly reduced overall egg numbers after a blood meal (Figure 4B). The mechanism underlying this phenotype is currently under investigation.
5. Conclusions
The results presented here make a strong case for the role of SLC7 AA transporters as upstream components of the TOR signaling cascade in the fat body of mosquitoes. AA transport across the fat body plasma membrane is necessary for successful vitellogenesis and reproduction. Inhibition of these transport processes could give rise to a new generation of insecticides for mosquito and disease control and eradication.
Highlights.
Aedes aegypti has eleven putative SLC7 transporters encoded in its genome
Gene expression of SLC7 transporters is highly dynamic during postembryonic development and vitollegenesis
RNAi-mediated knockdown of SLC7 proteins results in reduced TOR signaling in the female fat body
RNAi-mediated knockdown of SLC7 protein resulted in reduced egg numbers after a blood meal
SLC7 transporters are part of the TOR signaling cascade in the mosquito fat body
Acknowledgments
This research was supported by a grant from the NIH/NIGMS to IAH (5SC2GM092300-02).
Abbreviations
- AA
amino acids
- CAT
cationic amino acid transporter
- FBCM
fat body culture media
- HAT
heterodimeric amino acid transporter
- SLC7
solute carrier 7 family
- TOR
target of rapamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect biochemistry and molecular biology. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. The Journal of experimental biology. 2006;209:3071–3078. doi: 10.1242/jeb.02349. [DOI] [PubMed] [Google Scholar]
- Avruch J, Long XM, Spycher C, Han ZS, Rose AM, Muller F. TOR deficiency in C-elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Current Biology. 2002;12:1448–1461. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- Boudko DY, Kohn AB, Meleshkevitch EA, Dasher MK, Seron TJ, Stevens BR, Harvey WR. Ancestry and progeny of nutrient amino acid transporters. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1360–1365. doi: 10.1073/pnas.0405183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Deitsch KW, Chen JS, Raikhel AS. Indirect Control of Yolk Protein Genes by 20-Hydroxyecdysone in the Fat-Body of the Mosquito, Aedes-Aegypti. Insect biochemistry and molecular biology. 1995;25:449–454. doi: 10.1016/0965-1748(94)00082-a. [DOI] [PubMed] [Google Scholar]
- Dissanayake SN, Ribeiro JM, Wang MH, Dunn WA, Yan G, James AA, Marinotti O. aeGEPUCI: a database of gene expression in the dengue vector mosquito, Aedes aegypti. BMC Res Notes. 2010;3:248. doi: 10.1186/1756-0500-3-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Aimanova KG, Gill SS. Characterization of a blood-meal-responsive proton-dependent amino acid transporter in the disease vector, Aedes aegypti. The Journal of experimental biology. 2009;212:3263–3271. doi: 10.1242/jeb.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DCI. Intracellular amino acid sensing and mTORC1-regulated growth: New ways to block an old target? Curr Opin Invest Dr. 2010;11:1360–1367. [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10626–10631. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. The Journal of biological chemistry. 2005;280:20565–20572. doi: 10.1074/jbc.M500712200. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Boudko DY, Shiao SH, Voronov DA, Meleshkevitch EA, Drake LL, Aguirre SE, Fox JM, Attardo GM, Raikhel AS. AaCAT1 of the Yellow Fever Mosquito, Aedes aegypti A NOVEL HISTIDINE-SPECIFIC AMINO ACID TRANSPORTER FROM THE SLC7 FAMILY. Journal of Biological Chemistry. 2011;286:10803–10813. doi: 10.1074/jbc.M110.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes & development. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hays AR, Raikhel AS. A novel protein produced by the vitellogenic fat body and accumulated in mosquito oocytes. Development Genes and Evolution. 1990;199:114–121. doi: 10.1007/BF02029559. [DOI] [PubMed] [Google Scholar]
- Hidayat S, Yoshino K, Tokunaga C, Hara K, Matsuo M, Yonezawa K. Inhibition of amino acid-mTOR signaling by a leucine derivative induces G1 arrest in Jurkat cells. Biochemical and biophysical research communications. 2003;301:417–423. doi: 10.1016/s0006-291x(02)03052-8. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ. Clustal W and clustal X version 2.0. Bioinformatics (Oxford, England) 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Jin X, Aimanova K, Ross LS, Gill SS. Identification, functional characterization and expression of a LAT type amino acid transporter from the mosquito Aedes aegypti. Insect biochemistry and molecular biology. 2003;33:815–827. doi: 10.1016/s0965-1748(03)00081-x. [DOI] [PubMed] [Google Scholar]
- Maestro JL, Cobo J, Belles X. Target of Rapamycin (TOR) Mediates the Transduction of Nutritional Signals into Juvenile Hormone Production. Journal of Biological Chemistry. 2009;284:5506–5513. doi: 10.1074/jbc.M807042200. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch EA, Assis-Nascimento P, Popova LB, Miller MM, Kohn AB, Phung EN, Mandal A, Harvey WR, Boudko DY. Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. The Journal of experimental biology. 2006;209:3183–3198. doi: 10.1242/jeb.02374. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Nicklin P, Bergman P, Zhang BL, Triantafellow E, Wang H, Nyfeler B, Yang HD, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O’Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science (New York, NY) 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP, Zhang HB, Stallock JP, Ng JC, Reinhard C. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes & development. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Meleshkevitch EA, Miller MM, Popova LB, Harvey WR, Boudko DY. Synergy and specificity of two Na+-aromatic amino acid symporters in the model alimentary canal of mosquito larvae. The Journal of experimental biology. 2008;211:1594–1602. doi: 10.1242/jeb.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. The Journal of biological chemistry. 2006;281:11167–11176. doi: 10.1074/jbc.M601517200. [DOI] [PubMed] [Google Scholar]
- Paulsen IT, Ren QH, Chen KX. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic acids research. 2007;35:D274–D279. doi: 10.1093/nar/gkl925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Deitsch KW, Sappington TW. Culture and analysis of the insect fat body. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman & Hall; London: 1997. pp. 507–522. [Google Scholar]
- Remm M, Koressaar T. Enhancements and modifications of primer design program Primer3. Bioinformatics (Oxford, England) 2007;23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- Shearn A, Martin JF, Hersperger E, Simcox A. minidiscs encodes a putative amino acid transporter subunit required non-autonomously for imaginal cell proliferation. Mechanisms of development. 2000;92:155–167. doi: 10.1016/s0925-4773(99)00338-x. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]