Abstract
Clinical studies suggest that stress-related biobehavioral factors can accelerate progression of hematopoietic cancers such as acute lymphoblastic leukemia (ALL), but it is unclear whether such effects are causal or what biological pathways mediate such effects. Given the network of sympathetic nervous system (SNS) fibers that innervates the bone marrow to regulate normal (non-leukemic) hematopoietic progenitor cells, we tested the possibility that stress-induced SNS signaling might also affect ALL progression. In an orthotopic mouse model, Nalm-6 human pre-B ALL cells were transduced with the luciferase gene for longitudinal bioluminescent imaging and injected i.v. into male SCID mice for bone marrow engraftment. Two weeks of daily restraint stress significantly enhanced ALL tumor burden and dissemination in comparison to controls, and this effect was blocked by the β-adrenergic antagonist, propranolol. Although Nalm-6 ALL cells expressed mRNA for β1- and β3-adrengeric receptors, they showed no evidence of cAMP signaling in response to norepinephrine, and norepinephrine failed to enhance Nalm-6 proliferation in vitro. These results show that chronic stress can accelerate the progression of human pre-B ALL tumor load via a β-adrenergic signaling pathway that likely involves indirect regulation of ALL biology via alterations in the function of other host cell types such as immune cells or the bone marrow microenvironment.
Keywords: restraint stress, sympathetic nervous system, β-adrenergic receptor, propranolol, acute lymphoblastic leukemia, pediatrics, hematopoiesis, bioluminescent imaging
1. Introduction
Clinical and epidemiological studies have shown that stress-related biobehavioral factors are associated with accelerated progression of several types of cancer, including solid epithelial tumors and hematopoietic tumors such as leukemia (Antoni et al., 2006; Chida et al., 2008). Most experimental research on the biological mechanisms of such effects has been performed in the context of solid epithelial tumors (Armaiz-Pena et al., 2009; Thaker & Sood, 2008), which involve different molecular pathways from those in hematopoietic cancers (Vogelstein & Kinzler, 2002). Among the hematopoietic cancers that have been examined in experimental animal models, most studies have focused on leukemia and have found contradictory effects of stress on survival.
Early studies of stress effects on leukemia in animal models focused on infection with murine viruses that induced leukemic disease specific to the species. One study found a protective effect of chronic shock avoidance stress on the incidence and survival of Rauscher virus-induced leukemia (Jensen, 1968; Rasmussen, 1969). Another study found that restraint stress delayed Friend virus-induced incidence of erythroleukemia but only in males (Gotoh et al., 1986). In contrast, a more recent series of studies involving experimental injection of a leukemic rat NK cell line found that an acute period of forced swim stress or adrenaline injection at the time of leukemia cell injection increased subsequent mortality rates via a β-adrenergic receptor–dependent mechanism (Avraham et al., 2006; Ben-Eliyahu et al., 1999; Inbar et al., 2011). The heterogeneity of results across separate reports may stem from differences in the leukemia model systems used (e.g., viral induction of leukemia, progression of already established NK leukemia), differences in the outcomes analyzed (e.g., tumor incidence, survival time), and/or the specific stress paradigms utilized (e.g., repeated shock, restraint, acute swim stress).
As previous animal models of leukemia have used time to morbidity or mortality rate as their outcomes, it remains unclear whether animals died sooner because stress increased the total leukemia tumor burden or because stress increased vulnerability to the lethal downstream sequelae of leukemia tumor burden, which may include infection, hemorrhage, and asphyxia. Most human leukemias stem from a malignant myeloid or lymphoid progenitor cell that colonizes the bone marrow at the expense of normal progenitor cells, resulting in the failure of normal hematopoiesis and the onset of life-threatening lymphopenia, thrombocytopenia, and anemia (Sawyers et al., 1991). Accurately quantifying the total tumor burden of such “liquid tumors” has historically been difficult, because, like normal lymphocytes, leukemia cells originating in the bone marrow may circulate throughout the body and home to a variety of distant tissue sites, including lungs and the central nervous system. However, recent advances in bioluminescence-based imaging allow repeated longitudinal measurement of growing tumor burdens within individual hosts and have provided new insights into the biological pathways by which stress can affect the growth of solid epithelial tumors such as breast cancer (Sloan et al., 2010).
We sought to harness this bioluminescent approach in the present study to directly quantify the effects of stress on tumor burden and dissemination in an orthotopic mouse model of human leukemia. Human leukemias are broadly grouped into four types based on the kinetics of disease development and the malignant progenitor cell type. These include chronic myeloid leukemia, acute myeloid leukemia, chronic lymphoblastic leukemia, and acute lymphoblastic leukemia (Leukemia & Lymphoma Society, 2010). Of the four types, acute lymphoblastic leukemia (ALL) is the most common type of cancer in young children, and pre-B cell ALL is the most prevalent specific form of leukemia in children and adolescents (Kolenova et al., 2010; Pui et al., 2008; Pui, 2009). Thus, we utilized a well-established mouse xenograft model of pre-B ALL (Sipkins et al., 2005; Colmone et al., 2008) that recapitulates key features of human leukemia pathogenesis, including orthotopic bone marrow colonization, hematopoietic failure, systemic dissemination, and neurological-motor deficits. Given the well-described sympathetic nervous system (SNS) innervation of the bone marrow (Felten & Felten, 1991; Nance & Sanders, 2007), the presence of β-adrenergic receptors on both leukemia cells (Mamani-Matsuda et al., 2004) and normal lymphocytes (Nance & Sanders, 2007), β-adrenergic regulation of hematopoietic stem and progenitor cell function (Katayama et al., 2006; Mendez-Ferrer et al., 2008), and the involvement of β-adrenergic mechanisms in the progression of other tumors (Glasner et al., 2010; Inbar et al., 2011; Melamed et al., 2005; Sloan et al., 2010; Thaker et al., 2006), we also examined the role of β-adrenergic signaling as a potential mediator of stress effects on pre-B ALL tumor burden and dissemination.
2. Methods
2.1. Animals
Male SCID mice (Charles River Laboratories), 6–8 weeks of age, were maintained under BSL2 barrier conditions and housed on an individually ventilated cage (IVC) rack in dual filter disposable cages (Innovive, Inc.), in groups of 4–5 per cage, with corn cob bedding and ad libitum access to food and water on a 12:12 light/dark cycle at 22°C. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
2.2. In vivo model of acute lymphoblastic leukemia
Human Nalm-6 pre-B ALL cells were transduced with the FUhlucW lentiviral vector containing firefly luciferase under the control of the constitutively active ubiquitin-C promoter (Morizono et al., 2005) and cultured in RPMI-1640 with L-glutamine (Cellgro-Mediatech, Inc, #10-040-CV) supplemented with 10% FBS (Atlanta Biologicals, #S11550H), 100 IU penicillin/mL, 100μg streptomycin/mL (Cellgro-Mediatech, Inc., #30-002-CI), at 37°C, 5% CO2. 5 × 106 cells were injected into the lateral tail vein for bone marrow engraftment as previously described (Sipkins et al., 2005). Tumor burden and dissemination of the cells were tracked in live mice by repeated noninvasive optical imaging of leukemia-specific luciferase activity using an IVIS Lumina II system coupled to a desktop computer running Living Image 4.0 software (Caliper Life Sciences). After anesthetization with 2% isofluorane and intravenous injection of 150 mg/kg luciferin, mice were photographed under bright-field illumination and images were overlaid with luminescence data gathered over the maximum exposure period without pixel saturation (0.5–300 seconds).
Flow cytometry was used to examine the proportion of human Nalm-6 ALL cells to normal mouse leukocytes in femoral bone marrow based on Nalm-6 cell expression of human CD10 (Ganju et al., 1996). Marrow was collected with cold phosphate buffered saline without calcium and magnesium (PBS) (Cellgro-Mediatech, Inc., #21-031-CV), supplemented with 1% FBS, and subjected to red blood cell lysis buffer (BD Biosciences, #555899). White blood cells were incubated with PE-conjugated antibodies against human CD10 (BD Biosciences, #555375), washed, and analyzed on a FACSAria II High-Speed Cell Sorter with FACSDiva software (BD Biosciences) for analysis of total live lymphocytes from gating based on forward- versus side-scatter profiles.
2.3. Chronic restraint stress
Mice were randomly assigned to home cage control conditions or 2 hours per day restraint for 14 consecutive days commencing on the day after leukemia cell injection. Mice were restrained in a confined space that prevented them from moving freely but did not press on them (Thaker et al., 2006). This paradigm has been shown to induce chronic stress as shown by neuroendocrine activation (Thaker et al., 2006; Manni et al., 2008), weight loss (Smagin et al., 1999), and anxiety-like behaviors (Hermann et al., 1994). Four experiments were completed, with n = 4 or 5 mice per group (stress vs. control) for each experiment.
2.4. β-adrenergic receptor blockade
Propranolol hydrochloride (Sigma, #P8688) in PBS vehicle, or an equivalent volume of vehicle alone for placebo, was administered via Alzet osmotic mini-pumps (DURECT Corporation) implanted subcutaneously on the lateral dorsal side of the mouse near the scapula to deliver a dose of 2mg/kg/d beginning 8 days before initiation of restraint stress. Three experiments with propranolol blockade were conducted and included the following groups: (1) stress + propranolol; (2) stress + placebo; (3) control + placebo. For each experiment, n = 4 or 5 mice per group. To test for an effect of propranolol alone, the last of the three experiments utilized a fourth group, (4) control + propranolol (n = 5).
2.5. β-adrenergic receptor gene expression
To assess expression of mRNA for human β1-, β2-, and β3-adrenergic receptors in Nalm-6 ALL cells, total RNA from 3 × 106 cells was extracted (Qiagen RNeasy Mini Kit, #74104) and cleared of contaminating DNA with on-column DNase digestion (Qiagen RNase-Free DNase Set, #79254). Resulting RNA was assayed by quantitative one-step RT-PCR (Qiagen Quantitect Probe RT-PCR, #204443) using a sequence-specific primer-probe set for each gene (Applied Biosystems TaqMan Gene Expression Assays; ADRB1, #Hs02330048_s1; ADRB2, #Hs00240532_s1; ADRB3, #Hs00609046_m1). Two independent RT-PCR runs were completed using triplicate determinations for each gene on each run according to the manufacturer's specified protocol on a Bio-Rad iCycler Real-Time Detection System, with adrenergic receptor threshold cycle numbers normalized to values of β-actin mRNA amplified in parallel (ACTB, #Hs99999903_m1) and expressed relative to values from a no-template control.
2.6. β-adrenergic receptor signaling
To determine the functionality of β-adrenergic receptors on Nalm-6 ALL cells, intracellular cyclic adenosine monophosphate (cAMP) signaling was measured at 0, 5, and 15 minutes after stimulation with 10 μM norepinephrine (Sigma, #A9512), as previous studies indicate optimal leukocyte activation of cAMP flux by 5 min with that concentration (Cole et al., 1998; 1999). Vehicle-treated cells served as a negative control, and cells stimulated with forskolin (1 μM) served as a positive control. Three independent experiments were conducted, and cAMP levels for each condition at each time point were assayed in duplicate by ELISA according to the manufacturer's instructions (R&D Systems, #KGE002B) on a colorimetric microplate reader (Molecular Devices).
2.7. In vitro proliferation
A potential direct effect of β-adrenergic signaling on Nalm-6 ALL proliferation was tested by treating cells in vitro with norepinephrine at 1μM or 10μM. Proliferation was assessed by hemocytometry at 2, 6, 12, 24, 48, 72, and 96 hours after treatment. In each of three independent experiments, duplicate samples were prepared for each condition at each time point.
2.8. Statistical analysis
Data are presented as mean ± SE, and all statistical analyses were carried out using SAS version 9.2 (SAS Institute Inc.). All distributions were examined for outliers and non-normality, and log transformations were applied to normalize distributions where necessary. Mixed-effects linear models (SAS PROC MIXED) were used to analyze datasets pooled across experiments to assess the overall effect of chronic restraint stress on the longitudinal growth trajectory of ALL, to determine whether those effects were modified by pharmacologic intervention with propranolol, to determine whether norepinephrine increased intracellular cAMP levels, and to determine whether norepinephrine acted directly to increase proliferation of ALL in vitro.
3. Results
3.1. The Nalm-6 acute lymphoblastic leukemia model
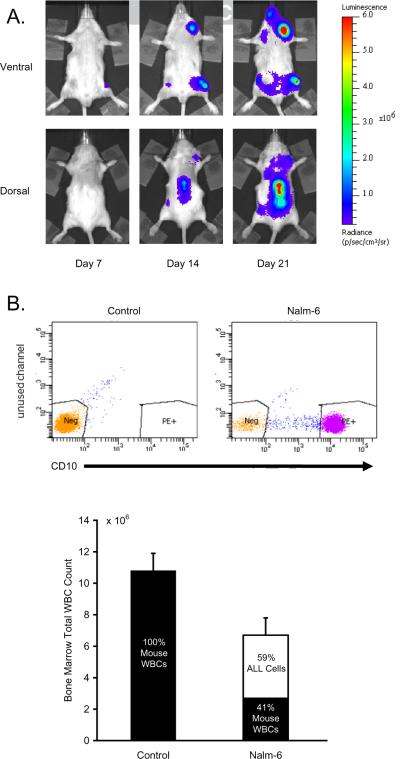
To assess the effect of chronic stress on tumor progression in a mouse model of human pre-B cell ALL, we used in vivo bioluminescent imaging to track the colonization of bone marrow and whole body dissemination of luciferase-tagged Nalm-6 cells over a course of 3 weeks following tumor cell inoculation (Figure 1A). Recapitulating human ALL dynamics, Nalm-6 cells engrafted in femoral bone marrow and other typical ALL target tissues, including lungs, spleen, liver, spinal column, and calvarium. Consistent with progressive hematopoietic failure, examination of femoral bone marrow revealed a reduction in the number of total white blood cells, with flow cytometric analysis indicating that 59% ± 8% of these cells were human Nalm-6 ALL cells rather than normal mouse leukocytes (Figure 1B).
Figure 1.
Nalm-6 ALL Model. (A) Nalm-6 pre-B ALL cells were localized and quantified by periodic imaging of leukemia-specific bioluminescence signal from ventral and dorsal sides of SCID mice. (B) Femoral bone marrow white blood cells (WBC) and CD10+ Nalm-6 ALL cells were quantified by flow cytometry.
3.2. Effects of chronic restraint stress
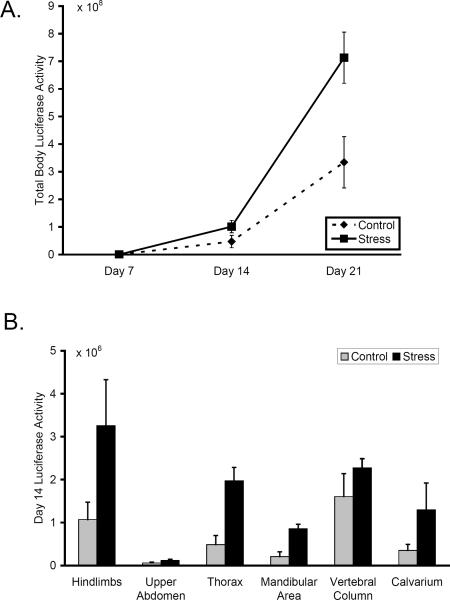
Chronic restraint stress significantly increased Nalm-6 ALL tumor burden in comparison to control mice (mixed effect longitudinal analysis pooling across 4 independent experiments, average difference 90% ± 38% in whole-body bioluminescence signal, p < .001) (Figure 2A). Stress had no detectable effect on ALL progression at Day 7, but by Day 14, tumor burden in animals subject to chronic restraint stress increased by an average of 111% ± 28% relative to unstressed controls (p = .003). As presented in Figure 2B, sub-analyses at this timepoint showed that chronic restraint stress increased tumor burden in multiple separate body regions, including the hindlimbs (p = .004), thorax (p = .001), mandibular area (p = .046), and calvarium (p = .027). Stress had no significant effect on tumor burden in the upper abdomen (p = .436) and vertebral column (p = .241). At Day 21, 7 days after the cessation of restraint stress, whole body tumor burden continued to show elevation in stressed animals (average difference: 92% ± 16%, p < .001). Separate body regions continued to show the same pattern of effects at Day 21; hindlimbs (p < .001), thorax (p = .048), mandibular area (p = .034), calvarium (p = .063), upper abdomen (p = .459), vertebral column (p = .484) (data not shown).
Figure 2.
Effect of chronic restraint stress on ALL progression. (A) 21-day longitudinal growth curves for total body Nalm-6 tumor burden in control and restraint-stressed animals. (B) Nalm-6 tumor burden in separate body areas at Day 14 in control and restraint-stressed animals. Data in each panel is representative of 4 independent experiments.
3.3. Role of β-adrenergic signaling
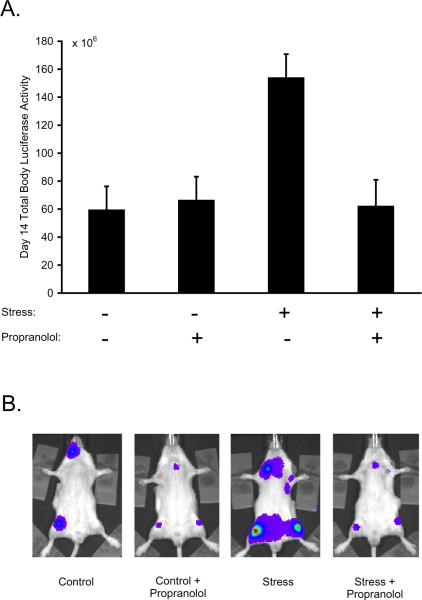
To determine whether β-adrenergic receptor activation mediated stress-enhanced ALL growth and dissemination, restraint-stressed mice were treated with the non-selective β-adrenergic antagonist, propranolol, beginning 8 days before initiation of restraint stress and continuing throughout the experimental period. As shown in Figure 3, there was a significant stress × propranolol interaction effect on total ALL tumor burden (p = .006). Propranolol significantly abrogated stress-enhanced ALL progression by the end of the stress period (average suppression: 97% ± 19%, p = .025). In propranolol-treated mice, stress-exposed animals showed no significant difference in total leukemia growth from control animals (average difference: 3% ± 19%, p = .923). In the absence of stress, propranolol had no significant effect on total Nalm-6 tumor progression compared to placebo (average difference: 44% ± 43%, p = .349). We found no evidence that the effect of propranolol on stress-enhanced tumor burden differed significantly across separate body regions (stress × propranolol × region interaction, p = .427).
Figure 3.
β-adrenergic signaling in stress-enhanced ALL progression. (A) Nalm-6 ALL was quantified in control mice vs. stress mice that were treated with or without the β-blocker, propranolol; data representative of 3 independent experiments. (B) Representative images of mice in the control (+ placebo), control + propranolol, stress (+ placebo) and stress + propranolol groups.
3.4. Direct β-adrenergic regulation of Nalm-6 ALL function
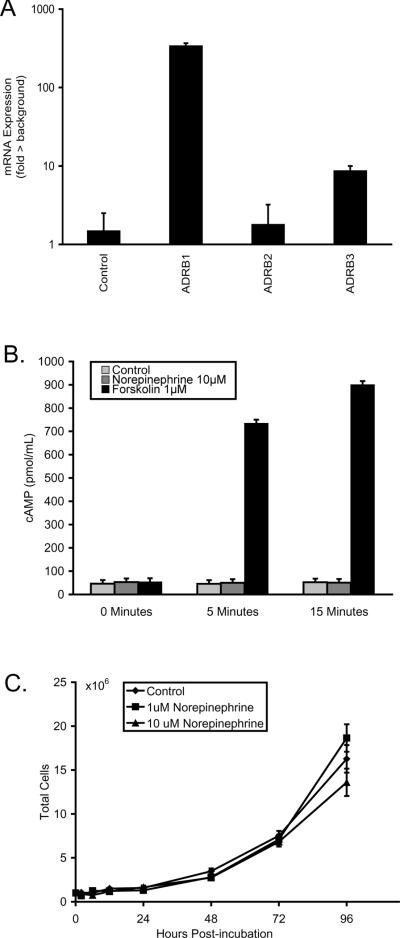
To determine whether β-adrenergic signaling might directly regulate Nalm-6 ALL cell biological function, we first examined β-adrenergic receptor expression. RT-PCR analysis showed expression of mRNA encoding β1- and β3-adrenergic receptors in Nalm-6 ALL cells, but minimal expression of mRNA for β2-adrenergic receptors (Figure 4A). To determine whether Nalm-6 cells responded functionally to β-adrenergic receptor stimulation, we examined intracellular cAMP flux after norepinephrine stimulation in vitro. As presented in Figure 4B, norepinephrine-stimulated cells showed no significant cAMP flux relative to unstimulated controls (all differences < 43% ± 14%, all p > .137). In contrast, forskolin induced significant accumulation of cAMP (all differences > 1857% ± 240%, all p < .001), indicating a robust capacity for cAMP signaling in Nalm-6 ALL cells. To definitively determine whether β-adrenergic signaling directly affected Nalm-6 ALL cell proliferation, we carried out 4-day longitudinal growth curve assays using physiologically relevant concentrations of norepinephrine (1 μM and 10 μM). No significant difference in proliferation was detected between norepinephrine-treated cells and vehicle-treated control cultures (condition × time interaction p = .279; Figure 4C).
Figure 4.
Nalm-6 ALL β-adrenergic receptor gene expression and functionality. (A) Gene expression for β1-, β2-, and β3-adrenergic receptors (ADRB1, ADRB2, ADRB3, respectively) was quantified in Nalm-6 human ALL by qRT-PCR. (B) cAMP flux in Nalm-6 ALL cells after stimulation with norepinephrine or forskolin. (C) 4-day longitudinal proliferation of Nalm-6 ALL cells in vitro after stimulation with norepinephrine.
4. Discussion
Chronic restraint stress significantly enhanced pre-B ALL tumor burden and dissemination in a well-established mouse xenograft model of the most prevalent form of human pediatric leukemia. Pharmacologic inhibition studies showed that stress effects were mediated by β-adrenergic signaling in vivo. However, we found no evidence that those in vivo growth and dissemination effects stemmed from direct stimulation of Nalm-6 ALL cell proliferation by β-adrenergic signaling. Thus, the effect of chronic stress on ALL progression in the present model appears to be indirectly mediated by other host cell types that interact with Nalm-6 human ALL cells. Such effects could potentially include SNS regulation of anti-tumor immune responses (Avraham et al., 2006; Ben-Eliyahu et al., 1999; Inbar et al., 2011) and/or SNS regulation of bone marrow stromal cells such as osteoblasts that play a key role in supporting growth and differentiation of healthy hematopoietic cells (Katayama et al., 2006; Mendez-Ferrer et al., 2008). Identification of the specific cellular and molecular mediators of such SNS effects represents an important topic for future research. However, the present data do suggest that pharmacologic antagonism of stress-induced β-adrenergic signaling could represent a novel adjuvant therapeutic strategy for inhibiting such effects.
Mechanistic investigations have documented a key role of β-adrenergic signaling in mediating stress effects on progression of several types of solid tumor (Sloan et al., 2010; Stefanski & Ben-Eliyahu, 1996; Thaker et al., 2006). However, the only previous data on the role of the SNS in mediating stress effects on hematopoietic tumors come from a line of research on malignant NK cell proliferation in rats, in which the non-selective β-adrenergic antagonist, nadolol, blocked the effects of an acute swim stress at the time of cancer injection on survival rates (Inbar et al. 2011). That line of research also showed that non-selective β-adrenergic antagonism increased survival rates in the absence of acute swim stress. In contrast, the present study found no effect of β-adrenergic antagonism on ALL progression in the absence of stress. Differences in the specific types of leukemia cells examined and/or the interaction between those cells and the host microenvironment (e.g., bone marrow stromal cells, immune cells, etc.) may account for the differing effects of β-adrenergic inhibition on basal tumor burden growth (i.e., in the absence of stress). Thus, although it will be important to determine if the present in vivo results apply to other ALL subtypes, the results are consistent with a growing body of literature that suggests SNS regulation of tumor biology may represent a relatively general physiologic influence on cancer progression, and, therefore, a broadly applicable target for health protective interventions in the context of human cancer (Antoni et al., 2006).
Findings from the present in vitro studies suggested that β-adrenergic blockade of stress-enhanced ALL progression was not likely due to a direct effect of adrenergic ligands on Nalm-6 ALL cell signaling or proliferation. Such findings are consistent with previous experimental investigations of ovarian cancer (Thaker et al., 2006) and breast cancer (Madden et al., 2011) that showed β-adrenergic signaling influenced cancer cell progression in vivo but did not directly affect cell proliferation in vitro. One potential indirect pathway through which β-adrenergic signaling might regulate Nalm-6 tumor burden involves NK cell-mediated killing of leukemia cells (e.g., as observed for the malignant rat CRNK-16 cell line; Avraham et al., 2006; Inbar et al., 2011). Previous studies indicate that human Nalm-6 ALL and primary ALL samples are generally resistant to human NK cell-mediated killing (Romanski et al., 2005), suggesting that other mechanisms may contribute to the present results. However, it remains possible that murine NK cell responses to human ALL cell lines may have been modulated in the present model system. Future studies could address this issue further by analyzing stress effects on human ALL progression in SCID-Beige mice that lack functional NK cells.
An alternative indirect pathway by which β-adrenergic signaling may affect ALL cell biology involves sympathetic innervation of the stem cell niche in bone marrow. SNS activity regulates both endogenous circadian mobilization and pharmacological cytokine-prompted mobilization of normal (non-leukemic) progenitor cells through β-adrenergic signaling in adjacent stromal cells (Katayama et al., 2006; Mendez-Ferrer et al., 2008). Thus, it is plausible that bone marrow stromal β-adrenergic signaling may similarly regulate proliferation and mobilization of malignant pre-B cells in ALL. Identification of the specific stromal cell type(s) sensitive to β-adrenergic signaling and definition of the biological interactions by which they support Nalm-6 ALL cell proliferation and dissemination represent important areas for further study.
Previous reports have shown that children and adolescents with ALL manifest high rates of psychological distress as a result of both initial diagnosis and subsequent medical procedures to treat the disease (Kazak et al., 1995; Willingham-Piersol et al., 2008). Adolescent survivors of ALL often manifest higher levels of anxiety in the wake of the disease than their healthy peers (Shelby et al., 1998). Given that the recurrence of ALL remains a significant challenge to medical management of the disease (Nahar & Müschen, 2009), the present results raise the possibility that cancer-related anxiety might contribute to disease recurrence and that pharmacologic inhibition of such effects at the level of the β-adrenergic receptor may represent an effective strategy for managing those effects in patients with pre-B ALL who are in remission or still have active disease.
In summary, the present study demonstrates that chronic stress can enhance the progression of human pre-B cell acute lymphoblastic leukemia in an orthotopic mouse model through an indirect pathway that is regulated by β-adrenergic signaling. These effects suggest novel strategies for protecting ALL patients from the potential biological effects of cancer-related anxiety and other types of stress on leukemia relapse and progression.
*Highlights.
A new light-based tracking system is used to document β-adrenergic-mediated stress effects on human tumor growth in an orthotopic mouse model of pediatric leukemia.
Acknowledgements
We thank David Stout and Waldemar Ladno at the UCLA Crump Preclinical Imaging Center. Flow cytometry was performed with the assistance of Iris Williams in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by NIH awards CA16042 and AI28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. This project was also supported in part by NIH grants T32-MH19925, CA138687, CA116778, and funding from the UCLA Cousins Center for Psychoneuroimmunology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–5. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Inbar S, Rosenne E, Ben-Eliyahu S. Autologous control of a highly malignant syngeneic CRNK-16 leukemia in the rat: a role for NK cells. Cancer Immunol Immunother. 2006;55:1348–57. doi: 10.1007/s00262-006-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–8. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Cole SW, Jamieson BD, Zack JA. cAMP up-regulates cell surface expression of lymphocyte CXCR4: implications for chemotaxis and HIV-1 infection. J Immunol. 1999;162:1392–400. [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–6. [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–5. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Felten SY, Felten DL. Innervation of lymphoid tissue. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Second Academic Press; San Diego: 1991. pp. 27–68. [Google Scholar]
- Ganju RK, Shpektor RG, Brenner DG, Shipp MA. CD10/neutral endopeptidase 24.11 is phosphorylated by casein kinase II and coassociates with other phosphoproteins including the lyn src-related kinase. Blood. 1996;88:4159–65. [PubMed] [Google Scholar]
- Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Lee G, Yoshikura H. Effects of physical restraint on leukemogenesis by Friend virus. Jpn J Cancer Res. 1986;77:985–91. [PubMed] [Google Scholar]
- Hermann G, Beck FM, Tovar CA, Malarkey WB, Allen C, Sheridan JF. Stress-induced changes attributable to the sympathetic nervous system during experimental influenza viral infection in DBA/2 inbred mouse strain. J Neuroimmunol. 1994;53:173–80. doi: 10.1016/0165-5728(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6:e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MM. The influence of stress on murine leukemia virus infection. Proc Soc Exp Biol Med. 1968;127:610. doi: 10.3181/00379727-127-32754. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Boyer BA, Brophy P, Johnson K, Scher CD, Covelman K, Scott S. Parental perceptions of procedure-related distress and family adaptation in childhood leukemia. Child Health Care. 1995;24:143–58. doi: 10.1207/s15326888chc2403_1. [DOI] [PubMed] [Google Scholar]
- Kolenova A, Hikkel I, Ilencikova D, Hikkelova M, Sejnova D, Kaiserova E, Cizmar A, Puskacova J, Bubanska E, Oravkinova I, Gencik M. Minimal residual disease detection using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the non-MRD-based ALL IC-BFM 2002 protocol for childhood ALL: Slovak experience. Neoplasma. 2010;57:552–61. doi: 10.4149/neo_2010_06_552. [DOI] [PubMed] [Google Scholar]
- Leukemia & Lymphoma Society . Facts 2010–2011. The Leukemia & Lymphoma Society; White Plains, NY: 2010. [Google Scholar]
- Madden KS, Szpunar MJ, Brown EB. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747–58. doi: 10.1007/s10549-011-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamani-Matsuda M, Moynet D, Molimard M, Ferry-Dumazet H, Marit G, Reiffers J, Mossalayi MD. Long-acting beta2-adrenergic formoterol and salmeterol induce the apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol. 2004;124:141–50. doi: 10.1046/j.1365-2141.2003.04746.x. [DOI] [PubMed] [Google Scholar]
- Manni L, Di Fausto V, Fiore M, Aloe L. Repeated restraint and nerve growth factor administration in male and female mice: effect on sympathetic and cardiovascular mediators of the stress response. Curr Neurovasc Res. 2008;5:1–12. doi: 10.2174/156720208783565654. [DOI] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–52. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Nahar R, Müschen M. Pre-B cell receptor signaling in acute lymphoblastic leukemia. Cell Cycle. 2009;8:3874–7. doi: 10.4161/cc.8.23.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH. Acute lymphoblastic leukemia: introduction. Semin Hematol. 2009;46:1–2. doi: 10.1053/j.seminhematol.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen AF., Jr Emotions and immunity. Ann N Y Acad Sci. 1969;164:458–62. doi: 10.1111/j.1749-6632.1969.tb14060.x. [DOI] [PubMed] [Google Scholar]
- Romanski A, Bug G, Becker S, Kampfmann M, Seifried E, Hoelzer D, Ottmann OG, Tonn T. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol. 2005;33:344–52. doi: 10.1016/j.exphem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Denny CT, Witte ON. Leukemia and the disruption of normal hematopoiesis. Cell. 1991;64:337–50. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Shelby MD, Nagle RJ, Barnett-Queen LL, Quattlebaum PD, Wuori DF. Parental reports of psychosocial adjustment and social competence in child survivors of acute lymphocytic leukemia. Child Health Care. 1998;27:113–29. [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr., Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–8. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol Behav. 1996;60:277–82. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin Cancer Biol. 2008;18:164–70. doi: 10.1016/j.semcancer.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. The genetic basis of human cancer. 2nd New York; McGraw Hill: [Google Scholar]
- Willingham-Piersol L, Johnson A, Wetsel A, Holtzer K, Walker C. Decreasing psychological distress during the diagnosis and treatment of pediatric leukemia. J Pediatr Oncol Nurs. 2008;25:323–30. doi: 10.1177/1043454208323293. [DOI] [PubMed] [Google Scholar]