Abstract
Sphingosine 1-phosphate (S1P) is a key immune mediator regulating migration of immune cells to sites of inflammation. S1P actions are mediated by a family of five G protein-coupled receptors. Sensory neurons express many of these receptors, and in vitro S1P has excitatory effects on small-diameter sensory neurons, many mediated by the S1P receptor 1 (S1PR1). This study investigated the role of S1P in regulating the sensitivity of DRG neurons. We found that in vivo perfusion of the normal L5 DRG with S1P increased mechanical sensitivity. Microelectrode recordings in isolated whole ganglia showed that large- and medium-diameter cells, as well as small-diameter cells, increased firing in the presence of S1P. To further determine the role of S1PRs, we examined the effects of in vivo S1PR1 knockdown in the L4 and L5 sensory ganglia. Small interfering RNA directed against S1PR1 did not affect baseline mechanical sensitivity in normal animals, in which S1P levels are expected to be low. However, when the L5 ganglion was locally inflamed, a procedure that leads to rapid and sustained mechanical hypersensitivity, S1PR1 siRNA injected animals showed significantly less hypersensitivity than animals injected with scrambled siRNA. Reduced expression of S1PR1, but not S1PR2 or S1PR3, was confirmed with qPCR methods. The results indicate that the S1PR1 receptors in sensory ganglia cells may play an important role in regulating behavioral sensitivity during inflammation.
Keywords: sphingosine 1-phosphate, S1PR1, inflammation, dorsal root ganglion
INTRODUCTION
Sphingosine 1-phosphate (S1P) is released from various activated immuno-competent cells under inflammatory conditions and is critically involved in their chemotaxis and migration. S1P is the endogenous ligand for a family of five G protein-coupled receptors that were originally named Edg receptors, now known as S1P receptors (S1PR1-5) [7, 8]. In vitro, S1P can enhance excitability of small-diameter capsaicin-sensitive rat sensory neurons, increasing the number of action potentials evoked by injected current. Similarly, S1P augments the current conducted by TRPV1 [6]. G-protein coupled receptors mediate these effects. Expression of S1PR1, 2, 3, and 4 has been observed with PCR methods from both whole DRG and cultured sensory neurons [13]. The S1PR1 receptor plays an important but not exclusive role in mediating these excitatory effects; approximately 50% of small diameter cells express this receptor and respond to the S1PR1-specific ligand, SEW2871, while another 25% are excited by S1P but not SEW2871 [3]. Thus, the S1P system may be an important contributor to the general phenomenon of sensory neuron sensitization by inflammation.
Here, we examined possible roles of S1P and S1PR1 in pain behaviors in vivo. To examine the functional role of the receptor in the context of inflammation, we used a pain model in which the L5 DRG is inflamed by local application of the immune stimulator zymosan in Incomplete Freund’s Adjuvant. This gives a rapid and prolonged increase in mechanical sensitivity of the hindpaw, as well as macrophage infiltration, activation of satellite glial cells as measured by GFAP expression, elevation of pro-inflammatory cytokines, and increased neuronal spontaneous activity[12]. Previous in vitro studies of S1P effects on sensory neurons have focused on small-diameter capsaicin-sensitive neurons, but larger diameter myelinated neurons may also play important roles in mediating pain behaviors in various rodent pain models[4], including the model used in this study [9]. Therefore we also examined effects of S1P on excitability of large- and medium-diameter cells.
MATERIAL AND METHODS
Animals and Behavior testing
Adult male Sprague Dawley rats weighing 150 – 200 g (Harlan, Indianapolis, USA) were housed in groups of two under a 12-h light/dark cycle, with food and water ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and was in accord with the EC Directive 86/609/EEC. Mechanical sensitivity of the hindpaw was examined with von Frey filaments. Initially (Fig 1), we tested 6 different regions of the hindpaw including the heel region, and fit the percent withdrawal responses with the Hill equation [11]. In later experiments (Fig. 3), mechanical sensitivity was tested in the heel region of the paw, using the up-and-down method [2]; this test point is sensitized in the pain model used [9].
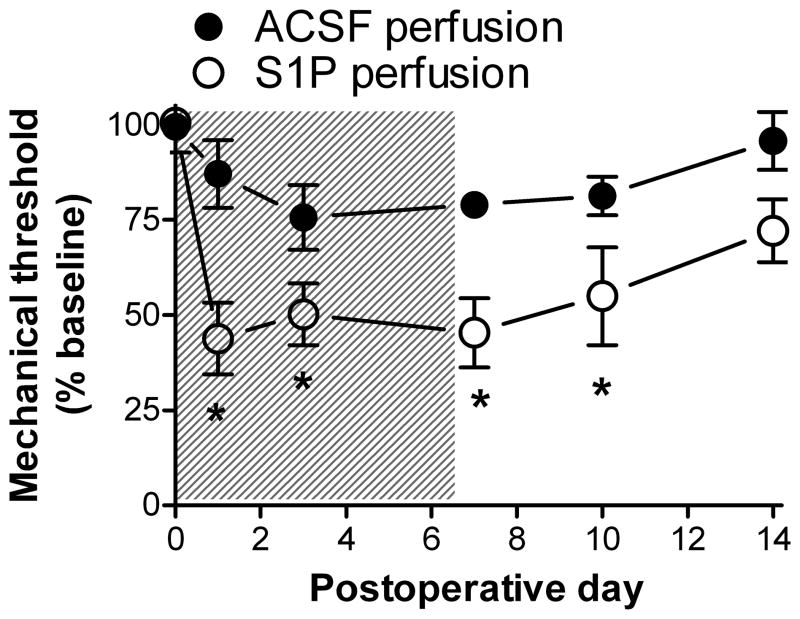
Figure 1.
In vivo local perfusion of the L5 DRG with S1P increases mechanical sensitivity of the ipsilateral hindpaw. Perfusion with 100 μM S1P or vehicle (ACSF) at 1μL/hour occurred during the first 7 days (shaded area). The baseline plotted on day 0 is the average of 3 measurements made on preceding days. Data were normalized to the average baseline value for all animals, which did not differ between the two groups. *, significant difference between S1P and ACSF groups (2 way ANOVA with Bonferroni posttest). In all figures the significance level is indicated by the number of symbols (see Methods). N = 4 animals per group.
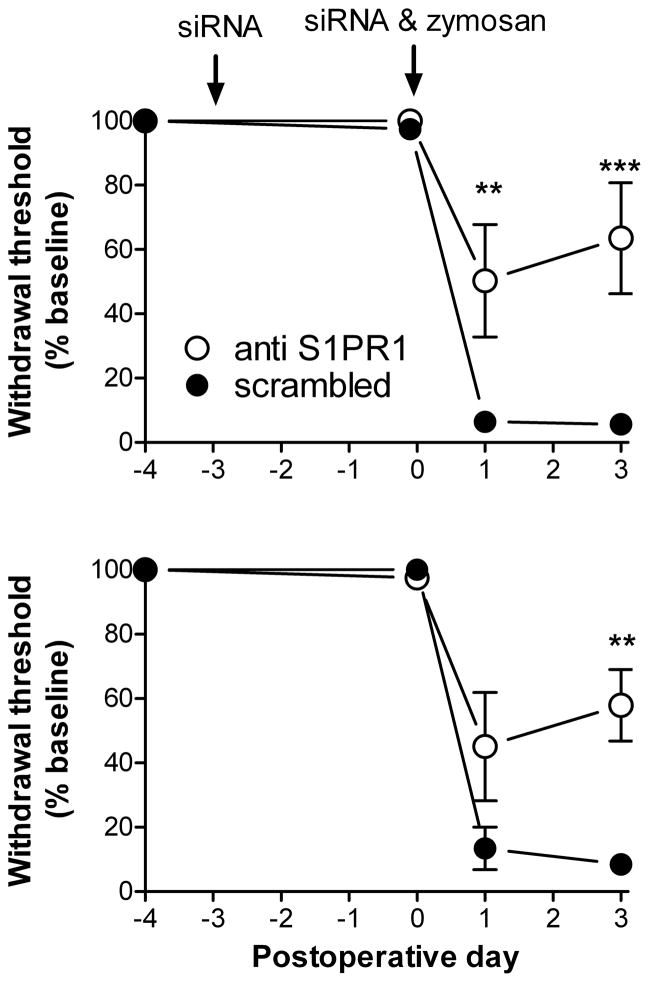
Figure 3.
Knockdown of S1PR1 in vivo reduces hypersensitivity after DRG inflammation. Baseline mechanical sensitivity was measured on POD -6 and POD -5; the average is plotted on POD -4. Data were normalized to this average baseline value for all animals, which did not differ between the two groups. Top: S1PR1 siRNA or scrambled control siRNA was injected into the L4 and L5 DRG on POD -3 (arrow). Mechanical sensitivity was measured on POD 0, and immediately afterwards the L5 DRG was inflamed (“zymosan”) and second injections of siRNA were made (2nd arrow). *, significant difference in mechanical sensitivity (2 way ANOVA with Bonferroni posttest). N = 4 animals per group. RNA was isolated immediately after POD 3 measurements. Bottom: similar results were obtained injecting the siRNA into the cauda equina (N = 6 per group; only 2 per group measured on POD1).
In vivo DRG inflammation, drug and siRNA application
For experiments perfusing the DRG with S1P in vivo, a small hole was drilled in the transverse process, into which silicon tubing (Silas, Dow Corning) was introduced connected to an osmotic pump ( Cupertino, CA, USA) implanted beneath the skin on the lower back. The pump was filled with S1P (100 μM; Avanti Polar Lipids, Alabaster, AL, USA ) or vehicle (artificial cerebrospinal fluid; ACSF). For some experiments, the L5 DRG was inflamed by injecting the immune activator zymosan (2 mg/ml, 10 μl, in Incomplete Freund’s Adjuvant) beneath the L5 intervertebral foramen under isoflurane anesthesia, as previously described [9]. For in vivo siRNA application, siRNA was made up with transfection reagent and 5% glucose (final concentration), and 3 μL aliquots were injected into the L4 and L5 DRG, 80 pmoles of siRNA per injection. The transfection reagent was cationic linear polyethylenimine (PEI) optimized for in vivo experiments (“in vivo JetPEI”, Polyplus Transfection, WVR Scientific, USA) used at a nitrogen/phosphorus ratio of 8. The injection was made through a small glass needle made by pulling an electrode from 1.2 mm o.d. borosilicate glass, breaking off the tip to 75 μm o.d., and bending 90° by heating. The needle was connected to a tuberculin syringe and inserted close to the DRG through a small hole cut into the overlying membrane close to the site where the dorsal ramus exits the spinal nerve. siRNA constructs were from Dharmacon (Lafayette, CO, USA). We chose to inject siRNA into both L4 and L5 DRG because there may be some spread of the zymosan/IFA from L5 into L4, and because the hindpaw receives innervation from both L5 and L4.
RNA isolation and qPCR
DRGs were isolated under isoflurane anesthesia. The outer sheath was dissected away and the DRG placed onto dry ice. RNA isolation was done with a spin column method (Norgen Biotek Corp, Thorold, Ontario, Canada, Cat #24100). RNA was stored at −80°C until use. After quality assessment (OD ratios A260/A280 > 2.0), RNA was treated with DNase (Invitrogen, Cat. # 18068-015) and cDNA was synthesized (iScript™ cDNA Synthesis Kit Bio-Rad Cat #170-8891). Pre-amplification of the 6 genes examined (Fig. 4 and text) was conducted with TaqMan® Gene Expression Assays (14 cycles, 45 nM primers, all probes were FAM-labeled except for HPRT (hypoxanthine guanine phosphoribosyl transferase) which was VIC-labeled) and TaqMan® PreAmp Master Mix (ABI, Cat. #4391128). Next, preamplified products were diluted 5-fold and qPCR reactions were run in duplicate on an Applied Biosystems 7500 Fast Real-Time PCR System, using the same TaqMan® Gene Expression Assays as for the preamplification except with 900 nM primers and Taqman® Gene Expression Master Mix (ABI, Cat #4369514). Efficiencies were 1.95–1.99 as determined by standard curves using RNA isolated from rat tissues expressing the genes of interest, and were incorporated into the calculation of expression ratios. Taqman assays used were: S1PR1:Rn02758712_s1; S1PR2:Rn02130568_s1; S1PR3:Rn02758880_s1; GFAP:Rn00566603_m1; Cd163:Rn01492519_m1; HPRT:Rn01527840_m1. All procedures for standard curve generation were approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
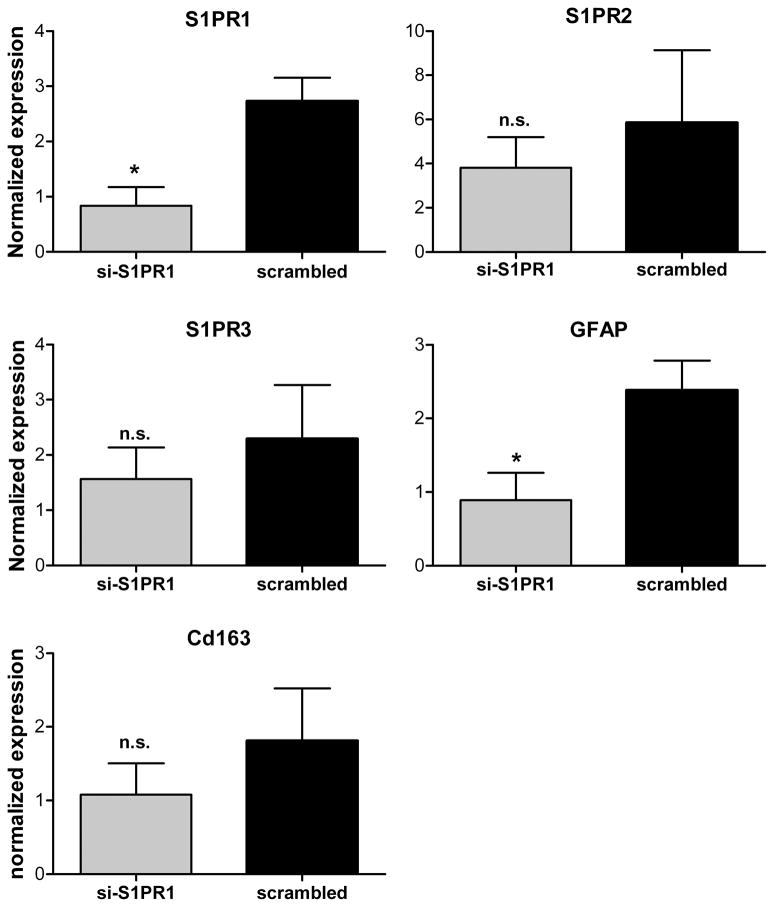
Figure 4.
qPCR validation of S1PR1 knockdown. RNA samples were taken on POD3 from the same animals as shown in Fig. 3 (top). Expression of the indicated genes was measured using qPCR protocols (see methods section). For each animal, gene expression (normalized to HPRT expression) was compared as the ratio of the expression in the L4+L5 DRG (site of the siRNA injection) to the same ratio in the L1+T12 DRG (remote from the siRNA injection). *, significant difference between anti-S1PR1 and scrambled injected animals (t-test).
Microelectrode recording
Intracellular microelectrode recording in acutely isolated whole DRG with the epineurium and ventral root removed, was as previously described [9]. This preparation allows neurons to be recorded without enzymatic dissociation, and surrounding satellite glia cells and neighboring neurons remain intact. Small soluble factors (e.g., inflammatory mediators) are presumably washed away, but changes in intrinsic neuronal properties are preserved. The DRG was continuously perfused with ACSF (in mM: NaCl 130, KCl 3.5, NaH2PO4 1.25, NaHCO3 24, Dextrose 10, MgCl2 1.2, CaCl2 1.2, pH = 7.3, bubbled with 95% O2/5% CO2) at 36 – 37°C. In some experiments, S1P was applied to the DRG for 20 minutes preceding any recordings and included throughout the recording period. Bovine serum albumin (BSA) in ACSF, used to make up S1P stock solutions, was used as the vehicle control. Cells were classified by capacitance (based on 16 msec current pulses giving 10 – 20 mV hyperpolarizations) and shape of action potentials (evoked by depolarizing 16 msec current pulses). Longer (300 msec) suprathreshold depolarizations were then used to determine if the cell fired multiple AP. Cells with unstable membrane potential, nonovershooting AP, or depolarized membrane potential (above −45 for large cells, −40 for small and medium cells) were not included. Large, medium, and small diameter cells were analyzed separately.
Data analysis
Data were tested with the D’Agostino and Pearson omnibus normality test which indicated that parametric statistics could be used. The statistical test used is indicated in the text or figure legend. Significance was ascribed for p<0.05. Levels of significance are indicated by the number of symbols, e.g., *, p = 0.01 to <0.05; **, p = 0.001 to 0.01; ***, p < 0.001. Data are presented as average ± S.E.M.
RESULTS
Perfusion of the L5 DRG with S1P increases mechanical sensitivity
In light of previous experiments showing increased excitability of sensory neurons exposed to S1P, we determined whether perfusing the L5 DRG with S1P in vivo would increase behavioral sensitivity of the hindpaw. The DRG was perfused with 100 μM S1P by inserting a small tube into a hole drilled into the transverse process attached to a 7 day osmotic pump with a flow rate of 1μL/hour. As shown in Fig. 1, this significantly increased the ipsilateral mechanical sensitivity (von Frey test) compared to animals perfused with vehicle (ACSF). The sensitization outlasted the perfusion by several days. No contralateral effects were seen at this dose although a higher dose (1 mM) did give contralateral effects (data not shown).
Large and medium diameter cells also increase action potential firing in response to S1P exposure
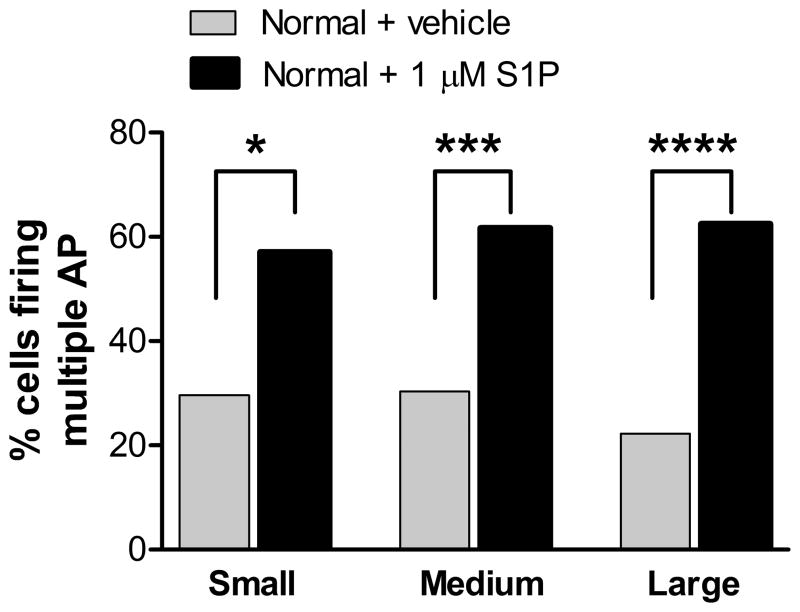
Previous studies showed that small diameter sensory neurons in acute culture increased the number of action potentials fired in response to injected current after exposure to 1 μM S1P. We used microelectrode recordings in an isolated whole DRG preparation to examine large and medium diameter cells as well. As shown in Fig. 2, cells of all three size classes from DRGs perfused with 1 μM S1P throughout the recording period were significantly more likely to fire multiple action potentials in response to suprathreshold current pulses. The incidence of spontaneous activity showed a trend towards increasing in medium and large cells exposed to S1P, but these did not reach significance: medium cells, 4.5% incidence in vehicle vs. 12.8% in S1P, p = 0.11; large cells, 1.4% in vehicle vs. 7.3% in S1P, p = 0.07. S1P exposure did not cause signficant changes in rheobase, threshold, action potential duration, or input resistance.
Figure 2.
S1P increases action potential firing in all sizes of sensory neurons in an isolated DRG. Microelectrode recordings were made in sensory neurons in isolated L5 whole DRG preparation. S1P (1μM) or vehicle control (BSA) was present in the ACSF perfusing the DRG for the entire recording period and for at least 20 minutes prior to the first recording. The percentage of cells firing multiple action potentials in response to suprathreshold current pulses was significantly higher in cells exposed to S1P (Fishers exact test). N values per group (left to right): 27, 35, 40, 32, 72, 109.
In vivo knockdown of S1PR1 reduces hypersensitivity induced by local DRG inflammation
Because previous experiments showed that S1PR1 was a key receptor mediating excitatory effects on sensory neurons in vitro, we used siRNA targeted to this receptor to examine its role in vivo. After establishing baseline levels of mechanical sensitivity (2 measurements, the average is plotted as POD −4 in Fig. 3), siRNA was injected into the L4 and L5 DRG. The siRNA constructs were either directed against the S1PR1, or as a negative control, a scrambled version of the same sequence. These constructs produced a specific knockdown of S1PR1 by ~75% using a Western blot analysis in vitro[3]. Preliminary experiments examining spread of the dye-labeled siRNA indicated that the siRNA did not spread significantly into the spinal cord or adjacent DRGs using this injection method. Three days after the first siRNA injection, mechanical sensitivity was again tested. No effect of the siRNA on mechanical sensitivity was observed, as might be expected since S1P levels are low in normal, non-inflamed tissues [8]. In order to examine possible roles of the receptor during inflammation, we next inflamed the L5 DRG by depositing a drop of zymosan in Incomplete Freund’s Adjuvant over the top of the DRG (POD0 in Fig. 3). At the same time, second injections of the siRNA were done. Animals injected with the scrambled control siRNA developed a pronounced mechanical hypersensitivity, evident by POD1 after DRG inflammation and still pronounced on POD3. These results were similar to those previously reported in the absence of any siRNA injection[9]. However, in animals injected with S1PR1 siRNA, this mechanical hypersensitivity was significantly ameliorated, on both POD1 and POD 3 (Fig. 3). The contralateral hypersensitivity is only modestly increased in this model, and no significant differences between the two groups in contralateral sensitivity were observed. Very similar effects on behavior were obtained in another set of experiments with the same design, except that the siRNA was injected into the cauda equina regions adjacent to the L4 – L6 DRG instead of directly into the DRG (Fig. 3, bottom).
This model also exhibits increased withdrawal responses to a cotton wisp stroked mediolaterally across the plantar surface of the hindpaw (a test of light touch-evoked tactile allodynia), which is rarely observed in normal animals. Combining data from both siRNA injection methods, it was observed that 5 of 8 animals gave such an allodynic response in the scrambled control group on POD 3, whereas only 1 of 8 animals in the S1PR1 siRNA-injected group exhibited this response, though this did not reach significance (p = 0.1).
After the POD3 behavioral measurements, the siRNA-injected L4 and L5 DRG were removed and combined for each animal, and RNA was isolated. Higher level DRGs (ipsilateral L1 and T12) were also isolated to confirm that the knockdown of S1PR1 was localized to L4/L5 as suggested by preliminary observations of dye-labeled siRNA. Knockdown of S1PR1 in the L4 and L5 DRG was confirmed by qPCR measurements of S1PR1 expression (Fig. 4). The expression of S1PR1 in L4 and L5, normalized to HPRT and then normalized to the same expression ratio in the L1 and T12 DRG within the same animal, was significantly lower in animals treated with S1PR1 siRNA compared to scrambled siRNA. Expression of GFAP mRNA, a marker of satellite glia activation, was also significantly lower. By the same measure, there was no significant effect of S1PR1 siRNA on S1PR2 or S1PR3, or on Cd163, a macrophage marker.
DISCUSSION
We observed marked effects of S1PR1 knockdown on mechanical sensitivity after localized DRG inflammation, but no effects in the absence of inflammation. The lack of effect in normal animals, prior to inflammation, is most likely due to a lack of S1P to significantly activate the receptor, consistent with the general observation that S1P is produced by activated immune cells, increasing during inflammation [8]. S1PR1 is expressed in DRG, and SEW2871 can modulate sensory neurons acutely isolated from normal, non-inflamed DRG [13]. Hence the S1PR1 is normally present in DRG neurons, so the lack of S1PR1 knockdown effects prior to inflammation is mostly likely due to a lack of S1P, not due to lack of the receptor in normal DRG. In addition, we observed expression of, but no significant regulation of, S1P1 – 4 via qPCR or microarray comparing normal DRG with inflamed (POD3) DRG (unpublished observations).
We observed excitatory effects of S1P applied to neurons in an acutely isolated whole DRG preparation, confirming prior results obtained in cultured small diameter neurons and extending these observations to medium and large diameter neurons under somewhat more physiological conditions (37°C, lack of enzymatic dissociation, and retaining the in vivo arrangement of surrounding satellite glial cells and neighboring neurons). The finding that large and medium diameter cells also exhibit increased action potential firing when exposed to S1P was not immediately predicted from the prior results in small diameter cells, because in those cells S1P works in part by increasing the TTX-resistant Na+ current [13] which is found in only a minority of myelinated cells. Further studies may indicate that the reduction of K current by S1P observed in small cells [13] is the predominant S1P effect in medium and large cells, or that S1P may modulate the TTX-sensitive sodium current in medium and large diameter neurons.
DRG perfusion with S1P in vivo led to mechanical hypersensitivity. This is consistent with the excitatory effects of S1P on sensory neurons in vitro, though this experiment cannot distinguish between direct S1P effects on neurons and indirect effects such as activation of immune cells, which would also be expected to increase behavioral sensitivity. Since PEI-complexed siRNA is internalized within hours [5], it seems unlikely that observed effects of the S1PR1 siRNA were due to knockdown of the receptor in blood-derived immune cells that migrated into the DRG after inflammation. Hence the marked reduction in mechanical sensitivity observed after knocking down S1PR1 in vivo provides evidence for an important role for the receptor in cells that are resident in the normal DRG. The neuronal receptor is one likely candidate to account for these behavioral effects, given the known excitatory effects of S1P on neurons including the larger myelinated neurons shown to be important in this model. However, receptors expressed in nonneuronal cells, such as glial and endothelial cells, could also play a role. The observation that GFAP expression (a marker of satellite glia activation) was also lower in S1PR1 siRNA-treated DRG could be an indirect effect of reduced excitability of neurons, since neuronal activity plays a role in activating satellite glia [10]. Alternatively or in addition, it may be that satellite glia themselves express the S1PR1, as has been observed in CNS astrocytes [1].
In summary, locally knocking down the S1PR1 receptor in the DRG in vivo significantly reduced mechanical hypersensitivity induced by local DRG inflammation while having no effect on sensitivity of normal animals. In vitro, S1P caused increased action potential firing in sensory neurons of all size classes. The results suggest that S1P receptors in resident DRG cells may play important roles in regulating pain behaviors after inflammation.
Acknowledgments
Funded by NIH grants NS060853, NS045594, and NS055860. Funding agencies had no additional research roles.
Footnotes
Author contributions: W.X. performed surgeries, microelectrode recording, behavioral and microscopy experiments. J.A.S. drafted the manuscript, performed RNA isolation, and data analysis. J.K. performed qPCR experiments and data analysis. All authors participated in experimental design, data analysis, and manuscript writing
Disclosure: none of the authors declares any conflict of interest. All authors approved the final manuscript. The Uniform Requirements for Manuscripts Submitted to Biomedical Journals of the ICMJE were adhered to.
Contributor Information
Wenrui Xie, Email: Xiewe@ucmail.uc.edu.
Judith A. Strong, Email: judith.strong@uc.edu.
Joanne Kays, Email: kaysj@iupui.edu.
Grant D. Nicol, Email: gnicol@iupui.edu.
References
- 1.Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53:621–30. doi: 10.1002/glia.20324. [DOI] [PubMed] [Google Scholar]
- 2.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 3.Chi XX, Nicol GD. The sphingosine 1-phosphate receptor, S1PR, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons. J Neurophysiol. 2010;104:2741–8. doi: 10.1152/jn.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–28. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 5.Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, Czubayko F, Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–66. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- 6.Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubi-Robles M, Quarta S, Biasio W, Kuner R, Gibbins IL, Kress M, Haberberger RV. Genetic evidence for involvement of neuronally expressed S1P receptor in nociceptor sensitization and inflammatory pain. PLoS One. 2011;6:e17268. doi: 10.1371/journal.pone.0017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–8. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–70. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 9.Xie W, Strong JA, Kim D, Shahrestani S, Zhang J-M. Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience. 2012;206:212–223. doi: 10.1016/j.neuroscience.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–857. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie W, Strong JA, Zhang JM. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151:447–59. doi: 10.1016/j.pain.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol. 2006;96:1042–52. doi: 10.1152/jn.00120.2006. [DOI] [PubMed] [Google Scholar]