Abstract
Oral delivery is the most common method for drug administration. However, poor solubility, stability, and bioavailability of many drugs make achieving therapeutic levels via the gastrointestinal (GI) tract challenging. Drug delivery must overcome numerous hurdles, including the acidic gastric environment and the continuous secretion of mucus that protects the GI tract. Nanoparticle drug carriers that can shield drugs from degradation and deliver them to intended sites within the GI tract may enable more efficient and sustained drug delivery. However, the rapid secretion and shedding of GI tract mucus can significantly limit the effectiveness of nanoparticle drug delivery systems. Many types of nanoparticles are efficiently trapped in and rapidly removed by mucus, making controlled release in the GI tract difficult. This review addresses the protective barrier properties of mucus secretions, how mucus affects the fate of orally administered nanoparticles, and recent developments in nanoparticles engineered to penetrate the mucus barrier.
Keywords: Oral delivery, mucus barrier, mucoadhesion, mucus penetrating particles
1. Introduction
Oral delivery is the most widely used and most readily accepted form of drug administration. The human intestinal epithelium is highly absorptive and is composed of villi that increase the total absorptive surface area in the gastrointestinal (GI) tract to 300–400 m2 [1]. Enterocytes (absorptive) and goblet cells (mucus secreting) cover the villi, which are interspersed with Follicle Associated Epithelium (FAE). These lymphoid regions, Peyer’s patches, are covered with M cells specialized for antigen sampling. M cells are significant for drug delivery, since they are relatively less protected by mucus [2] and have a high transcytotic capacity [3].
Despite these potential advantages, oral formulations face several common problems, particularly for peptides and proteins: (i) poor stability in the gastric environment, (ii) low solubility and/or bioavailability and (iii) the mucus barrier can prevent drug penetration and subsequent absorption. To overcome these limitations, nanoparticle formulations are being developed that encapsulate and protect drugs and release them in a temporally or spatially controlled manner. The nanoparticle surface can also be modified to enhance or reduce bioadhesion to target specific cells.
The mucus layers that protect epithelial surfaces have been highlighted as significant barriers to nanoparticle penetration [4–10]. Mucus has evolved to protect exposed epithelial surfaces by efficiently trapping pathogens and foreign particulates and rapidly clearing them. Mucus is continuously secreted both to remove pathogens and to lubricate the epithelium as material passes through, decreasing the residence time of nanoparticles that fail to penetrate the loosely adherent layer of GI mucus. This article reviews the properties and function of mucus in the GI tract, including how the barrier properties and composition change with GI diseases; these changes can affect the fate of nanoparticle-based drug delivery systems aimed at providing improved drug pharmacokinetics and/or targeting. Strategies for improving drug delivery to the GI tract by using mucoadhesive nanoparticles, and by disrupting the mucus barrier, are also discussed. Lastly, the recent development of mucus penetrating particles and their potential for further improving drug delivery to the GI tract is discussed.
2. Role of mucus in the GI tract
2.1 Mucus composition, thickness, and pH in the GI tract
Mucus is a complex hydrogel composed of proteins, carbohydrates, lipids, salts, antibodies, bacteria, and cellular debris. The main protein component of mucus is mucins, which can be either secreted or cell-bound. In total, there are at least twenty proteins encoded in the MUC gene family [5], of which, MUC2, MUC5AC, and MUC6 are the secreted mucin types found throughout the GI tract [11]. Secreted mucin monomers link together via disulfide bonds to form large molecules, 0.5–40 MDa in size [12]. Typically comprising 2–5% of mucus by wet weight, mucin molecules entangle and cross-link adhesively and reversibly to form a dynamic viscoelastic gel with shear-thinning properties. The protein backbone contains large amounts of serine, threonine, and proline residues, with O-linkage to oligosaccharides. There are also N-linked oligosaccharides (2–3%) located near the ends of mucin monomers [13], but O-linked oligosaccharides comprise 40–80% of the mucin dry weight [14]. These oligosaccharides vary in size and branching depending upon the location in the body within an individual [15]; long, branched oligosaccharide side chains on mucins may protect against proteolytic degradation by digestive proteases [16]. Heavily glycosylated regions of mucins are separated by “naked” protein regions with both adsorbed and covalently linked lipids [13]. These hydrophobic, lipid-coated domains contribute significantly to adhesive trapping interactions with mucus and to mucin-mucin interactions that govern the viscoelastic properties of the mucus gel. Although mucins are considered primarily responsible for the gel properties of mucus [16], mucus viscoelasticity is further regulated by water, lipid, and ion content, as comprehensively reviewed elsewhere [17].
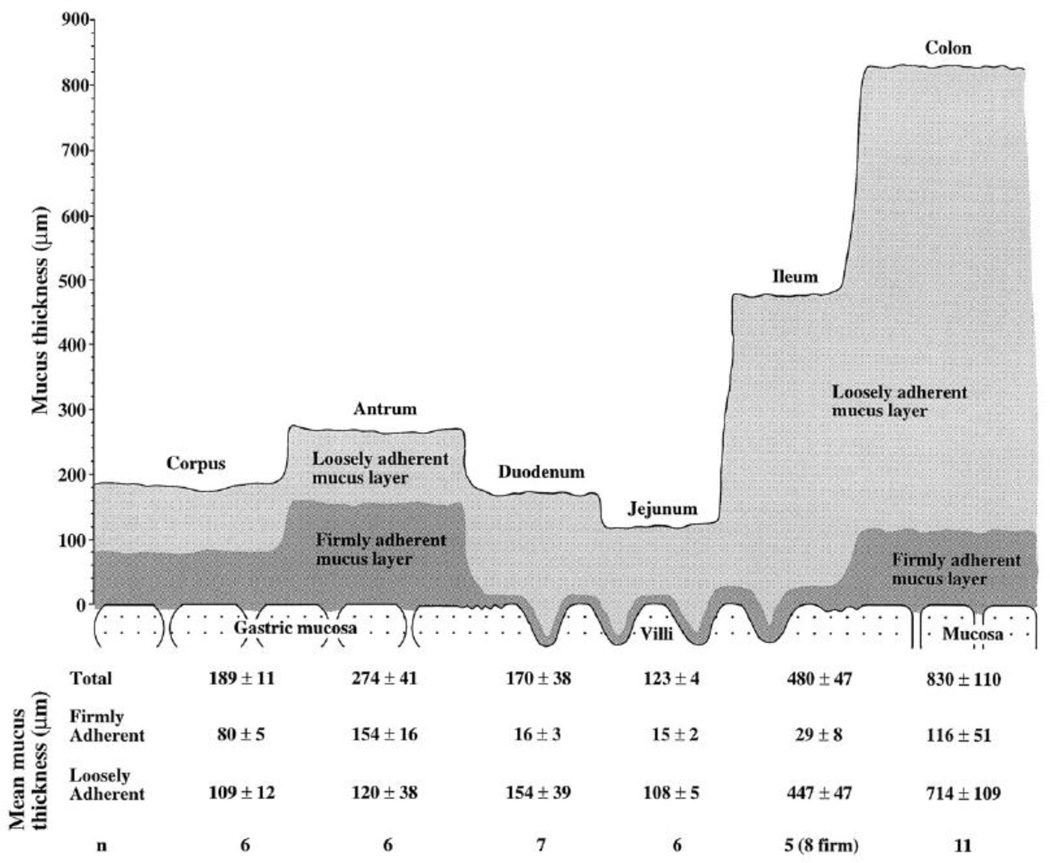
The viscoelastic properties of GI mucus are essential for its protective and lubricating properties. For undigested boluses of food to be peristaltically transported through the GI tract without damaging the epithelium, a slippage plane forms between loosely and firmly adherent mucus layers [13, 18]. Figure 1 illustrates the thickness of the loosely and firmly adherent layers throughout the GI tract of the rat. These distinct layers, although different in absolute thickness, are also present in the human GI tract. The firmly adherent layer includes the cell-bound mucins and the collective glycolipids and glycoproteins that make up the glycocalyx. Five cell-bound mucins have been identified in the GI tract: MUC1, 3, 4, 12 and 13 [11]. Cell-bound mucins have cytoplasmic tails that participate in signal transduction; it has been hypothesized that the secreted mucin layer may signal the underlying epithelium via contact with the cell-bound mucins [11, 15].
Figure 1.
Schematic figure showing the thicknesses of the loosely adherent and firmly adherent mucus layers in vivo in the rat gastrointestinal tract. The loosely adherent mucus layer is removable by careful suction, whereas the firmly adherent mucus layer is not. The table presents values for mucus thickness as means ± SE for each group. Figure obtained from [18].
To protect the mucosal epithelium, there is constant turnover of the adherent layer and the glycocalyx in the GI tract that serves to remove potentially damaging compounds and organisms introduced through the diet [11]. Therefore, a regulated balance occurs between mucin synthesis, degradation, and removal, which affect the overall thickness of the mucus barrier. The thickness varies throughout the human GI tract, in a way that is likely related to a balance between protective capability and nutrient absorption rate [18]. In humans, mucus is thickest in the stomach (180 µm; range 40–450 µm) and the colon (110–160 µm) [19–21], which may increase protection from acid in the stomach and bacteria in the colon [15]. In the small intestine, the thickness also varies depending on diet; adequate fiber intake leads to an increased loosely adherent layer [13] in addition to generally increased mucus turnover [22]. In addition to mucus thickness, pH also varies throughout the GI. In the stomach, mucus pH varies from 1–2 on the luminal surface to nearly neutral pH at the epithelial surface (the mechanism for maintaining this pH gradient is discussed in the next section). The pH is rapidly yet only partially neutralized in the duodenum and steadily increases through the rest of the GI tract, reaching a pH of 7–8 in the colon and rectum [23].
2.2 The barrier and protective properties of GI mucus
The question of how the stomach is able to digest food without digesting itself is one of the clearest examples of the dynamic barrier properties of mucus secretions. The gastric mucosa sustains a gradient from pH 1–2 to pH 7 over a mucus thickness of only about 200 µm. In maintaining this gradient, gastric mucus acts as a selective barrier to diffusion of acid, based on interactions that change depending on pH. Above pH 4 (e.g. when food buffers pH above the normal acidic state), acid secreted by parietal cells of the stomach causes channels to form in the mucus layer, allowing acid to quickly reach the lumen and re-acidify the contents for optimum digestive and antimicrobial activity. Below this pH, mucus slows acid diffusion [24], and bicarbonate ions continuously secreted by the epithelium neutralizes acid before it reaches the epithelium. These characteristics allow for acid to be secreted by glands in the epithelium, and simultaneously act as a barrier to slow the rate at which secreted acid approaches the epithelial surface from the lumen [15]. The constant presence of acid and enzymes in the stomach actively degrades mucus, which must be counterbalanced by continual mucus secretion [16]. Remarkably, an adult human secretes ~10 L of water into the GI tract per day, of which 9.8 L are reabsorbed [13]; water secretion and reabsorption are both driven osmotically by active transport of salts and nutrients. Water secretion into the mouth and stomach helps lubricate and protect their epithelia. Ulcers form when the mucus barrier is compromised, thereby allowing acid and digestive enzymes to contact the gastric epithelium [11]. Reabsorption of this water greatly speeds transport and absorption of nutrients and small particles that can penetrate the mucus that is continuously secreted by the rest of the GI tract [25].
Lubrication is another important protective property of mucus in the GI tract. Mucus maintains an unstirred adherent layer throughout the GI tract, even when shearing forces caused by undigested material passing through remove much of the loosely adherent layer. The mechanisms for how this layer adheres firmly to the epithelium are poorly understood, but likely involve adhesive and physical interactions with the glycocalyx [4]. The shear-thinning properties of mucus allow the superficial, loosely adherent layer of mucus to coat and lubricate undigested material, forming a slippage plane (Figure 2). Because of this lubricating action and the retention of an unstirred firmly adherent layer, the epithelium is minimally damaged by peristaltic propulsion of ingested material [4, 17].
Figure 2.
Summary schematic illustrating the fate of an ingested food bolus propelled through the GI tract via peristaltic contractions. Mucins in the loosely adherent layer adhere to the food, wrapping it in a ‘blanket’ of mucus. The shear thinning properties of the secreted mucins allow the bolus to pass without perturbing the firmly adherent layer and the epithelium. Enzymes and emulsifying lipids that can pass through the mucus will begin to digest the food, extracting nutrients. Water is continuously removed from any undigested material as it passes through the small intestine and colon.
GI mucus also enables colonization by commensal or ‘good’ bacteria while acting as a barrier to pathogenic bacteria. The mucus barrier constrains most bacteria to the superficial luminal surface; few bacteria penetrate to the epithelial surface. In addition, secreted mucins help prevent adhesion of bacteria to epithelial cells by displaying many of the same glycosylation patterns present on epithelial cell surfaces that are recognized by bacterial adhesins. In this way, bacteria are trapped in the superficial layer of mucus before reaching the epithelial surface [13, 26]. Mucus also traps pathogens and foreign particles via non-specific interactions, such as hydrophobic interactions. The substantial hydrophobic adhesive barrier character in GI tract mucus is due to lipids that compose 20% or more of the dry weight [27, 28]; a small fraction of fatty acids are covalently bound to mucins in the ‘naked’ protein domains, and the majority are simply adsorbed [29]. Contact angle measurements on the gastric mucosal surface of many mammals, including humans, demonstrate that the luminal surface is made hydrophobic by a monolayer of surface-active phospholipids (see Figure 3) [30]. Lipids also contribute to the viscoelasticity of mucus, which is drastically reduced when lipidic components are removed [31]. The glycosylated portions of mucins are capable of non-specific hydrogen bonding and electrostatic interactions [32]. Mucus gel entanglement itself creates a ‘sieve’ that can sterically block particulates that are too large to penetrate the mucus mesh spacing (pore size) [4, 33].
Figure 3.
Schematic model depicting the possible role of an extracellular lining of zwitterionic phospholipids in generating the hydrophobic barrier of the stomach to luminal acid. Figure adapted from [30].
Mucus can also trap biologically active molecules that can incite inflammatory or healing processes following their release, such as trefoil factors. After their release, these highly conserved peptides promote wound healing and mucosal repair at sites of epithelial damage [15, 34]. GI mucus also contains a diverse range of secreted antimicrobial molecules (defensins, lysozymes, etc.) and immunoglobulins [35]. Pathogens and toxins that manage to bypass these protective mechanisms and breach the mucus barrier will induce more rapid mucus secretion, which serves to remove offending material [13, 35]. For a more comprehensive review of mucus barrier properties, see [4].
3. Mucoadhesive nanoparticle systems
Most orally administered particles are not retained and undergo direct transit through the GI tract [36, 37]. Mucoadhesion has been commonly employed in attempting to improve the residence time of particles in the GI tract. Non-specific mucoadhesion of microparticles in the GI tract is a well-known phenomenon; in 1962, Florey observed in cats that particles of India ink become coated with intestinal mucus such that they do not come into contact with the intestinal epithelium [38]. Gruber et al. observed in dogs that, irrespective of particle size, density, or composition, the particles became wrapped in a plug of mucin resembling a slug [39]. Similarly, Tirosh and Rubinstein concluded that both mucoadhesive (polycarbophil) and non-adhesive (Eudragit® RL-100) microparticles (~150 µm) had equivalent intestinal transit times in the rat intestine, subsequently being expelled coated with a mucus plug as shown in Figure 4 [40]. It is important to distinguish that the Eudragit® particles were considered “non-adhesive” because they were not positively charged (see next section for discussion on the effect of surface charge); however, this hydrophobic polymer most likely adhered to mucus. In addition to electrostatic interactions, mucoadhesion can arise from hydrophobic interactions, van der Waals interactions, and polymer chain interpenetration [41]. Consequently, mucoadhesive nanoparticles could have limitations for oral delivery, including the possibility of adhering nonspecifically to unintended surfaces. It is likely that rather than reaching the more slowly cleared firmly adherent mucus layer, mucoadhesive nanoparticles will become trapped in the loosely adherent mucus layer and become vulnerable to rapid clearance [42]. Significant work has been undertaken in attempt to overcome these limitations in vivo, including the use of specialized polymeric, pH-responsive, and lipid-based formulations.
Figure 4.
Agglomerated Eudragit® particles coated with a mucus plug collected immediately following discharge from the proximal jejunum of the perfused rat colon (magnification ×60). Figure obtained from [40].
3.1 Mucoadhesive polymeric systems
3.1.1 Improving oral bioavailability with mucoadhesive particles
Small molecule drugs, proteins, or peptides can be encapsulated and protected from the harsh gastric environment by polymeric nanoparticles. Additionally, nanoparticle surface characteristics can be tailored to optimize mucoadhesion, cellular uptake, immune system interactions, and cell targeting. Particles synthesized from commonly used polymers, such as poly(lactic acid) (PLA), poly(sebacic acid) (PSA), poly(lactic-co-glycolic acid) (PLGA) and poly(acrylic acid) (PAA) may achieve mucoadhesion via hydrogen bonding, polymer entanglements with mucins, hydrophobic interactions, or a combination of these mechanisms [5]. For example, 680–850 µm microspheres composed of mucoadhesive copolymers of fumaric acid and sebacic acid exhibited prolonged retention in the rat gut compared to a more weakly adhesive alginate particle based on fracture strength measurements and GI transit times; the copolymer formulation had ~50% increase in the area under the curve (AUC) for dicumarol as compared to the spray-dried drug and alginate formulation [43]. Similarly, Mathiowitz et al demonstrated that encapsulating insulin in a similar mucoadhesive polymer blended ~200 nm nanoparticle led to a 7-fold decrease in deviation from fasting blood glucose concentration as compared to insulin solution in rats [44]. The authors attributed this difference to reduced insulin degradation when it is encapsulated in the nanoparticles and to uptake of nanoparticles by Peyer’s patches. However, blood glucose levels were not statistically different for insulin solution as compared to saline only, which would indicate that the fragile protein was significantly degraded during GI transit. To determine whether mucoadhesion contributed to the improved blood glucose regulation, insulin encapsulated particles would have to be compared with ‘non-adhesive’ nanoparticles.
In addition to prolonging residence time, improved oral bioavailability can also be attributed to protection from proteolytic enzymes. Upon exposure to protease degradation, the stability of insulin and calcitonin was improved when encapsulated in polymeric nanoparticles [45, 46]. Alonso’s group has also observed that coating ~160 nm PLA nanoparticles with poly(ethylene glycol) (PEG) imparts additional protection against enzyme induced aggregation and degradation in simulated GI fluids in vitro [47]. The zeta potential of uncoated PLA was −44 mV, which increased to −14 mV when the particles were coated with PEG (PLA-PEG), thus indicating a moderate surface coating of neutrally charged PEG; it is important to note that this partial coating would not likely be adequate to produce mucus penetrating particles, as was shown for cervicovaginal mucus [9]. After oral gavage of 1 mL radio-labeled particles to rats, blood samples and lymph tissue were excised; 3–4 fold more radioactivity (a fraction of a percent of the original dose in total) was found in the blood at all time points up to 24 h in the case of PLA-PEG particles. The authors attributed this effect to increased uptake of PLA-PEG particles across the intestinal epithelium. However, it was shown that 5–15% of the radiolabel released from the particles after 4 h in intestinal and gastric fluid stimulants, indicating that a significant amount could release after 24 h. It is possible that the partial PEG coating on the PLA-PEG particles allowed a small fraction of nanoparticles to get in closer proximity of the epithelium before releasing the radiolabel, which could then be taken up into the bloodstream.
Another contributor to improved bioavailability is likely the direct uptake of particles by intestinal cells. Although the extent of absorption of particulate delivery systems by enterocytes versus M cells is controversial, it is clear that nanoparticle surface properties are very important for intestinal uptake. Evidence suggests that particle translocation can occur across enterocytes in the villus part of the intestine, but the overall endocytic activity is low [36]. For instance, des Rieux et al. reported a thousand-fold increase in the transport of 200 and 500 nm polystyrene particles in vitro when epithelial gut cells were co-cultured with cells that had been differentiated to achieve M cell-like features [48]. Thus, extensive effort has been focused on elucidating optimal surface and size characteristics for uptake into the Peyer’s patches.
3.1.2 Optimizing mucoadhesive nanoparticle characteristics
Studying the effect of polymer hydrophobicity, Eldridge and coworkers determined in mice that 1–10 µm hydrophobic microspheres administered by oral gavage (polystyrene, polymethylmethacrylate, and polyhydroxybutrate) were taken up by Peyer’s patches more efficiently than less hydrophobic lactide and glycolide polymer particles. Particles composed of hydrophilic cellulose polymers were absorbed 100-fold less than hydrophobic particles, further supporting the interpretation that the major determinant for particle absorption by Peyer’s patches is particle hydrophobicity [49]. However, it is also true that hydrophobic interactions play a major role in mucoadhesion [4]. It is important to reemphasize that Peyer’s patches are relatively less protected by mucus, perhaps supporting their immune-sensory role for sampling lumenal contents [2]. However, particles that adhere to GI mucus would not be able to efficiently reach the Peyer’s patches. It is possible that Peyer’s patch uptake could be optimized by a combination of mucus penetration and M-cell adhesion; unfortunately, the large hydrophilic particles used by Eldridge would likely be sterically trapped in GI mucus, thus eliminating the potential increase in hydrophilic particle uptake due to mucus penetration. Behrens and coworkers also determined that 200 nm hydrophobic polystyrene nanoparticles had enhanced uptake in Caco-2 cell culture, although the uptake was reduced 2-fold by the presence of mucus in MTX-E12 culture [50]. Similar to Eldridge, Behrens suggests that although nanoparticle hydrophobicity enhances cell association and subsequent uptake, hydrophobicity could also be a major obstacle for mucus penetration. It is not clear whether cells in culture produce an intact mucus layer equivalent to the mucus barrier in vivo, or whether culture buffers cause significant dilution of the mucus. Thus, it is possible that an intact mucus barrier would lead to a further decrease in hydrophobic particle uptake compared to a lack of a mucus barrier. However, it is clear that mucus affects nanoparticle uptake in cell culture models, so further testing of particle uptake in vivo is critical.
In addition to hydrophobicity/hydrophilicity, nanoparticle surface charge also affects oral drug delivery. The surface charge can be used to improve the proximity of nanoparticles to the epithelium to enhance drug absorption, as well as to increase particle uptake via Peyer’s patches. Many studies have indicated that mucoadhesive particles increase drug absorption compared to free drug; electrostatic interactions between a positively-charged particle surface and the extensive negatively charged sugar moieties on mucins are strongly mucoadhesive. For this reason, a considerable number of studies have been conducted using positively charged polymers such as chitosan for enhancing drug absorption [51–54]. Coating PLGA nanoparticles with chitosan improved the absorption of tetanus toxoid [55] and salmon calcitonin [56]. It is of note that Schipper and coworkers observed that exposure of perfused rat ileal tissue to chitosan caused an increase in mucus secretion and only modest absorption-enhancing effects for atenolol. Upon further testing with mucus covered HT29-H goblet cells, the binding of chitosan to the epithelial surface and subsequent absorption-enhancing effects were significantly improved if the mucus layer was removed prior to chitosan addition [57]. It is likely that particle adhesion to the outer mucus surface limits drug absorption; the improvement when comparing delivery of encapsulated drug to free drug is likely due to sustained release from particles, decreased drug degradation during GI tract transit, etc. Utilizing a drug-loaded particle that can penetrate the mucus barrier and release drug closer to the epithelium might further improve drug absorption [see section 3.2]. A study by Jani and coworkers addressing particle uptake by Peyer’s patches found that neutral surface charge was ideal for uptake of 130 and 950 nm polystyrene particles [58]. However, mucoadhesive particle uptake by Peyer’s patches is also limited by the presence of mucus [see section 3.2.1].
Aside from surface characteristics, size is an important characteristic for efficient uptake. Numerous studies investigating the effect of size have been conducted in various animal models and experimental systems, with the general consensus being that particles less than 1 µm in size can be transcytosed by M cells [48, 58–60]. However, the optimal size is also an area of contention and conflicting reports; care must be taken in interpreting uptake and translocation results due to the various limitations and the numerous experimental variables of in vitro cell cultures and ex vivo intestinal loop models [61]. For example, Eyles and coworkers found that when 5 times the total volume of 870 nm polystyrene particles (0.5 mL compared to 0.1 mL) was administered by gavage to the rat stomach, almost 5-fold more polystyrene particles were found in the blood 15 minutes after dosing [62]. This phenomenon is potentially important for explaining the large uptake seen in many experiments with mucoadhesive particles since dilution with a large fluid volume can degrade the mucus barrier in a way that may not be relevant for administering oral drugs in humans. Eyles and coworkers also observed in the same study that administering the particles in water (hypotonic) led to 5-fold greater uptake of particles into the blood 15 minutes after dosing as compared to particles administered in saline (isotonic) [62]. In this case, significantly greater particle uptake by Peyer’s patches was probably caused by osmotically driven fluid absorption.
3.1.3 Targeted mucoadhesive nanoparticle systems
In addition to “passive” targeting of lymphoid tissue, the use of targeting ligands to enhance particle uptake has been investigated using ligands that bind specific receptors expressed on enterocytes or on M cell surfaces. Coating nanoparticles with these ligands is intended to enhance the binding specificity and decrease the elimination rate due to mucus turnover [63, 64], although the extent to which these particles could penetrate the mucus barrier to adhere to enterocytes was unclear. Many different types of ligands have been described including lectins [65], invasins [66] and vitamin B12 derivatives [67]. Lectins are naturally occurring proteins or glycoproteins that bind reversibly to specific sugars, and are involved in many cell recognition and adhesion processes. Several accounts have reported increases in lectin-conjugated particle uptake, thought to be caused by increasing interactions with mucus [68, 69] and epithelial cells [70]. For example, Hussain et al. conjugated tomato lectin to 500 nm polystyrene particles administered by 0.1 mL oral gavage daily for 5 days [71]. After washing the tissues with phosphate buffered saline, particles were extracted from intestinal tissue and Peyer’s patches; the authors determined that 12% of the dose was associated with the intestine (enterocytes) as compared to <1% associated with the Peyer’s patches. However, later experiments by Atuma et al. determined that not even suction could remove the adherent mucus layer in rat intestines [18]. This would imply that the majority of particles were likely trapped in the adherent mucus layer, not absorbed by the enterocytes. Additionally, Hussain and coworkers determined that total systemic circulation of lectin-conjugated particles reached 23%, which was attributed to the fact that the majority of the intestinal surface is non-lymphoid tissue that was absorbing the particles [71]. However, they did not take into consideration that the particles were administered in water, which has been shown to increase uptake by Peyer’s patches into systemic circulation [62]. Uptake by M cells in the Peyer’s patches could be further increased by the presence of lectin, since three lectin types are known to bind to M cells in the rat [72]. Indeed, when the binding was blocked by incubating the particles with a potent inhibitor of tomato lectin, the particle uptake was reduced to 0.5%, which is typical of polystyrene uptake by lymphoid tissue in rats [71].
Wheat germ agglutinin (WGA) is another commonly used lectin, which targets N-acetyl-D-glucosamine and sialic acid found ubiquitously throughout the intestinal tract [73]. Yin and coworkers hypothesized that direct lectin interaction with the glycocalyx would make 200 nm PLGA nanoparticles less affected by the secreted mucus layer turnover, while also potentially triggering endocytosis by the intestinal epithelium [74]. They believed that this effect led to increased bioavailability of encapsulated immunomodulating peptide (thymopentin) in immunosuppressed rats, as measured by a ~2-fold increase in the ratio of CD4+/CD8+ T cell populations as compared to uncoated PLGA nanoparticles, free thymopentin, and the control. Also, the WGA-coated PLGA particles administered orally had a similar effect as thymopentin solution administered by I.V. In another study further characterizing the effects of WGA-conjugated PLGA, Yin and coworkers, based on fluorescent photomicrographs, stated that these nanoparticles adhered to intestinal villous epithelium as well as Peyer’s patches after daily administration. This adhesion was found to increase with increasing surface density of WGA. However, fluorescent microscopy is insufficient for determining particle uptake by cells; confocal microscopy is more appropriate. As stated previously, washing the tissues prior to imaging does not remove the adherent mucus layer on the villous epithelium; it is more likely that the particles were trapped in this mucus layer on top of the epithelium rather than internalized. Systemic uptake was as high as 13% at 1 day and 15% after dosing for 7 days, which was 1.5–3 fold higher than unconjugated particles [75]. The high overall systemic uptake of both coated and uncoated particles could have been due to large gavage volumes, but the specific value is not stated. It is possible that lectin interaction with Peyer’s patches also leads to enhanced uptake, because one potential drawback of lectin-based targeting for drug delivery applications is the potential for immunostimulatory effects [42].
Immunostimulatory effects can be advantageous for vaccination, prompting the development of nanoparticles that induce M cell uptake. Taking inspiration from nature, it was noted that pathogenic bacteria such as Salmonella and Shigella species are able to invade the mucosal immune system via surface invasin proteins. These proteins both allow bacteria to adhere to the mucosa and be internalized by the epithelium [76]. Salman and coworkers coated 280 nm poly(methyl vinyl ether-co-maleic anhydride) (PVM/MA) nanoparticles with flagella-enriched Salmonella extract, with the intention of increasing adhesion in the Peyer’s patches of rats [77]. It was demonstrated that the coated particles exhibited competitive binding with Salmonella dosed orally; the particles administered 30 mins prior decreased the epithelial binding of the bacterium. However, the gavage volume was not stated; a high volume could explain how these particles were able to pass through the mucus barrier and reach the epithelium. Hussain and coworkers coated 500 nm polystyrene nanoparticles with invasin-C192, which is found on the surface of Yersina bacteria. After a single 0.1 mL gavage in rats, 13% of the invasin coated nanoparticles were found in the systemic circulation, compared to 2% of uncoated control particles. As a control, invasin coated nanoparticles were coated in porcine mucin, which would interfere with their cell adhesive capability, resulting in <2 % systemic uptake [76]. The authors were unable to explain why coating the particles in porcine mucin would interfere with systemic uptake, but uptake was not decreased by interaction with mucin while the particles passed through the rat GI tract. It is possible that the relatively high density of M cells and relatively low mucin secretion in rats [see section 5] likely contributes to all of the studies described that result in such high particle uptake with mucoadhesive particles.
Other groups have used materials that target particular areas of the GI tract based on their degradation kinetics by preserving the encapsulated material until reaching the target site. NiMOS (nanoparticles in microspheres oral system) contain pDNA inside type B gelatin nanoparticles, which are then encapsulated within poly(episilon-caprolactone) (PCL) microparticles [78, 79]. The outer PCL coat (2–5 µm in diameter) is degraded by intestinal lipases, which releases the encapsulated 200 nm nanoparticles. It is hypothesized that the nanoparticles are then taken up by the cells of the small and large intestine. In Figure 5, green fluorescent protein- (GFP) expressing plasmid DNA was administered orally as either free plasmid, encapsulated in gelatin nanoparticles alone, or in the NiMOS system. It is evident that GFP expression occurred only in the small intestine of rats when the plasmid DNA was encapsulated in NiMOS [79]. In a mouse model for TNBS-induced ulcerative colitis, NiMOS containing murine IL-10-expressing plasmid DNA were given in one oral dose. The expression of IL-10 acts to maintain immunological balance by inhibiting production of proinflammatory cytokines. The successful transfection, determined by significantly increased IL-10 mRNA transcript levels in the intestines, prevented progression of colitis as measured by colon length and weight, body weight, and myeloperoxidase (MPO) activity [80]. These results are encouraging, but mucus in the human GI tract will likely be a more significant barrier than in the mouse [see section 5].
Figure 5.
Qualitative enhanced green fluorescent protein (GFP) expression in the small and large intestinal tract of male Wistar rats cryosections after oral administrate of saline (A), naked GFP plasmid (B), gelatin nanoparticles encapsulating GFP plasmid (C) and the NiMOS encapsulating GFP plasmid (D). The bright-field and epifluorescence images of the tissue cryosections were obtained from small intestine and large intestine of rats after 5 days following a single 100 µg dose of plasmid administered orally in the control and NiMOS formulations. The letter “L” denotes the luminal side of the small and large intestine. Figure obtained from [79].
Another novel degradation-specific formulation being tested is siRNA encapsulated in a novel thioketal polymer. This polymer degrades in the presence of reactive oxygen species (ROS), which are present at relatively high concentrations in inflamed tissues. Specifically in the GI tract, biopsies taken from patients suffering from ulcerative colitis [81], colon cancer [82], and helicobacter pylori [83] infections have 10–100 fold increased mucosal ROS concentrations. The particles are also coated with 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), a positively charged surfactant, increasing mucoadhesion in the intestines. Wilson and coworkers loaded the 600 nm particles with TNF-α-siRNA to treat mice with dextran sulfate sodium (DSS) induced ulcerative colitis [84]; TNF-α is integral in the onset and persistence of intestinal inflammation. After receiving DSS or normal water for seven days, the mice were given daily 0.2 mL gavages for six days. On the seventh day, mice were sacrificed and assessed by histology, MPO activity, and weight loss. Indeed, the thioketal particles were associated with 2-fold lower MPO activity, improved histology, and reduced weight loss; however, it was clear that after day 3 of daily gavaging, the initial weight gain was beginning to reverse. At the time of sacrifice, the weight of the mice was almost back to the starting weight, and likely would have kept declining, whereas the weight of the control mice was increasing. It is likely that the effectiveness of this treatment was limited by the mucus barrier, considering the increased mucus secretion associated with inflammation. As inflammation progressed, the mucus barrier thickness would increase, sequestering the particles further from the epithelium.
Lamprecht et al. determined that polystyrene particles (0.1–10 µm) dosed by 0.5 mL gavage on two consecutive days selectively adhered to inflamed tissue in a rat model of TNBS-induced colitis [85]. The authors determined seventy-two hours after the second gavage 0.1 µm particles had the highest deposition in inflamed tissue at 15% of the original dose (6.5 fold higher than deposition in controls) and that 1 and 10 µm particles had 3–4 fold increase in deposition over control tissues. Lamprecht went on to quantify the amount of particles remaining after mucus removal by ‘extensive’ washing of the tissues, which removed 62% of 0.1 µm, 69% of 1 µm, and 85% of 10 µm particles. Thus, the authors concluded that the nonspecific targeting to the inflamed epithelium was largely due to increased mucus secretion by the inflamed tissues.
3.2 Mucoadhesive pH-responsive systems
Delivery with pH-responsive particles has been of particular interest in the GI tract, because pH varies throughout the GI tract [see section 2]. The pH gradient trends from high acidity in the stomach to a neutral or slightly alkaline pH in the distal colon, although portions of the intestines can reach near neutral pH [86]. A widely adopted approach is to use polymers that exhibit pH-dependent swelling [87]. Acrylic-based polymers such as poly(methacrylic acid) (PMAA) retain a collapsed state in the low pH of the stomach, and swell as they transit through the intestines. Blends of this polymer with polyethylacrylate (PMAA-PEA) and polymethacrylate (PMAA-PMA) can be tailored to dissolve in specific pH ranges corresponding to specific locations in the GI tract [88]. For more details, the reader is referred to more comprehensive reviews of pH-responsive materials and nanoparticles [89, 90].
Lin and coworkers designed novel “multi-ion-crosslinked” nanoparticles composed of chitosan, poly-γ-glutamic acid, tripolyphosphate, and Mg ions loaded with insulin that slowly release insulin until reaching pH 7. They were able to demonstrate in vitro that these particles delivered insulin across Caco-2 cell monolayers, indicating promise for delivery of insulin in the small intestine [91]. However, the mucus barrier is absent in this type of in vitro system. In similar work, Sonaje and coworkers investigated the use of pH-responsive chitosan/poly-γ-glutamic acid acid nanoparticles loaded with a rapid-acting insulin analog in rats. The orally delivered nanoparticles were shown to release slowly at low pH and rapidly at higher pH, thereby potentially localizing their delivery to the small intestine. Compared to a subcutaneous dose of fast-acting insulin, the particles resulted in a slower and lower spike in insulin levels (peak of 40 µIU/mL at 3 h compared to a peak of >100 µIU/mL in minutes) and thus a less dramatic decrease in blood glucose levels (minimum of 60% of base level as compared to 20% of the base level). When compared to the standard subcutaneous insulin treatment, the particles produced a similar blood glucose response, indicating that the nanoparticles could be a promising non-invasive alternative [92].
Although human trials have not been completed with pH-responsive nanoparticles, trials conducted with ingested pH-responsive pellets could provide important insight. McConnell and coworkers prepared theophylline pellets (1–1.4 mm) coated with a pH-responsive polymer (Eudragit® S) that dissolves above pH 7, intending to target the colon for drug release. The results in 8 human fasted volunteers were very mixed: there was no dissolution detected in 1 volunteer, the AUC ranged from 8.8–55.0 µg h/mL, and the drug release started in the small intestine [93]. Ibekwe et al. also tested 8 mm Eudragit® S coated tablets in healthy subjects in various states of feeding or fasting while monitoring the intestinal pH and pellet degradation time. Intestinal transit time and GI tract pH varied between subjects, 1 out of 8 pellets did not degrade in the fasted state, and 3 out of 8 pellets did not degrade in both the fed and pre-fed regimens [94]. It was difficult to draw conclusions about the effect of transit time, feeding state, and pH on the dissolution of the pellets. Although dissolution would be more rapid for nanoparticles, these studies illustrated variations in pH both along the GI tract and between patients that will significantly affect pharmacokinetics of pH-responsive systems.
3.3 Mucoadhesive lipid-based systems
Lipids are present in significant amounts throughout the GI tract [see section 2], which makes lipid-based systems attractive for oral delivery. There are many formulation types, such as liposomes, mixed micelles, micro- and nanoemulsions, and solid lipid nanoparticles [95]. The main advantage of lipid-based systems is the increased solubility and bioavailability of the encapsulated drugs [96]. A major drawback, however, is that lipid formulations are not as stable in the gut as polymeric systems [97]. It is generally thought that the performance is not based on the inherent stability or initial dispersion size, but by how the formulation is processed in the GI tract [96]. Lipid formulations have been used extensively for many different types of drugs and as synthetic vectors for nucleic acid and DNA delivery, and have been extensively reviewed [95–98].
4. Mucus penetrating systems
4.1 Disrupting the mucus barrier
An alternative approach to studies employing nanoparticles designed to be mucoadhesive is to develop methods that enhance nanoparticle-based drug delivery by disrupting barrier properties of the mucus lining. Although Peyer’s patches are reported to be less protected by the mucus barrier than the rest of the GI tract, this lymphoid tissue only accounts for 1% of the total surface area [42]. The mucus barrier thus limits delivery to the non-lymphoid tissue, as well as potentially binding and eliminating the majority of nanoparticles targeted to the Peyer’s patches. Eyles et al. discovered that the way nanoparticles are introduced can significantly influence their fate in the GI tract. They investigated the effect of increasing the volume administered to rats by gavage over a range of 0.1 to 0.5 mL. Interestingly, polystyrene nanoparticles administered in 0.1 mL volume were found mostly trapped on the intestinal mucus surface, whereas nanoparticles administered in 0.5 mL volume appeared to “channel” through the mucus barrier. This “viscous fingering” occurs when a lower viscosity fluid, on being pressure driven through a higher viscosity fluid, forms channels filled with low viscosity fluid (Figure 6) [13]. This enhancement in particle penetration led to a 6-fold increase in particles in the blood after 15 minutes [62].
Figure 6.
Snapshot of the concentration field during the displacement of a more viscous fluid (dark) by a fully-miscible, less viscous fluid (light). Figure obtained from [128].
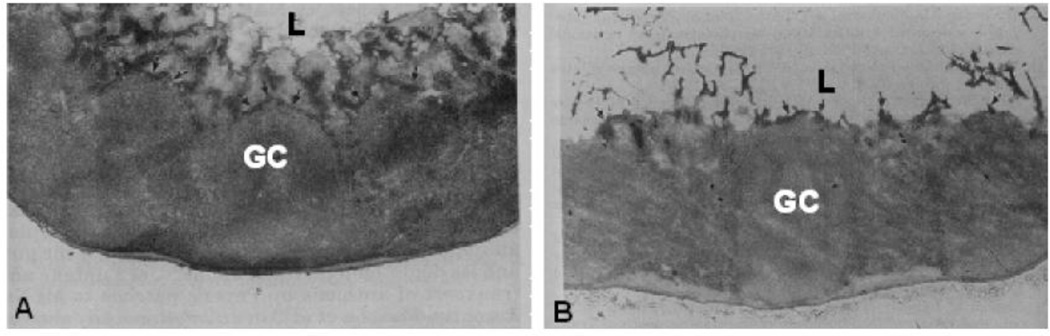
More typically, investigators use mucolytics and so-called “permeation enhancers” to improve delivery in the GI tract. It is of note that mucolytics are generally used in the lung with diseases, such as cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD), where mucus is abnormally viscoelastic [6, 99]; the effects of mucolytics on the healthy mucosa, thereby transiently diminishing the barrier properties and normal rheological function, are unclear. N-acetyl-L-cysteine (NAC), a commonly used mucolytic, was shown to cause a 6-fold increase in the absorption of 3.2 µm polystyrene particles in both the Peyer’s patches and mesenteric lymph nodes in a ligated rat intestine model [100]. Figure 7 shows the effect of the 10% NAC treatment on the GI mucus layer as compared to the normal mucus barrier. As is evident in Figure 7B, the NAC treatment disrupts the mucus barrier present in Figure 7A, increasing the number of particles that can access the Peyer’s patches. However, the authors referenced other studies in which bacterial translocation and attachment was increased by the removal of the mucus barrier, citing the important protective properties and the need for preserving the integrity of the mucus lining. For example, a 30% depletion of mucus by pilocarpine in an ex vivo rat intestinal model led to a 3-fold increase in E. Coli translocation [26].
Figure 7.
Distribution of the mucus gel layer in front of the Peyer’s patch in (A) a control group and (B) the N-acetylcysteine (NAC) group. Periodic acid-Schiff staining shows a uniform, continuous layer of mucus gel in front of the Peyer’s patch that is then completely lacking after NAC treatment. L, lumen; GC, germinal center. Magnification, ×25. Figure adapted from [100].
It has been suggested that mucolytics may create a mucus-free surface that could facilitate the attachment of particles conjugated to mucosal cell targeting ligands, such as tomato lectin [101]. Lehr et al. observed that these lectin-conjugated particles bind strongly to mucosal cell surfaces, but this binding is reduced in the presence of mucus [70]. Indeed, others have questioned this proposed method and the effects of undermining the protective function of the mucus barrier in the presence of proteolytic enzymes and acid [16]. Overall, there is significant evidence that mucus is a substantial barrier to nanoparticulates, and these barrier properties cannot be undermined without compromising the mucosa.
4.2 Particles that penetrate the mucus barrier
Although GI mucus is thought to be a significant barrier to nanoparticle translocation, it is possible to engineer nanoparticles capable of penetrating the mucus barrier. Viruses evolved a method to accomplish this feat. Olmsted and coworkers determined that many viruses are capable of penetrating mucus [102], by (i) being small enough to avoid being blocked sterically by the mucin mesh, (ii) possessing surfaces without mucoadhesive hydrophobic areas, and (iii) densely coating exposed surfaces with negative and positive charges yielding a net-neutral, highly hydrophilic surface [4, 5]. Similarly, bacterial strains grown with more hydrophilic surface lectins were able to penetrate mucin gels much more readily than those grown with hydrophobic surfaces [103]. For the GI tract in particular, the hydrophobic mucus barrier properties are due to both the “naked” (non-glycosylated) regions of mucin glycoproteins [29] and the lipid constituents [30]. Larhed and coworkers demonstrated the hydrophobic barrier properties of GI mucus could even slow the diffusion of small molecules like testosterone [104, 105]; this effect was the most pronounced for “native” pig intestinal mucus as compared to reconstituted mucin purified from the native samples. The authors demonstrated that the pig intestinal mucus contained 37% (w/w) lipids and only 5% (w/w) mucin proteins by dry weight, and that adding additional lipid and proteins to the reconstituted mucus led to diffusion rates that were similar to the “native” mucus. Also, as the concentration of purified mucin increased, the diffusion rate of testosterone exponentially decreased, illustrating the importance of both the mucin and lipid components to the barrier properties
Non-specific hydrophobic interactions similarly affect particle penetration through mucus [60], considering the majority of conventional nanoparticles used for GI tract delivery are composed of hydrophobic polymers. Thus, particles composed of polystyrene, for example, are likely to be mucoadhesive. Frey and coworkers investigated the binding of 1 µm polystyrene beads to ligated rabbit intestinal loops after 1 hr particle exposure. These particles were coated with cholera toxin, which binds specifically to glycolipids in membranes of all cells. However, the particles only bound to M-cells and not to enterocytes as seen in Figure 8, suggesting that the particles were not able to penetrate the mucus coating the epithelium [2]. Although the lack of penetration could have been a size effect, a similar observation was made using particles smaller than 300 nm. When investigating the trafficking of poly(lactic acid) nanoparticles in ligated mouse intestinal loops, Primard and coworkers also discovered that the nanoparticles were mainly retained in the mucus layer over non-lymphatic tissue, shown in Figure 9 [106]. This trapping effect was evident even though 500 uL of volume (enough to “fill the loop”) was used, which could cause significant dilution of the mucus barrier.
Figure 8.
Simultaneous uptake of equal numbers of CTB-coated (red) and control microparticles (green) into rabbit Peyer’s patch domes after 1 h exposure. Fluorescence microscopy of a representative cryostat section shows that both types of particles were taken up into the dome, but not into adjacent villi. Scale bar, 100 µm. Figure obtained from [2].
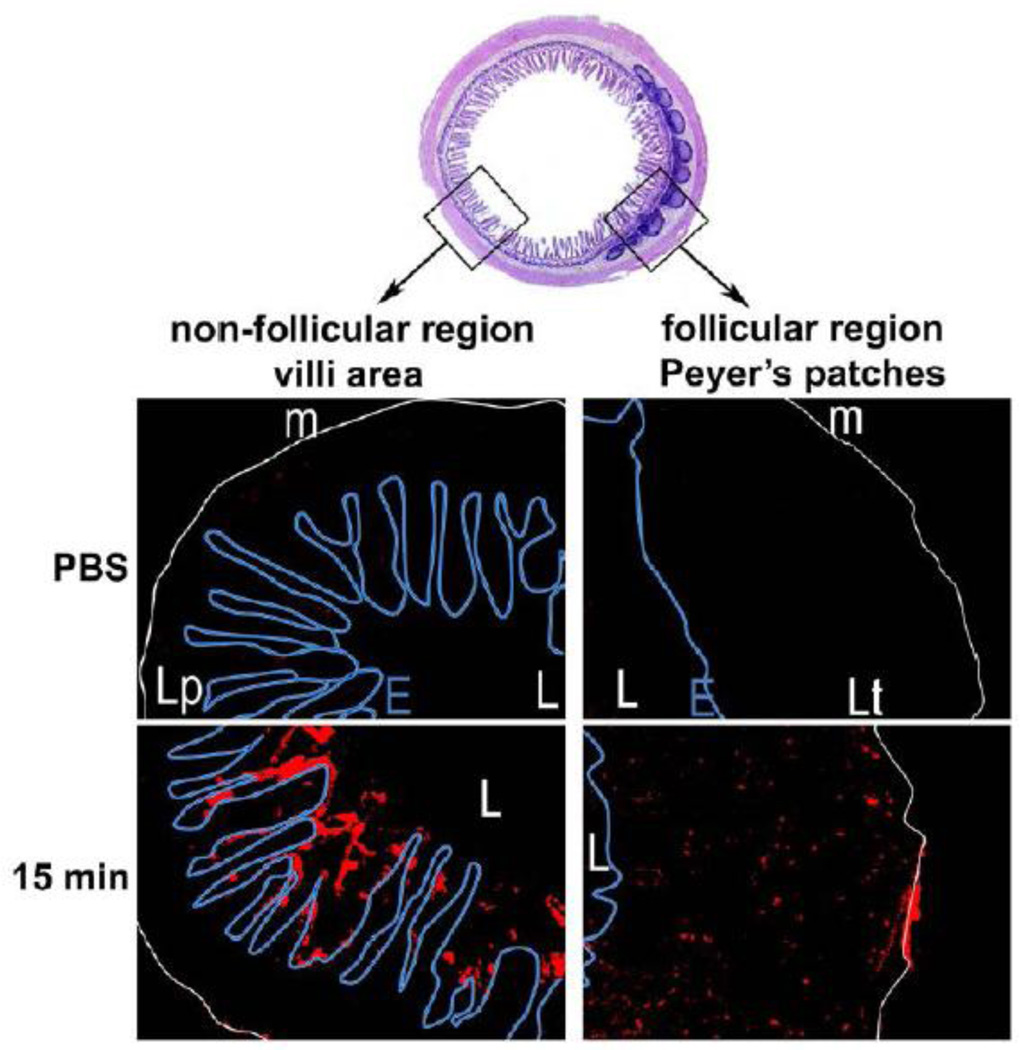
Figure 9.
Fluorescent micrographs obtained from transversal cryosections of mice ileum intestine. In vivo intestinal ligated loop was incubated with 10 mg/mL of CellTrace BODIPY nanoparticles (NPs) diluted in PBS for 15, 30, 45, and 60 min (only showing 15 mins, trends similar across all time points). Micrographs were obtained from villus and Peyer’s patch (PP) areas. The letter L indicates the intestinal lumen, the muscularis mucosa is underscored with a white line (m), and the epithelial barrier is indicated by a blue line (E), around the Lamina propia (Lp in villus area) or the lymphoid tissue (Lt in PP area). Original magnification ×10. Figure adapted from [106].
Despite previous results by other authors that indicated a homogeneous layer of nanoparticles associated with the gut wall, Kreuter and coworkers found that 200–300 nm nanoparticles composed of another hydrophobic polymer, polycyanoacrylate, were irregularly distributed throughout the intestine of mice [37]. They also found large variations in nanoparticle distribution between animals, which was suggested as indicative of nanoparticles traveling in a ‘slug’ of mucin (as illustrated in Figs 2 and 4). In contrast, Szentkuti observed that polystyrene nanoparticles as large as 415 nm were capable of rapidly contacting the rat colonic epithelium; these particles were able to traverse 30–50 µm of mucus in just 2 minutes [107]. However, according to the Stokes-Einstein equation for Brownian diffusion, particles of this size in water (the main component of mucus) at body temperature would diffuse at ~1 µm2/sec. These polystyrene particles would have had to diffuse at a rate of 8–20 µm2/sec, which is highly unlikely. Considering the large volume (200 µL of PBS) was administered by enema in a ligated colon section with pellets present, the fluid pressure may have caused viscous fingering, rapidly transporting particles through watery channels to the epithelium.
The variation in animal models is further confounded by discordant results in various in vitro models. In many cases, reconstituted mucin solutions are used without the additional lipid, protein, and cellular components that are present in native mucus. Norris and coworkers observed significant permeability of polystyrene nanoparticles less than 300 nm in size with various surface charges through reconstituted mucin gel in a diffusion chamber [60]. However, a significant portion of the mucin used was able to diffuse through the membrane pores between the two chambers, which would further reduce the barrier properties of the mucin gel. More recently, Lieleg and coworkers also investigated the diffusion of polystyrene particles with different surface charges in reconstituted pig gastric mucin gels. Interestingly, they used 0.25–1% mucin gels, which is lower concentration than the typical 2–5% used [108]. This work focused heavily on the role of surface charge and mucin gel pH, which was found to significantly affect the particle diffusion. However, no mention of hydrophobic interactions was made, despite the fact that gastric mucus in vivo has considerable hydrophobic character due to the presence of lipids that are not present in purified mucin gels [30]. Crater and coworkers compared the transport rates of polystyrene particles with various surface charges in “native” pig intestinal mucus and purified reconstituted intestinal mucin. Overall, they found that positively charged polystyrene nanoparticles were slowed as compared to those with a negative surface charge, but the overall diffusion rates were not slowed more than 20–40 fold as compared to their theoretical diffusion rates in water; additionally, the nanoparticle diffusion in “native” mucus and reconstituted mucin gel were very similar [109]. Considering what has been previously found regarding comparisons of purified mucin gels with native mucus, it is possible that the procedure for obtaining the “native” mucus removed some of the components that confer the significant hydrophobic barrier properties of GI mucus that would render polystyrene particles mucoadhesive.
To our knowledge, there has not been a demonstration of inherently mucus penetrating nanoparticles in GI mucus, in vitro or in vivo. Rather, “penetration” has occurred in systems where the native mucosa is physically or chemically altered, present on top of cell culture unlikely to reproduce the true complexity, or stripped of components that are vital to the barrier properties. To reliably ascertain that particles will be able to penetrate mucus in vivo, carefully obtained, unaltered samples must be freshly obtained and used with minimal dilution, as has been done with cervicovaginal mucus (CVM) and Cystic Fibrosis (CF) sputum [6–10, 33, 99, 110–112]. Although it has been clearly demonstrated that polystyrene nanoparticles are highly mucoadhesive due to their hydrophobic core (regardless of surface charge), Lai and coworkers were able to engineer a mucus penetrating coating by covalently attaching a high density of low molecular weight polyethylene glycol (PEG) [9, 33, 111, 112]. These mucus penetrating particles (MPP) penetrate healthy, undiluted human CVM at rates only a few fold slower than their theoretical diffusion in pure water. This mucoinert coating has been translated to engineering biodegradable nanoparticles [8], nanoparticles capable of penetrating highly viscoelastic cystic fibrosis sputum [7], and biodegradable particles composed entirely of “generally regarded as safe” (GRAS) ingredients [10]. Using these mucoinert particles as “probes”, this group has also determined the pore size (mesh spacing) for CVM [112] and investigated the effects of agents that alter the mucus microstructure [111]. Figure 10 shows the ensemble effective diffusivity of biodegradable MPP in undiluted CVM, which is 100-fold higher than that of conventional mucoadhesive nanoparticles composed of polystyrene, PLGA, and poly(sebacic acid) (PSA). The same potential for improved delivery exists for these mucus penetrating particles in the GI tract. By penetrating into the more slowly cleared adherent mucus layer, MPP may provide increased GI tract residence time over mucoadhesive (conventional) particles (CP), leading to prolonged drug absorption (Figure 11).
Figure 10.
Geometric ensemble effective diffusivity (<Deff>) at a time scale of 1 sec for polystyrene (PS), poly(lactic-co-glycolic acid) (PLGA), poly(sebacic acid) (PSA), and poly(sebacic acid)-co-poly(ethylene glycol) (PSA-PEG) in CVM. × denotes individual sample <Deff> values (n = 3); – denotes the average. Figure adapted from [8].
Figure 11.
A summary schematic illustrating the fate of mucus penetrating particles (MPP) and conventional mucoadhesive particles (CP) administered to the GI mucosal surface. MPP readily penetrate the loosely adherent mucus layer and enter the firmly adherent mucus layer. In contrast, CP are immobilized in the loosely adherent layer. Because MPP can enter the firmly adherent layer and thus are in closer proximity to cells, cells will be exposed to a greater dose of drug released from MPP compared to drug released from CP. As the loosely adherent layer is cleared, CP are removed, whereas MPP are retained longer within the firmly adherent layer and continue to release drugs to cells. Figure adapted from [5].
5. Alterations to the mucus barrier in disease states
Another important consideration for oral drug delivery is that the barrier properties of mucus are altered in disease states. Despite the highly protective properties of GI mucus, breaches do occur. On a daily basis, the GI tract is confronted with the formidable task of allowing transfer and uptake of nutrients and fluids, while being confronted with potentially harmful substances and pathogenic bacteria. It is remarkable that the GI tract is able to maintain a healthy homeostasis, considering the enormous surface area (300–400 m2 in humans) and colonization by ~1014 bacteria of more than 500 different species [1]. Although it is often unclear whether a disease state is first established, followed by an alteration in the mucosal barrier versus a direct breach of an intact barrier, it is known that histochemical alterations in mucins occur in many GI diseases [113]. In the case of inflammatory disorders, the changes are not in MUC gene expression or polymorphism, but in glycosylation patterns on mucins [11]. The degree of sulfation and sialylation, and the length of the oligosaccharide chains vary with Irritable Bowel Disease (IBD), which can change the viscoelastic properties, resistance to bacterial degradation and adhesion, and general interactions with the environment of the mucus barrier [34]. Glycosylation changes have been implicated in the early events of gastritis and ulcers; the concomitant diminished barrier properties allow Helicobacter pylori penetration and attachment, which then leads to reversible alteration in glycosylation that favors further attachment and colonization of the bacteria [114]. In the case of ulcerative colitis (UC), the barrier properties of the mucus layer are diminished in multiple ways. The overall thickness of the adherent mucus layer in the colorectum is reduced due to overall depletion of goblet cells [115, 116], accompanied by reduced expression of the protective trefoil peptides [117]. Additionally, altered lipid composition [118, 119] may be responsible for the observed decrease in surface tension and hydrophobicity in clinical specimens obtained from UC patients [120]. Although it is not clear which factor contributes most to UC pathogenesis, it was recently found in a phase IIa/b studies that lipid supplementation in the colon led to mucosal healing in 60% of patients [121]. It has also been reported that some intestinal inflammatory diseases could modify the permeability of the epithelium, thus allowing nanoparticles to be more easily transported. Similarly, bacterial invasion can induce up-regulation of particle transport via Peyer’s patches [42].
Oral delivery of nanoparticles is also being investigated for treatment and detection of cancers [122–124], so it is important to understand the effect of tumor presence on the mucosa. Mucins have a complex and integral role in tumor development. Mucosal cancer is often associated with alterations in both glycosylation and mucin expression, with mucus secretion rates and glycosylation varying depending on the tumor type and location [11]. It has been recently hypothesized that increased expression of mucins by cancer cells and aberrant glycosylation may impede drug uptake, which can contribute to drug resistance [125]. There is also extensive evidence that cell-bound mucins play a significant role in the progression of epithelial cancers; cancer cells overexpress transmembrane mucins that promote cell growth and survival [126]. For more comprehensive reviews on the role of mucins in cancer, see [15, 126].
6. Animal models
In general, in vivo studies investigating oral delivery of nanoparticles have mainly focused on therapeutic effects or pharmacokinetics, as opposed to understanding the fate of the particles and how the results might translate in humans. Thus, it is worth briefly discussing the relevance of commonly used animal models. Rats and mice are most commonly used, but these rodents do not produce as much mucin as humans, possibly reducing the barrier properties to drug delivery and nanoparticle distribution in the GI tract [37, 39, 127]. Additionally, rodents, particularly rabbits, have a high density of Peyer’s patches as compared to humans [39]. Rodents, as well as many other animals, have less acidic stomachs, which could significantly impact comparative formulation stability. Significant differences in bacterial colonization, stomach emptying times, intestinal transit times, mucus thicknesses, etc., as detailed in a more comprehensive review comparing the anatomy, physiology, and biochemistry of humans and commonly-used laboratory animals [23] must be considered. Care must be taken both when selecting an animal model, as well as when interpreting and comparing results for oral delivery.
7. Conclusions
Oral delivery is the most commonly used and readily accepted form of drug administration. Many small molecule drugs are successfully administered via the oral route, due to the high absorptive capacity of the GI tract. However, many drugs are not suitable for oral administration due to poor solubility, stability, and/or bioavailability. Encapsulating these drugs in nanoparticles can overcome these limitations, as well as allowing the potential for targeted, sustained delivery in the GI tract.
Significant barriers in the GI tract exist for nanoparticle formulations. Nanoparticles must withstand the acidic environment of the stomach, as well as the degradative enzymes in the intestines. Also, nanoparticles in the GI tract must penetrate the mucus barrier being secreted by the epithelium. The unique rheological and adhesive properties of mucus protect the epithelium from both mechanical forces and foreign pathogens and particles. Rapid mucus secretion and clearance rates efficiently remove foreign materials, limiting the residence time of orally administered nanoparticles.
Many promising studies have been completed with various drugs. However, there is a vast array of in vitro systems and animal models that have been used, which has produced discordant results regarding the optimum characteristics for efficient nanoparticle delivery in the GI tract. Additionally, there is significant evidence indicating that efficient oral drug delivery in the GI tract is limited by nanoparticles that adhere to the mucus barrier. Mucus penetrating particles can potentially improve oral drug delivery by penetrating the quickly cleared, loosely adherent mucus layer and be retained longer in the firmly adherent layer. Increased GI tract residence time and increased distribution over the epithelium could lead to more effective treatments.
Acknowledgments
This work was supported in part by the NIH (5R01HD062844, 5R33AI079740, 5R21AI094519) (J.H. and R.C), the Cystic Fibrosis Foundation (HANES07XX0) (J.H.), and fellowships from the National Science Foundation and the Howard Hughes Medical Institute (L.M.E).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The mucus penetrating particle technology described in this publication is being developed by Kala Pharmaceuticals. Dr. Hanes is co-founder of Kala, where he serves on the Board of Directors and as a consultant. Drs. Hanes and Cone own company stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Cl Ga. 2008;22:391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: Implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plapied L, Duhem N, des Rieux A, Preat V. Fate of polymeric nanocarriers for oral drug delivery. Curr Opin Colloid In. 2011;16:228–237. [Google Scholar]
- 4.Cone RA. Barrier properties of mucus. Advanced Drug Delivery Reviews. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine-Uk. 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, Hanes J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30:2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. P Natl Acad Sci USA. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that "Slip" through the Human Mucus Barrier. Angew Chem Int Edit. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Lai SK, Wang YY, Zhong WX, Happe C, Zhang M, Fu J, Hanes J. Biodegradable Nanoparticles Composed Entirely of Safe Materials that Rapidly Penetrate Human Mucus. Angew Chem Int Edit. 2011;50:2597–2600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corfield AP, Carroll D, Myerscough N, Probert CSJ. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 12.Carlstedt I, Sheehan JK. Structure and Macromolecular Properties of Mucus Glycoproteins. Monogr Allergy. 1988;24:16–24. [PubMed] [Google Scholar]
- 13.Cone R. Mucus. In: Mestecky L, Strober, Bienenstock, McGhee, editors. Handbook of Mucosal Immunology. London: Academic Press; 2005. pp. 49–72. [Google Scholar]
- 14.Bansil R, Turner BS. Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid In. 2006;11:164–170. [Google Scholar]
- 15.Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 16.Macadam A. The Effect of Gastrointestinal Mucus on Drug Absorption. Advanced Drug Delivery Reviews. 1993;11:201–220. [Google Scholar]
- 17.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Advanced Drug Delivery Reviews. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol-Gastr L. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 19.Sandzen B, Blom H, Dahlgren S. Gastric Mucus Gel Layer Thickness Measured by Direct Light-Microscopy - an Experimental-Study in the Rat. Scand J Gastroentero. 1988;23:1160–1164. doi: 10.3109/00365528809090185. [DOI] [PubMed] [Google Scholar]
- 20.Copeman M, Matuz J, Leonard AJ, Pearson JP, Dettmar PW, Allen A. The Gastroduodenal Mucus Barrier and Its Role in Protection against Luminal Pepsins - the Effect of 16,16-Dimethyl Prostaglandin-E(2), Carbopol-Polyacrylate, Sucralfate and Bismuth Subsalicylate. J Gastroen Hepatol. 1994;9:S55–S59. doi: 10.1111/j.1440-1746.1994.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 21.Kerss S, Allen A, Garner A. A Simple Method for Measuring Thickness of the Mucus Gel Layer Adherent to Rat, Frog and Human Gastric-Mucosa - Influence of Feeding, Prostaglandin, N-Acetylcysteine and Other Agents. Clin Sci. 1982;63:187–195. doi: 10.1042/cs0630187. [DOI] [PubMed] [Google Scholar]
- 22.Montagne L, Piel C, Lalles JP. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr Rev. 2004;62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 23.Kararli TT. Comparison of the Gastrointestinal Anatomy, Physiology, and Biochemistry of Humans and Commonly Used Laboratory-Animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 24.Bhaskar KR, Garik P, Turner BS, Bradley JD, Bansil R, Stanley HE, Lamont JT. Viscous Fingering of Hcl through Gastric Mucin. Nature. 1992;360:458–461. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- 25.Hastewell J, Williamson I, Mackay M. Cell Biology and Active-Transport Processes in the Colon. Advanced Drug Delivery Reviews. 1991;7:119–147. [Google Scholar]
- 26.Albanese CT, Cardona M, Smith SD, Watkins S, Kurkchubasche AG, Ulman I, Simmons RL, Rowe MI. Role of Intestinal Mucus in Transepithelial Passage of Bacteria across the Intact Ileum in-Vitro. Surgery. 1994;116:76–82. [PubMed] [Google Scholar]
- 27.Sarosiek J, Slomiany A, Takagi A, Slomiany BL. Hydrogen-Ion Diffusion in Dog Gastric Mucus Glycoprotein - Effect of Associated Lipids and Covalently Bound Fatty-Acids. Biochem Bioph Res Co. 1984;118:523–531. doi: 10.1016/0006-291x(84)91334-2. [DOI] [PubMed] [Google Scholar]
- 28.Witas H, Sarosiek J, Aono M, Murty VLN, Slomiany A, Slomiany BL. Lipids Associated with Rat Small-Intestinal Mucus Glycoprotein. Carbohyd Res. 1983;120:67–76. doi: 10.1016/0008-6215(83)88007-0. [DOI] [PubMed] [Google Scholar]
- 29.Gwozdzinski K, Slomiany A, Nishikawa H, Okazaki K, Slomiany BL. Gastric mucin hydrophobicity - effects of associated and covalently bound lipids, proteolysis, and reduction. Biochemistry International. 1988;17:907–917. [PubMed] [Google Scholar]
- 30.Lichtenberger LM. The Hydrophobic Barrier Properties of Gastrointestinal Mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 31.Murty VLN, Sarosiek J, Slomiany A, Slomiany BL. Effect of Lipids and Proteins on the Viscosity of Gastric Mucus Glycoprotein. Biochem Bioph Res Co. 1984;121:521–529. doi: 10.1016/0006-291x(84)90213-4. [DOI] [PubMed] [Google Scholar]
- 32.Khanvilkar K, Donovan MD, Flanagan DR. Drug transfer through mucus. Advanced Drug Delivery Reviews. 2001;48:173–193. doi: 10.1016/s0169-409x(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 33.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. P Natl Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirazi T, Longman RJ, Corfield AP, Probert CSJ. Mucins and inflammatory bowel disease. Postgrad Med J. 2000;76:473–478. doi: 10.1136/pmj.76.898.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 36.Galindo-Rodriguez SA, Allemann E, Fessi H, Doelker E. Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit Rev Ther Drug Carrier Syst. 2005;22:419–464. doi: 10.1615/critrevtherdrugcarriersyst.v22.i5.10. [DOI] [PubMed] [Google Scholar]
- 37.Kreuter J, Muller U, Munz K. Quantitative and Microautoradiographic Study on Mouse Intestinal Distribution of Polycyanoacrylate Nanoparticles. Int J Pharm. 1989;55:39–45. [Google Scholar]
- 38.Florey HW. Secretion and Function of Intestinal Mucus. Gastroenterology. 1962;43:326. [PubMed] [Google Scholar]
- 39.Gruber P, Longer MA, Robinson JR. Some biological issues in oral, controlled drug delivery. Advanced Drug Delivery Reviews. 1987;1:1–18. [Google Scholar]
- 40.Tirosh B, Rubinstein A. Migration of adhesive and nonadhesive particles in the rat intestine under altered mucus secretion conditions. J Pharm Sci. 1998;87:453–456. doi: 10.1021/js9703380. [DOI] [PubMed] [Google Scholar]
- 41.Woodley J. Bioadhesion - New possibilities for drug administration? Clin Pharmacokinet. 2001;40:77–84. doi: 10.2165/00003088-200140020-00001. [DOI] [PubMed] [Google Scholar]
- 42.des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. Journal of Controlled Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Chickering DE, Jacob JS, Desai TA, Harrison M, Harris WP, Morrell CN, Chaturvedi P, Mathiowitz E. Bioadhesive microspheres .3. An in vivo transit and bioavailability study of drug-loaded alginate and poly(fumaric-co-sebacic anhydride) microspheres. Journal of Controlled Release. 1997;48:35–46. [Google Scholar]
- 44.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Biologically erodable microsphere as potential oral drug delivery system. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 45.Lowe PJ, Temple CS. Calcitonin and Insulin in Isobutylcyanoacrylate Nanocapsules - Protection against Proteases and Effect on Intestinal-Absorption in Rats. J Pharm Pharmacol. 1994;46:547–552. doi: 10.1111/j.2042-7158.1994.tb03854.x. [DOI] [PubMed] [Google Scholar]
- 46.Damge C, Vranckx H, Balschmidt P, Couvreur P. Poly(alkyl cyanoacrylate) nanospheres for oral administration of insulin. J Pharm Sci. 1997;86:1403–1409. doi: 10.1021/js970124i. [DOI] [PubMed] [Google Scholar]
- 47.Tobio M, Sanchez A, Vila A, Soriano I, Evora C, Vila-Jato JL, Alonso MJ. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloid Surface B. 2000;18:315–323. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 48.Rieux Ad, Ragnarsson EGE, Gullberg E, Préat V, Schneider Y-J, Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. European Journal of Pharmaceutical Sciences. 25:455–465. doi: 10.1016/j.ejps.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled Vaccine Release in the Gut-Associated Lymphoid-Tissues .1. Orally-Administered Biodegradable Microspheres Target the Peyers Patches. Journal of Controlled Release. 1990;11:205–214. [Google Scholar]
- 50.Behrens I, Pena AIV, Alonso MJ, Kissel T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharmaceut Res. 2002;19:1185–1193. doi: 10.1023/a:1019854327540. [DOI] [PubMed] [Google Scholar]
- 51.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28:2233–2243. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Advanced Drug Delivery Reviews. 2010;62:28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi H, Thongborisute J, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Advanced Drug Delivery Reviews. 2005;57:1583–1594. doi: 10.1016/j.addr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. Journal of Controlled Release. 2002;78:15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 56.Kawashima Y, Yamamoto H, Takeuchi H, Kuno Y. Mucoadhesive DL-lactide/glycolide copolymer nanospheres coated with chitosan to improve oral delivery of elcatonin. Pharm Dev Technol. 2000;5:77–85. doi: 10.1081/pdt-100100522. [DOI] [PubMed] [Google Scholar]
- 57.Schipper NGM, Varum KM, Stenberg P, Ocklind G, Lennernas H, Artursson P. Chitosans as absorption enhancers of poorly absorbable drugs 3: Influence of mucus on absorption enhancement. European Journal of Pharmaceutical Sciences. 1999;8:335–343. doi: 10.1016/s0928-0987(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 58.Jani P, Halbert GW, Langridge J, Florence AT. The Uptake and Translocation of Latex Nanospheres and Microspheres after Oral-Administration to Rats. J Pharm Pharmacol. 1989;41:809–812. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 59.Roger E, Lagarce F, Garcion E, Benoit JP. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine-Uk. 2010;5:287–306. doi: 10.2217/nnm.09.110. [DOI] [PubMed] [Google Scholar]
- 60.Norris DA, Puri N, Sinko PJ. The effect of physical barriers and properties on the oral absorption of particulates. Advanced Drug Delivery Reviews. 1998;34:135–154. doi: 10.1016/s0169-409x(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 61.Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Advanced Drug Delivery Reviews. 2001;50:107–142. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 62.Eyles J, Alpar HO, Field WN, Lewis DA, Keswick M. The Transfer of Polystyrene Microspheres from the Gastrointestinal-Tract to the Circulation after Oral-Administration in the Rat. J Pharm Pharmacol. 1995;47:561–565. doi: 10.1111/j.2042-7158.1995.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 63.Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Advanced Drug Delivery Reviews. 2004;56:459–480. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Advanced Drug Delivery Reviews. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Harokopakis E, Hajishengallis G, Michalek SM. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson GF, Halbert GW. The in vitro cell association of invasin coated polylactide-co-glycolide nanoparticles. Pharmaceut Res. 2000;17:1420–1425. doi: 10.1023/a:1007503123620. [DOI] [PubMed] [Google Scholar]
- 67.Russell-Jones GJ, Arthur L, Walker H. Vitamin B12-mediated transport of nanoparticles across Caco-2 cells. Int J Pharm. 1999;179:247–255. doi: 10.1016/s0378-5173(98)00394-9. [DOI] [PubMed] [Google Scholar]
- 68.Clark MA, Hirst BH, Jepson MA. Lectin-mediated mucosal delivery of drugs and microparticles. Advanced Drug Delivery Reviews. 2000;43:207–223. doi: 10.1016/s0169-409x(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 69.Irache JM, Durrer C, Duchene D, Ponchel G. Preparation and characterization of lectin-latex conjugates for specific bioadhesion. Biomaterials. 1994;15:899–904. doi: 10.1016/0142-9612(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 70.Lehr C-M, Bouwstra JA, Kok W, Noach ABJ, de Boer AG, Junginger HE. Bioadhesion by Means of Specific Binding of Tomato Lectin. Pharmaceut Res. 1992;9:547–553. doi: 10.1023/a:1015804816582. [DOI] [PubMed] [Google Scholar]
- 71.Hussain N, Jani PU, Florence AT. Enhanced Oral Uptake of Tomato Lectin-Conjugated Nanoparticles in the Rat. Pharmaceut Res. 1997;14:613–618. doi: 10.1023/a:1012153011884. [DOI] [PubMed] [Google Scholar]
- 72.Owen RL, Bhalla DK. Cytochemical Analysis of Alkaline-Phosphatase and Esterase-Activities and of Lectin-Binding and Anionic Sites in Rat and Mouse Peyers Patch M-Cells. Am J Anat. 1983;168:199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- 73.Nagata Y, Burger MM. Wheat Germ Agglutinin. Journal of Biological Chemistry. 1974;249:3116–3122. [PubMed] [Google Scholar]
- 74.Yin YS, Chen DW, Qiao MX, Lu Z, Hu HY. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. Journal of Controlled Release. 2006;116:337–345. doi: 10.1016/j.jconrel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Yin YS, Chen DW, Qiao MX, Wei XY, Hu HY. Lectin-conjugated PLGA nanoparticles loaded with thymopentin: Ex vivo bioadhesion and in vivo biodistribution. Journal of Controlled Release. 2007;123:27–38. doi: 10.1016/j.jconrel.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 76.Hussain N, Florence AT. Utilizing bacterial mechanisms of epithelial cell entry: Invasin-induced oral uptake of latex nanoparticles. Pharmaceut Res. 1998;15:153–156. doi: 10.1023/a:1011981610840. [DOI] [PubMed] [Google Scholar]
- 77.Salman HH, Gamazo C, Campanero MA, Irache JM. Salmonella-like bioadhesive nanoparticles. Journal of Controlled Release. 2005;106:1–13. doi: 10.1016/j.jconrel.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 78.Bhavsar MD, Amiji MM. Polymeric nano- and microparticle technologies for oral gene delivery. Expert Opin Drug Del. 2007;4:197–213. doi: 10.1517/17425247.4.3.197. [DOI] [PubMed] [Google Scholar]
- 79.Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS) Journal of Controlled Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Bhavsar MD, Amiji MM. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther. 2008;15:1200–1209. doi: 10.1038/gt.2008.67. [DOI] [PubMed] [Google Scholar]
- 81.LihBrody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Digest Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 82.Peng YC, Hsu CL, Tung CF, Chou WK, Huang LR, Hung DZ, Hu WH, Yang DY. Chemiluminescence assay of mucosal reactive oxygen species in gastric cancer, ulcer and antral mucosa. Hepato-Gastroenterol. 2008;55:770–773. [PubMed] [Google Scholar]
- 83.Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepato-Gastroenterol. 2001;48:743–751. [PubMed] [Google Scholar]
- 84.Wilson DS, Dalmasso G, Wang LX, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharmaceut Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 86.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of Gastrointestinal Ph Profiles in Normal Ambulant Human-Subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao WW, Chan JM, Farokhzad OC. pH-Responsive Nanoparticles for Drug Delivery. Mol Pharmaceut. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dai S, Tam KC, Jenkins RD. Aggregation behavior of methacrylic acid/ethyl acrylate copolymer in dilute solutions. Eur Polym J. 2000;36:2671–2677. [Google Scholar]
- 89.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 90.Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33:1088–1118. [Google Scholar]
- 91.Lin YH, Sonaje K, Lin KM, Juang JH, Mi FL, Yang HW, Sung HW. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. Journal of Controlled Release. 2008;132:141–149. doi: 10.1016/j.jconrel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 92.Sonaje K, Lin KJ, Wey SP, Lin CK, Yeh TH, Nguyen HN, Hsua CW, Yen TC, Juang JH, Sung HW. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: Oral delivery using pH-Responsive nanoparticles vs. subcutaneous injection. Biomaterials. 2010;31:6849–6858. doi: 10.1016/j.biomaterials.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 93.McConnell EL, Short MD, Basit AW. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. Journal of Controlled Release. 2008;130:154–160. doi: 10.1016/j.jconrel.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 94.Ibekwe VC, Fadda HM, McConnell EL, Khela MK, Evans DF, Basit AW. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharmaceut Res. 2008;25:1828–1835. doi: 10.1007/s11095-008-9580-9. [DOI] [PubMed] [Google Scholar]
- 95.Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert RO, Martin F, Muller-Goymann C. Phospholipids and Lipid-Based Formulations in Oral Drug Delivery. Pharmaceut Res. 2010;27:1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 96.Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Advanced Drug Delivery Reviews. 2008;60:625–637. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 97.O'Neill MJ, Bourre L, Melgar S, O'Driscoll CM. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011;16:203–218. doi: 10.1016/j.drudis.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 98.He CX, He ZG, Gao JQ. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin Drug Del. 2010;7:445–460. doi: 10.1517/17425241003596337. [DOI] [PubMed] [Google Scholar]
- 99.Hida K, Lai SK, Suk JS, Won SY, Boyle MP, Hanes J. Common Gene Therapy Viral Vectors Do Not Efficiently Penetrate Sputum from Cystic Fibrosis Patients. Plos One. 2011;6:e19919. doi: 10.1371/journal.pone.0019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan J, Iiboshi Y, Cui L, Wasa M, Okada A. Role of intestinal mucus on the uptake of latex beads by Peyer's patches and on their transport to mesenteric lymph nodes in rats, JPEN. J Parenter Enteral Nutr. 1999;23:19–23. doi: 10.1177/014860719902300119. [DOI] [PubMed] [Google Scholar]
- 101.Gupta PK, Leung S-HS, Robinson JR. Bioadhesives/Mucoadhesives in drug delivery to the gastrointestinal tract. In: Lenaerts V, Gurny R, editors. Bioadhesive drug delivery systems. Boca Raton: CRC Press Inc.; 1990. [Google Scholar]
- 102.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paerregaard A, Espersen F, Jensen OM, Skurnik M. Interactions between Yersinia-Enterocolitica and Rabbit Ileal Mucus - Growth, Adhesion, Penetration, and Subsequent Changes in Surface Hydrophobicity and Ability to Adhere to Ileal Brush-Border Membrane-Vesicles. Infect Immun. 1991;59:253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Larhed AW, Artursson P, Bjork E. The influence of intestinal mucus components on the diffusion of drugs. Pharmaceut Res. 1998;15:66–71. doi: 10.1023/a:1011948703571. [DOI] [PubMed] [Google Scholar]
- 105.Larhed AW, Artursson P, Grasjo J, Bjork E. Diffusion of drugs in native and purified gastrointestinal mucus. J Pharm Sci. 1997;86:660–665. doi: 10.1021/js960503w. [DOI] [PubMed] [Google Scholar]
- 106.Primard C, Rochereau N, Luciani E, Genin C, Delair T, Paul S, Verrier B. Traffic of poly(lactic acid) nanoparticulate vaccine vehicle from intestinal mucus to sub-epithelial immune competent cells. Biomaterials. 2010;31:6060–6068. doi: 10.1016/j.biomaterials.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Szentkuti L. Light microscopical observations on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon. Journal of Controlled Release. 1997;46:233–242. [Google Scholar]
- 108.Lieleg O, Vladescu I, Ribbeck K. Characterization of Particle Translocation through Mucin Hydrogels. Biophys J. 2010;98:1782–1789. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crater JS, Carrier RL. Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport. Macromol Biosci. 2010;10:1473–1483. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 110.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. Altering Mucus Rheology to "Solidify" Human Mucus at the Nanoscale. Plos One. 2009;4:e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. P Natl Acad Sci USA. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Owen DA, Reid PE. Histochemical Alterations of Mucin in Normal Colon, Inflammatory Bowel-Disease and Colonic Adenocarcinoma. Histochem J. 1995;27:882–889. [PubMed] [Google Scholar]
- 114.Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, Graham DY, Genta RM. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433:419–426. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 115.Mccormick DA, Horton LWL, Mee AS. Mucin Depletion in Inflammatory Bowel-Disease. J Clin Pathol. 1990;43:143–146. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pullan RD, Thomas GAO, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of Adherent Mucus Gel on Colonic Mucosa in Humans and Its Relevance to Colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Longman RJ, Poulsom R, Corfield AP, Warren BF, Wright NA, Thomas MG. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J Histochem Cytochem. 2006;54:1335–1348. doi: 10.1369/jhc.5A6904.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U, Stremmel W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoelectrospray-tandem mass spectrometry. Scand J Gastroentero. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- 119.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, Lehmann WD, Fuellekrug J, Stremmel W, Ehehalt R. Alterations of Phospholipid Concentration and Species Composition of the Intestinal Mucus Barrier in Ulcerative Colitis: A Clue to Pathogenesis. Inflamm Bowel Dis. 2009;15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 120.Schneider H, Braun A, Fullekrug J, Stremmel W, Ehehalt R. Lipid Based Therapy for Ulcerative Colitis-Modulation of Intestinal Mucus Membrane Phospholipids as a Tool to Influence Inflammation. Int J Mol Sci. 2010;11:4149–4164. doi: 10.3390/ijms11104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stremmel W, Braun A, Hanemann A, Ehehalt R, Autschbach F, Karner M. Delayed Release Phosphatidylcholine in Chronic-active Ulcerative Colitis A Randomized, Double-blinded, Dose Finding Study. J Clin Gastroenterol. 2010;44:E101–E107. doi: 10.1097/MCG.0b013e3181c29860. [DOI] [PubMed] [Google Scholar]
- 122.Jain A, Jain SK. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. European Journal of Pharmaceutical Sciences. 2008;35:404–416. doi: 10.1016/j.ejps.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 123.Kanthamneni N, Chaudhary A, Wang J, Prabhu S. Nanoparticulate delivery of novel drug combination regimens for the chemoprevention of colon cancer. Int J Oncol. 2010;37:177–185. doi: 10.3892/ijo_00000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng SS. New-concept chemotherapy by nanoparticles of biodegradable polymers: where are we now? Nanomedicine-Uk. 2006;1:297–309. doi: 10.2217/17435889.1.3.297. [DOI] [PubMed] [Google Scholar]
- 125.Wang X, Shah AA, Campbell RB, Wan KT. Glycoprotein mucin molecular brush on cancer cell surface acting as mechanical barrier against drug delivery. Appl Phys Lett. 2010;97:26370. [Google Scholar]
- 126.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Maincent P, Kawashima Y, Lehr CM. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299:775–781. [PubMed] [Google Scholar]
- 128.Jha B, Cueto-Felgueroso L, Juanes R. Fluid Mixing from Viscous Fingering. Phys Rev Lett. 2011;106:194502. doi: 10.1103/PhysRevLett.106.194502. [DOI] [PubMed] [Google Scholar]