Abstract
The subthalamic nucleus (STN) serves important functions in regulating movement, cognition, and motivation and is connected with cortical and basal ganglia circuits that process reward and reinforcement. In order to further examine the role of the STN on motivation toward food in non-deprived rats, these experiments studied the effects of pharmacological inhibition or μ-opioid receptor stimulation of the STN on the 2-hr intake of a sweetened fat diet, the amount of work exerted to earn sucrose on a progressive ratio 2 (PR-2) schedule of reinforcement, and performance on a differential reinforcement of low-rate responding (DRL) schedule for sucrose reward. Separate behavioral groups (N = 6–9) were tested following bilateral inhibition of the STN with the GABAA receptor agonist muscimol (at 0–5 ng/0.5 μl/side) or following μ-opioid receptor stimulation with the agonist D-Ala2, N-MePhe4, Gly-ol-enkephalin (DAMGO; at 0, 0.025 or 0.25 μg/0.5 μl/side). Although STN inhibition increased ambulatory behavior during 2-hr feeding sessions, it did not significantly alter intake of the sweetened fat diet. STN inhibition also did not affect the breakpoint for sucrose pellets during a 1-hr PR-2 reinforcement schedule or impact the number of reinforcers earned on a 1-hr DRL-20 sec reinforcement schedule in non-deprived rats. In contrast, STN μ-opioid receptor stimulation significantly increased feeding on the palatable diet and reduced the reinforcers earned on a DRL-20 schedule, although DAMGO microinfusions had no effect on PR-2 performance. These data suggest that STN inhibition does not enhance incentive motivation for food in the absence of food restriction and that STN μ-opioid receptors play an important and unique role in motivational processes.
Keywords: Subthalamic nucleus, motivation, opioids, food intake, reward
1. Introduction
The subthalamic nucleus (STN) is a central node of basal ganglia circuitry that has garnered much attention for its role in Parkinson’s disease. Neural activity within the STN increases following the death of the midbrain dopaminergic neurons that causes the movement disorder (for reviews of STN function in human and animal models of Parkinson’s disease, see [1, 2]). Deep brain stimulation of the STN (which is thought to functionally inhibit STN output) reduces the motor symptoms of Parkinson’s disease patients, often substantially improving function for as long as five years [3]. Although initially viewed as an exclusively motor-related structure, converging evidence from both animal and human studies suggests that the STN also regulates cognitive and motivational functions. Recordings from the rat, cat, and monkey demonstrate that STN neurons encode movements, task-relevant stimuli, and reward presentation [4–7]. In humans, deep brain stimulation of the STN improves motor function in Parkinson’s disease patients, but has also been shown to cause mild to moderate impairments of performance on cognitive tasks such as verbal fluency, attention, and working memory [e.g., 2, 8–11] . Other reported side effects of STN stimulation include depression and weight gain [2, 12]. Likewise, STN lesions or inactivation in rodents reverses spontaneous striatal glutamate output [13] and improves motor dysfunction that is caused by 6-hydroxydopamine-induced lesions of the dopamine inputs into striatum [14–16]. However, despite improved locomotor function in this rodent model of Parkinsonism, dopamine-depleted rats treated with lesions or inactivation of the STN also show alterations in goal-directed behavior, such as premature responding in tasks that require the animals to withhold responding or attend to a delay prior to the initiation of a response [14–17].
That manipulations of the STN affect mood, motivation, and/or reward processes is consistent with its anatomical connections with the limbic components of basal ganglia circuitry. The STN has been traditionally viewed as an important node within the “indirect” motor pathway of the basal ganglia (which regulate motor and limbic function), and its firing properties and functional output is influenced by mesolimbic dopamine afferents [18–20]. Recent evidence suggests that the STN may also affect basal ganglia function via a “hyperdirect” pathway, in which the STN receives input from frontal cortices and subsequently projects to the functional output of the striatum, the globus pallidus [21, 22]. Thus, the STN influences neural processing across basal ganglia dopaminergic-ventral striatum-pallidal circuits, all of which have been identified as critical regions for learning about and directing behavior toward motivationally-relevant stimuli, such as natural rewards or drugs of abuse [23–26].
Recent research has begun to examine the behavioral role that the STN plays in regulating motivated behavior. Consistent with the effects of lesions in Parkisonian rat models, STN lesion or inactivation in otherwise intact rats reduces response inhibition and increases impulsive behavior in hungry animals trained to nose-poke or lever-press for food reinforcement. Specifically, STN lesions have been argued to cause deficits in response inhibition and attentional processing [27, 28], and may additionally affect the motivational salience of food rewards and/or the cues that predict reward [8, 29–31]. Previous research has demonstrated that lesions, pharmacological inhibition, or deep brain stimulation of the rat STN increases the effort that food-deprived rats will expend to earn food reinforcement [8, 29–31]. This pattern of data suggests that the STN may play an important role in regulating the incentive salience of food rewards. Interestingly, similar treatments appear to reduce the effort that rats will expend to self-administer cocaine [32, 33], suggesting that STN manipulations may differentially regulate the salience of natural versus drug rewards.
Also consistent with a potential role for the STN in regulating incentive motivation for food is the presence of μ-opioid receptors within the nucleus [34, 35], which have been implicated in motivational processes within other brain regions. In the interconnected basal ganglia pathways that the STN regulates, enhancement of both “wanting” of palatable foods (as measured by food intake) and the “liking” of palatable solutions (as measured by orofacial hedonic responses) result from the stimulation of local μ-opioid receptors. Specifically, μ-receptor stimulation of the ventral tegmentum [36–38], nucleus accumbens [39], and ventral pallidum [40] all increase feeding, and specific zones within the nucleus accumbens and ventral pallidum appear to modulate the “liking” of palatable solutions [40, 41]. To our knowledge, there have been no studies examining whether opioid receptors of the STN might serve a similar function.
The purpose of the current experiments was two-fold. First, in most prior reports in which rats were shown to have increased incentive motivation for food following STN lesions or inhibition, the animals were tested in a food deprived state. This makes it difficult to determine if the resulting behavioral patterns were due to a general increase of motivational processes caused directly by STN manipulation (which should be evident even in ad libitum fed animals), or were rather due to an effect of STN manipulation exaggerating the normal increase in incentive processes when the animal is in a state of caloric need. Thus, these experiments examined the effects of STN manipulations in non-deprived rats. The first aim was to determine if appetitive or consummatory motivation toward palatable food was enhanced by pharmacological inhibition of the STN in rats that were not food deprived. Secondly, as μ-opioid receptors are heavily expressed in the STN, and are furthermore found throughout ascending pathways that sense and regulate food intake, it was of interest to determine whether μ-opioid receptors within the STN might regulate food intake and appetitive motivation similar to that seen in other nodes of basal ganglia circuitry. Individual groups of rats were given 2-hr daily exposure to a palatable sweetened fat diet (Experiments 1 & 2), were trained to lever press on a progressive ratio 2 (PR-2) schedule of reinforcement for sugar reward (Experiment 3), or were trained on a differential reinforcement of low rate of responding task (DRL-20 sec; Experiment 4). Once non-deprived rats stabilized their consumption or performance of the operant task, they were tested following STN infusions of the GABAA receptor agonist muscimol or with the μ-opioid receptor agonist DAMGO.
2. Materials and Methods
2.1. Subjects and Housing
Adult male Sprague-Dawley rats (approximately 300 g at experiment onset; Harlan, Madison, WI) were acclimated to dual housing in a colony room maintained at ~21 °C with a 12-hr light–dark cycle. Standard rat chow and water were available ad libitum except as noted below during operant training. All procedures were conducted in accordance to NIH animal care guidelines and approved by the Wake Forest University Animal Care and Use Committee.
2.2. Surgery
Following approximately 1-wk acclimation to the housing environment (Experiments 1 and 2) or after the animals had completed operant training (Experiments 3 and 4), each rat was anesthetized with a Ketamine-Xylazine cocktail (100 mg/kg-10mg/kg). Standard aseptic procedures were used to implant 11.5 mm indwelling stainless steel guide cannulas (23 gauge) bilaterally above the STN (with the skull flat; A-P: −3.9 mm posterior to bregma, M-L: ±2.5 mm, D-V: 7.1 mm below skull surface). Guide cannulas were affixed to the skull with the use of screws and dental acrylic, and stylets were placed to prevent obstruction. Rats recovered for at least 7 days prior to habituation to the feeding chambers (Experiments 1 & 2) or retraining in the operant chambers (Experiments 3 & 4).
2.3. Drugs
Muscimol (Tocris Biosciences) and [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO; Sigma-Aldrich) were dissolved in sterile saline for these experiments. Stock solutions of 200 ng/microliter muscimol and 5 micrograms/microliter DAMGO were aliquoted and stored at −20º C. On the day of testing, solutions were thawed and diluted in sterile saline to achieve the final doses (see below).
2.4. Histology
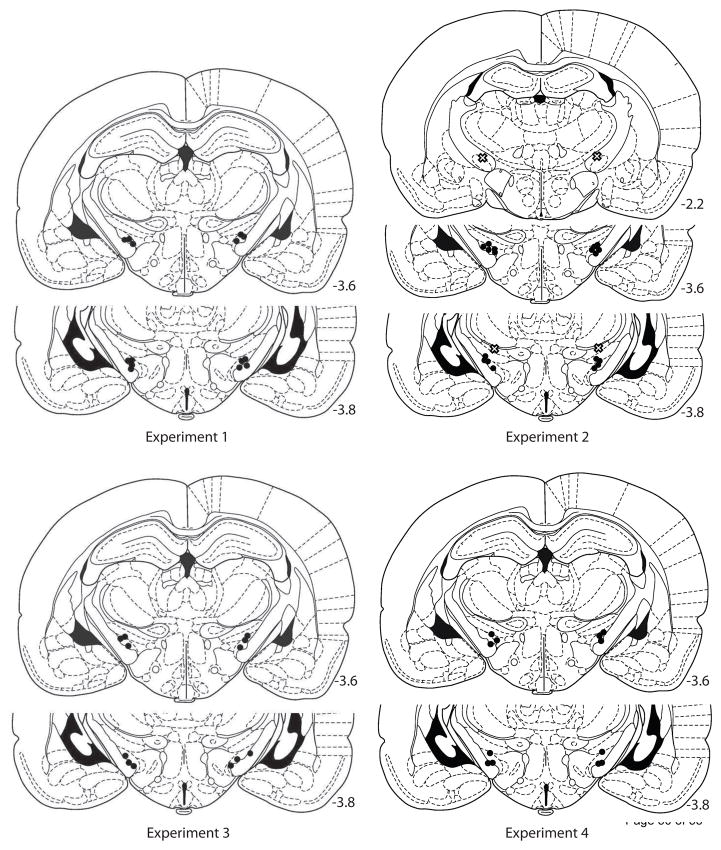
At the conclusion of each experiment, rats were deeply anesthetized with sodium pentobarbital and perfused through the heart with a 0.9% buffered NaCl solution, followed by 10.0% formalin. Brains were removed and allowed to sink in 10.0% sucrose formalin. They were then frozen and sliced into 60-μm sections with a cryostat through the level of the STN. Sections were stained with cresyl violet. The tips of the cannulas were confirmed by light microscopy and charted with reference to a brain atlas [42]. Only animals whose injectors were bilaterally placed within the STN were included in the behavioral analysis and described below (see Figure 1).
Figure 1.
Location of injector tips for animals included in the behavioral analysis for each experiment. For Experiment 2, “X”s represent the locations of two animals that were excluded from analysis due to bilateral misplacement of the injections (see text). Schematic drawings were adapted from Figures 28, 34, & 35 of The Rat Brain in Sterotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, copyright 1998 [42].
2.5. Experiments 1 & 2: Examining the effects of STN inhibition or μ-opioid receptor activation on the intake of a palatable sweetened fat diet
2.5.1. Apparatus
Food intake chambers were constructed from clear acrylic, with internal dimensions of 42 cm wide, 30.5 cm deep and 33 cm tall. Infra-red (IR) eyebeams were located along the wire floor at three locations (5 cm above the wire floor) to measure ambulation; four additional IR beams were placed at a height of 16 cm above the floor to index rearing behavior. IR beam interruption was continually recorded by Med-PC software (Med Associates, St. Albans, VT) during each experimental session. A water bottle was hung at one end of the chamber, and a food intake monitor (Med Associates, St. Albans, VT) was placed at the opposite end. Food intake monitors consisted of a food hopper that was suspended from a calibrated potentiometer which allowed for continuous monitoring of the mass/weight of the food hopper during the experimental sessions. Each day, the food hoppers were filled with a sweetened fat diet (278.3 g/kg vitamin free casein, 100.0 g/kg sucrose, 4.2 g/kg DL-methionine, 441.2 g/kg shortening, 77.7 g/kg safflower oil, 26.3 g/kg cellulose, 53.3 g/kg mineral mix, 15.2 g/kg vitamin mix and 3.8 g/kg choline chloride; kilocaloric value of diet=6.2 kcal/g; Teklad Diets, Madison, WI, USA). Non-deprived rats typically eat this diet when available, and its intake has been shown to be sensitive to injections of opiate drugs into other regions of the basal ganglia [43, 44]. The computer program recorded the mass/weight of the food hoppers at 10-sec intervals across each 2-hr feeding session. A speaker maintained an ambient level of white noise at 65 dB in the experimental room.
2.5.2. Feeding paradigm on a palatable fat/sucrose diet
Once recovered from surgery, rats were given at least six days of habituation to the palatable diet for 2-hr sessions in the feeding chambers. All sessions were conducted during the last half of the lights-on period. On the final two days of habituation, rats received mock intracranial injections. During the first mock infusion, injectors were lowered to the end of the guide cannula. The second mock injection utilized injectors that were lowered 1.0 mm below the end of the guides to the infusion site. No solutions were delivered on mock injection days. Experimental treatments began 48 hrs after the last mock injection.
On experimental treatment days, intake of the sweetened fat diet and locomotor activity was monitored for 2 hrs following the injection of vehicle or drug solutions into the STN. Injection cannulas (30 gauge) were lowered bilaterally into the STN and 0.5 μl of solution was delivered (at a rate of 0.32 μl per minute) by a Harvard Apparatus (Holliston, MA) microinfusion pump. Injectors remained in place for one minute to allow for diffusion, and rats were immediately placed in the feeding chambers. Dependent measures included the total amount of palatable food eaten across the 2-hr period, the number of approaches to the food intake monitor, ambulation within the chamber (assessed as the number of complete crossings of the chamber from end to end), number of rears recorded, and total water intake during each feeding session.
Individual animals received all drug treatments across multiple experimental days, the order of which was randomly determined for each rat. Rats in Experiment 1 (N = 7) received intra-STN infusions of 0, 1, 3, and 5 ng of muscimol in 0.5 μl of saline; rats in Experiment 2 (N = 9) received intra-STN infusions of 0, 0.025, and 0.25 μg of DAMGO. Muscimol doses were chosen based upon behaviorally effective doses from previous reports [28, 32]. Doses of DAMGO were chosen to be comparable to doses previously shown to be effective at increasing food intake when injected into other basal ganglia circuits [36, 37, 40, 45]. All drug treatment days were separated by a minimum of two drug-free days, on which rats received neither injections nor access to the sweetened fat diet. Although no overt stereotypy was observed during or immediately following STN infusions, some rats showed a tendency to gnaw on wood shavings when returned to their home cages following the food intake sessions [46]. To prevent its ingestion, rats were housed on wire floors for the 24 hrs following each drug treatment.
2.6. Experiment 3: Examining the effects of inhibition or μ-opioid receptor activation on progressive ratio performance for sugar pellets in non-deprived rats
2.6.1. Apparatus
Rats (N = 6) were trained in commercially constructed operant chambers (Med Associates, St. Albans, VT), enclosed in ventilated sound-attenuating cubicles. Each chamber was equipped with two retractable levers, a house light, and a food magazine into which 45-mg sugar pellets were dispensed. An IR sensor in the food magazine measured nose-poke activity. The chambers interfaced with a computer that recorded the time of each experimental event and controlled all reinforcement contingencies.
2.6.2. Progressive Ratio 2 paradigm
In this experiment, training preceded surgery. Following one week of acclimation to the laboratory and daily handling, food availability was restricted to allow for gradual reduction of body weight to 90% of free-feeding levels. During the final two days of food restriction, rats were given 2 g of sugar (45-mg pellets; BioServ, Frenchtown, NJ) with their daily chow to prevent neophobia during training.
Once the rats had achieved their target weights, they were habituated to the operant chambers with three daily sessions of a random time-30 sec reinforcement schedule (one pellet delivered on average every 30 sec, no levers present) for 30 min. On the subsequent and following days, both levers (one active and one inactive) were inserted into the chamber. Training proceeded for three sessions each on fixed-ratio 1, 3, and 5 schedules of reinforcement, at which point all rats had achieved reliable responding on the lever. Rats were then switched to a progressive ratio 2 (PR-2) schedule of reinforcement for seven sessions. In this schedule, the rat was reinforced for the first lever press and was then required to increase the number of responses by two lever presses for each subsequent pellet delivery. Thus, progressively more effort was required to earn each reinforcer. The number of responses required in the final completed ratio determined the break point, a well validated measure reflecting the strength of the reinforcer and the motivational state of the animal [47, 48]. At the end of 7 days with the PR-2 schedule, all rats had achieved high levels of lever response. They were returned to ad-libitum feeding and underwent surgery to implant guide cannulas above the STN.
One week following surgery, the non-food-deprived rats were returned to the chambers, and the session length for the PR-2 schedule was increased to 1 hr. Once the rats had achieved stable break points, they were habituated to the injection procedure (as described above). Each rat subsequently received five intra-STN drug infusions. Rats were treated with intra-STN injections of the saline vehicle, 3 ng muscimol, 5 ng muscimol, 0.025 μg DAMGO, or 0.25 μg DAMGO. During the first three days, half the rats received the vehicle or muscimol infusions and half the rats received vehicle or DAMGO injections, in random order for each rat. During the fourth and fifth experimental sessions, each rat received both doses of the remaining drug (DAMGO or muscimol) in a counterbalanced order. Following injections, rats were immediately placed into the chambers for a 1-hr PR-2 session. Each drug treatment was separated by at least 2 days of additional PR-2 training to stabilize baseline performance and allow wash-out of the drug. Dependent measures were the total number of bar presses on the active and inactive levers, nose pokes into the magazine, and the final completed reinforcement ratio (break point).
2.7. Experiment 4: Examining the effects of inhibition or μ-opioid receptor activation on DRL-20 sec performance for sugar pellets in non-deprived rats
A separate group of rats (N = 6) was housed, food restricted, and trained to lever press on an FR1 schedule as in experiment 3. Following 3 days of training on the FR1 schedule of reinforcement, rats were placed on a variable-interval (VI) 30 sec schedule of reinforcement. Once stable responding was observed (requiring 11 days of training), the rats were switched to a differential reinforcement of low rate responding (DRL-20 sec) schedule, in which rats were reinforced for the first lever press, and then obtained subsequent sugar reinforcement only if they withheld responding on the active lever for a full 20 seconds before pressing the lever again. Any press of the active lever reset the 20-second clock for reinforcement; presses on the inactive lever resulted in no programmed consequences. All training sessions lasted 30 min.
Rats stabilized responding on the active lever after 13 days of training on the DRL-20 schedule. They were then returned to ad libitum access to food prior to surgical implantation of guide cannulas above the STN. Following one week of surgical recovery, the non-deprived rats were placed back into the chambers, and the DRL-20 sec sessions were increased to 1 hr. Once stable responding on the DRL-20 sec schedule was achieved, rats underwent injections of muscimol and DAMGO, at the same doses and in the same manner as in Experiment 3. Dependent measures included the total number of presses on the active and inactive levers, the number of reinforcements earned, time between lever presses (as assessed by an inter-response time histogram), and the total number of nose pokes into the magazine.
3. Results
3.1 Experiments 1 & 2: Examining the effects of STN inhibition or μ-opioid receptor activation on the intake of a palatable sweetened fat diet
3.1.1. Experiment 1
Food intake was analyzed utilizing two-way repeated measures analysis of variance (ANOVA) comparing the amount of palatable diet consumed (in grams) as a function of drug dose and time within each 2 hr session (with intake quantified at 5 min intervals). Total ambulation, rearing, water intake, and head entry measures were summed across the entire feeding session and analyzed using repeated measures ANOVAs with drug dose as the independent variable.
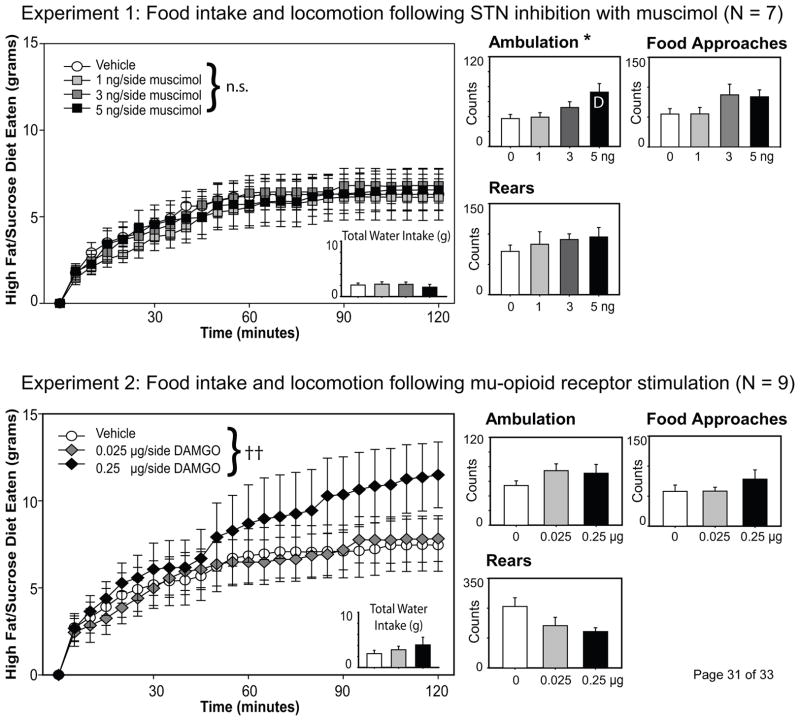
As can be seen in the top panels of Figure 2, inhibiting the STN with increasing doses of muscimol did not alter palatable food intake across the 2-hr feeding session. Although there was an expected effect of time on feeding [F(23,138) = 23.9, p < .001], there was no effect of drug treatment [F(3, 18) =0.40, p = .76], nor was there a significant drug X time interaction effect [F(69,414) = 0.75, p =.93], on the amount of palatable diet consumed. Water consumption also did not vary across drug treatment, F(3,18) = 0.76, p = .53.
Figure 2.
Food intake and locomotion following STN inhibition with the GABAA agonist muscimol (Experiment 1, top panels) or μ-opioid receptor stimulation of the STN with DAMGO (Experiment 2, bottom panels). Cumulative intake of the sweetened fat diet is shown at 5-min intervals across the 2 hr sessions; individual lines represent food intake for all animals under the specified dose of each drug. Although STN injection of 5 ng muscimol significantly increased ambulation as measured by the number of crossings within the food intake chamber, there was no effect of STN inhibition on food or water intake across the sessions. In contrast, the intake of a sweetened fat diet was significantly increased in the second hour of the feeding session following STN μ-opioid receptor stimulation. The double cross demarks a significance of p < .01 for at the drug X time interaction effect; * denotes p < .05 for drug effects on ambulation; D indicates difference from vehicle injection as determined by Tukey’s HSD.
However, STN inhibition significantly impacted ambulatory behavior within the feeding chamber [F(3, 18) = 3.69, p = .03]. Tukey’s HSD post-hoc analysis revealed that the 5 ng/side dose of muscimol significantly increased ambulation compared to the vehicle injection. There was also a trend toward increased approaches to the food monitor with the 3 and 5 ng doses of the drug [F(3, 18) = 2.88, p = .064]. In contrast, rearing behavior was not significantly affected by drug treatment, F(3, 18) = 0.75, p = .54.
3.1.2. Experiment 2
Activation of μ-opioid receptors within the STN elicited a significant drug X time interaction effect across the 2-hr feeding session [F(46, 368) = 2.15, p < .001], although the main effect of drug was not significant [F(2, 16) = 0.778, p = .476]. As can be seen in the lower panels of Figure 2, the 0.25 μg dose of DAMGO increased feeding on the palatable diet, although consumption does not differ from the vehicle or 0.025 μg doses until well into the second hour of testing. Mu-opioid receptor stimulation of the STN had no effect upon water intake or the number of head entries into the food monitor (both p’s > .10), but tended to increase ambulation [F(2,16) = 2.82, p = .089] and inhibit rearing behavior [F(2,16) = 3.27, p = .064].
Two rats from Experiment 2 were excluded from analysis due to bilateral misplacement of the injectors. As shown in Figure 1 (the X’s represent the injection site for these two animals), one rat had its injections placed anterior to the STN (internal capsule; Rat 1), while another rat’s infusions were immediately dorsal to the STN (zona incertia: Rat 2). Unlike animals with STN injections, neither rat showed evidence of increased feeding following DAMGO injections (Total intake Rat 1: Veh, 9.9 g; 0.025 DAMGO: 6.5 g; 0.25 DAMGO: 6.7 g; Total intake Rat 2: Veh 4.2; 0.025 DAMGO: 4.9 g; 0.25 DAMGO: 1.3 g). These data provide evidence that the pro-feeding effects of the injections were not due to diffusion of the drug outside of the STN, and are consistent with prior examinations of STN function with similar drug volumes [28].
3.2. Experiment 3: Examining the effects of inhibition or μ-opioid receptor activation on progressive ratio performance for sugar pellets in non-deprived rats
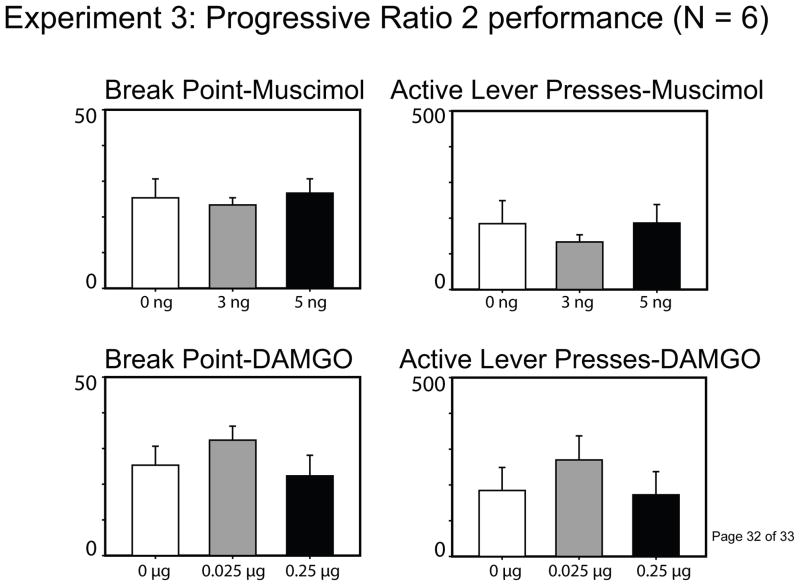
Separate repeated measures ANOVAs were conducted comparing each of the dependent measures following either muscimol or DAMGO injections. As can be seen in the left panels of Figure 3, neither inhibition [F(2, 10) = 0.43, p = .66] nor μ-opioid receptor stimulation [F(2,10) = 1.06, p = .38] of the STN altered the effort that sated rats exerted to earn sugar reinforcement within the PR-2 paradigm. The drug treatments also had no effect upon the total number of presses on the active or inactive levers, the number of nose pokes into the chamber, or the number of reinforcers earned (all p’s > .10; see Figure 3 for summary of total active lever presses; other data not shown).
Figure 3.
Progressive ratio 2 performance following injections of muscimol or DAMGO into the STN. Neither inhibition nor μ-opioid receptor stimulation affected the break point or the total number of active lever presses on a PR-2 schedule of reinforcement (all p-values > .05).
3.4. Examining the effects of inhibition or μ-opioid receptor activation on DRL-20 sec performance for sugar pellets in non-deprived rats
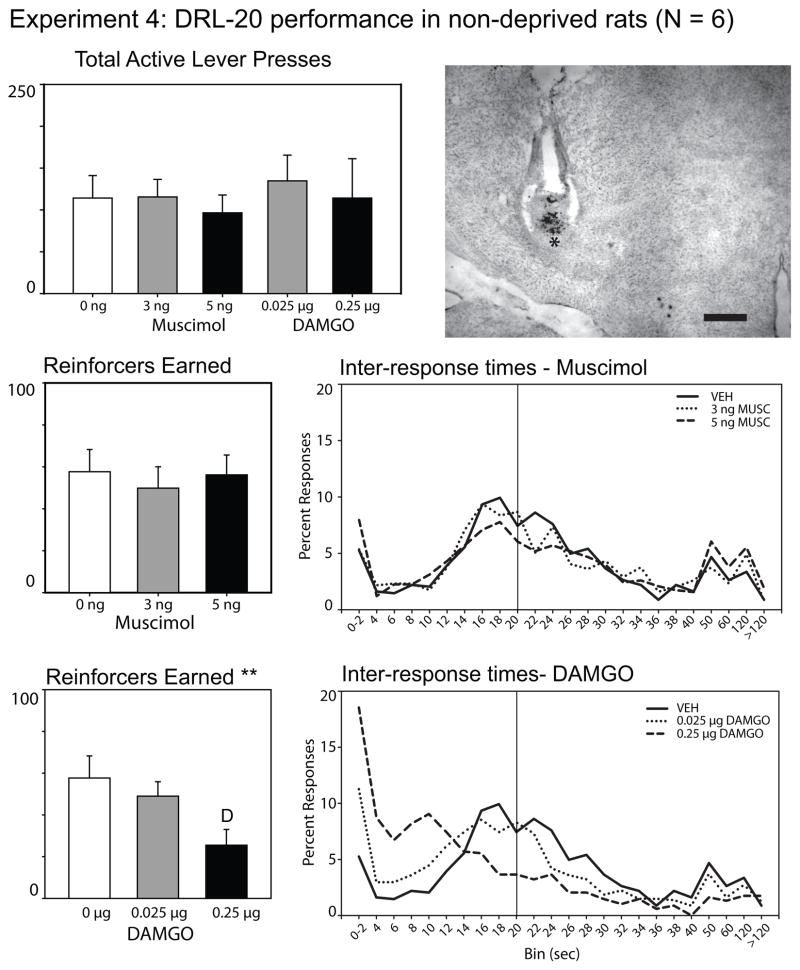
Prior to examining the effects of either STN inhibition with muscimol or μ-opioid receptor activation on the number of reinforcers earned during the DRL-20 sec schedule, a repeated measures ANOVA was run across all five drug treatment conditions to determine if there were any differences in the number of lever presses elicited following drug treatment during the 1-hr session. There were no significant differences on total lever presses across drugs (muscimol and DAMGO) and drug doses, either for the active lever [F(4, 20) = 0.34, p = .29; see top left panel, Figure 4] or for the inactive lever [F(4, 20) = 1.52, p = .23; data not shown, average number of inactive lever presses/session = 3.4 ± 0.73 St Err].
Figure 4.
Rat performance on a DRL-20 sec schedule of reinforcement following inhibition of the STN or the stimulation of its μ-opioid receptors. Total lever presses, reinforcers earned, and inter-response time (IRT) curves are presented for all conditions. IRT curves represent the rats’ ability to inhibit responses for the 20 sec interval. The curves show the proportion of inter-response times within 2 sec bins up to 40 sec for each drug condition; subsequent bins represent the % responses between the time points shown on the x-axis. The vertical line at 20 sec indicates the delay requirement between lever presses that was required to earn sugar reinforcement. Neither muscimol nor DAMGO injections changed the total number of lever presses during the 1-hr sessions (top panel). STN inhibition did not alter the number of reinforcers earned during the task, nor fundamentally affect the inter-response times of the animals. In contrast, μ-opioid receptor stimulation of the STN reduced the number of reinforcers earned, and shifted the IRT response curve to the left across increasing doses (bottom panels). ** indicates p < .01 for the effect of DAMGO on reinforcers earned; D indicates a significant difference from vehicle injection as determined by Tukey’s HSD. A photomicrograph of a representative cannula placement is shown in the top right panel. The injector tip is demarked by a “*”, and is located at the dorsal border of the STN. The scale bar to the left of the 3rd ventricle represents 500 micrometers.
As shown in Figure 4, inactivation of the STN with muscimol also had no effect upon the number of reinforcers earned in the DRL-20 sec schedule, F(2, 10) = 1.05, p = .39. Inter-response time (IRT) histograms were developed to examine the probability structure of the responses of the animals. As can be seen in the IRT histogram for the muscimol injections, neither 3 nor 5 ng of muscimol affected the temporal patterns of responding when injected into the STN. Approaches to the food hopper, as assessed by nose pokes into the hopper unit, were unaffected by STN inhibition, F(2, 10) = 0.65, p > .55 (data not shown).
In contrast, stimulation of μ-opioid receptors caused a significant decrease in the number of reinforcers earned during the 1-hr DRL-20 sec session, F(2, 10) = 8.93, p = .006. Post-hoc testing with Tukey’s HSD confirmed that the 0.25 μg dose of DAMGO significantly impaired the rats’ ability to earn reinforcement compared to vehicle injections. As can be seen in the IRT histograms (Figure 4), DAMGO consistently impaired the animals’ ability to wait the 20 seconds required to earn a reinforcer, as evidenced by a shift of the response curve to the left after receiving the 0.25 μg dose. Nose pokes into the food hopper were unaffected by DAMGO treatment, F(2, 10) = 0.65, p > .55 (data not shown).
4. Discussion
The goal of these experiments was to examine the effects of STN inactivation or μ-opioid receptor stimulation upon palatable feeding, progressive ratio performance, and impulsive-like behavior as measured in the DRL-20 sec schedule of reinforcement in rats that were not food restricted. Although muscimol injections into the STN increased locomotor activity at the highest dose tested, there were no effects of STN inhibition on the amount of palatable diet consumed in a 2-hr feeding session, the amount of effort that sated rats expended to earn sugar reinforcement in a PR-2 paradigm, or the number of reinforcers earned within a DRL-20 sec schedule of reinforcement. In contrast, stimulation of μ-opioid receptors within the STN increased intake of the sweetened fat diet in the second hour of the feeding session, but decreased successful performance of the DRL-20 sec schedule of reinforcement. Mu-opioid receptor stimulation of the STN had no effect upon progressive ratio performance. These novel data extend prior research by demonstrating STN μ-opioid receptor involvement in promoting consummatory behavior and influencing appropriate goal-directed responding.
4.1. STN inhibition and incentive motivation
That STN inhibition with muscimol did not affect consummatory behavior on the sweetened fat diet is consistent with a prior report by Baunez et al. [8] in which lesions of the same region did not alter the intake of rat chow or sugar in food restricted or sated rats in 2 or 24 hr feeding tests. The current data suggest that STN inhibition does not directly impact the consummatory phase of food-directed motivation, even when food consumption is driven by the availability of a palatable food source rather than hunger. Several laboratories have suggested that STN lesions, inhibition, or DBS may increase incentive motivation during appetitive phases of food-motivated behavior. Specifically, Baunez and colleagues [8, 32, 33] have shown that lesions or DBS of the STN enhance the effort that food-restricted rats exhibit in a progressive ratio task. Additionally, reports by Uslaner and Robinson [30, 31] and Bezzina and colleagues [29, 49] have similarly argued that STN lesions enhance incentive salience. However, in the present experiments, STN inhibition with muscimol did not affect appetitive motivation in sated animals as assessed by the effort that rats exerted within a progressive ratio task, nor did it alter the number of reinforcers earned by rats within a DRL-20 sec schedule. It is possible that we failed to observe evidence for increased incentive motivation following STN inactivation due to differences in the task parameters of progressive ratio and DRL schedules of reinforcement across laboratories. For instance, Baunez and colleagues used a variant of a progressive ratio 5 procedure, rather than the PR-2 schedule used here. However, it is also plausible that STN lesions or inhibition do not increase rats’ motivation for food per se, but instead may exaggerate the motivational value of food incentives in response to a state of energy need (as when they are food restricted).
Additional evidence for this hypothesis can be found in the literature. Uslaner and Robinson [31] trained STN-lesioned rats on a DRL-30 sec paradigm, and tested them during both sated and food restricted conditions. Although STN lesions did alter lever pressing patterns (by increasing burst responding and decreasing the number of presses during the peak of the IRT curve; a similar trend can be seen for the 5 ng muscimol IRT curve for the present experiment), lesioned rats did not earn fewer reinforcers than sham-lesioned controls when the rats were not food-deprived. However, following food restriction the STN-lesioned rats increased their total number of lever-presses compared to control rats, shortened their IRTs, and earned fewer reinforcers within the session [31]. The same authors reported that sign-tracking to a stimulus paired with food delivery, a purported measure of incentive salience, was enhanced by STN lesions in rats that were chronically food-restricted [30]. In another study that examined behavioral performance in both food-deprived and sated conditions, STN-lesioned rats tested with a five-choice serial reaction time task reduced their premature responding and perseverative panel pushes when they were fed ad libitum versus when they were food restricted [27]; these measures were unchanged for sham-lesioned animals across feeding conditions. Thus, the effects of STN lesions or inhibition on food-directed incentive motivation may be dependent upon the physiological state of the animal.
Similar to other regions of the basal ganglia that are involved in motivation, the STN receives direct input from both orexin-containing neurons of the lateral hypothalamus (Peyron et al., 1998) and from central thalamic regions that are heavily innervated by hypothalamic projections [50–52]. The STN is therefore well-positioned to regulate the function of downstream motivational circuits based upon hypothalamic inputs signaling current metabolic state. Furthermore, recent evidence suggests that the STN and the prefrontal cortex are part of a network that coordinates similar aspects of inhibitory control [see 53, 54]). The absence of STN processing in food-restricted rats may therefore prevent normal prefrontal-STN inhibitory control of behavior. This may be of particular relevance when hunger already serves to enhance the incentive value of food-associated cues and behavioral task requirements necessitate response inhibition for a successful outcome (as in DRL tasks). In the absence of food restriction, temporary STN inhibition may not itself enhance appetitive motivation for food.
4.2. Effects of μ-opioid receptor stimulation of the STN
Although STN inhibition with muscimol did not change consummatory or appetitive behavior directed toward food in non-deprived rats, stimulation of μ-opioid receptors within the nucleus did affect food-motivated behavior. Within the feeding chambers, rats increased their consumption of a palatable sweetened fat diet following the 0.25 μg dose of DAMGO. This is the first demonstration of a behavioral effect of μ-opioid receptor stimulation of the STN on food intake, and is consistent with prior reports that used similar local treatments within other brain regions that are involved in sensing food and directing behavior toward food. For instance, feeding is affected by μ-opioid receptor stimulation within the nucleus of the solitary tract and the parabrachial nucleus of the pons (which process sensory characteristics of food; [55, 56]), various nuclei of the hypothalamus (which regulate food intake based upon energy need; [57]), and mesolimbic pathways involved in reward processes such as the ventral tegmentum, broad regions of the striatum (including the nucleus accumbens), and the ventral pallidum [36–40]. The increase in consummatory behavior following μ-opioid receptor stimulation of the STN was not observed until the second hour of the food intake session. This pattern is similar to the delayed enhancement of rat chow intake following μ-opioid stimulation of brain stem regions [55, 56], and is also reminiscent of early reports examining the effects of morphine or DAMGO injections into the nucleus accumbens [39].
However, the overall pattern of behavioral effects following μ-opioid receptor stimulation of the STN is not identical to that observed following DAMGO administration in other basal ganglia regions that the STN regulates. For instance, μ-opioid receptor activation of the nucleus accumbens at the doses used here significantly enhances palatable food intake within a very short time frame (effects are observable by 30 minutes into the session; [43, 44]). Additionally, nucleus accumbens DAMGO infusions increase break point on a progressive ratio task similar to the one used here [58]. In contrast, DAMGO-elicited intake of the sweetened fat diet was enhanced only after a delay in these experiments, and we found no effect of μ-opioid receptor activation of the STN on PR-2 performance at equivalent drug doses. STN μ-receptor stimulation also reduced the number of reinforcers that rats earned on the DRL-20 sec schedule of reinforcement by decreasing the inter-response interval between each lever press. That response times were shortened argues against the possibility that the rats were inhibited in their locomotion following DAMGO treatment, particularly as the total number of lever presses across the 1 hr session was unchanged across drug and vehicle conditions. Although prior reports have shown that the STN and ventral striatal circuits regulate DRL performance, including reductions of DRL performance following excitotoxic lesions or 5-HT depletion of the nucleus accumbens core [31, 59–62], this is the first demonstration of an effect caused by opioid receptor activation within basal ganglia pathways on this task. Whether μ-opioid receptor stimulation of the STN impaired DRL performance by increasing impulsivity, impairing response inhibition, or by affecting temporal discrimination awaits further examination with a broader variety of behavioral tests. Regardless, the current data suggest that μ-opioid receptors within the STN serves a complementary but unique role with other basal ganglia circuits in the regulation of food-seeking and the prolonging of consummatory behavior in the presence of palatable foods.
4.3 Concluding remarks
Treatment of Parkinson’s disease with STN deep brain stimulation improves motor performance, but causes side effects including weight gain, cognitive impairments, and changes in mood [2, 8–12]. Investigation into the behavioral role of the STN in animal models is relatively recent, and has yet to lead to a comprehensive account of its functional role in cognitive and motivational function. The current experiments suggest that STN inhibition does not impact appetitive or consummatory aspects of motivation for food in non-deprived animals, at least as tested by the free consumption of a palatable diet and within the progressive ratio and DRL schedules examined here. However, that the STN serves an important function in motivated behavior was confirmed, as stimulation of μ-opioid receptors increased palatable feeding and reduced performance on the DRL-20 task. Paired with prior research, these experiments suggest that the behavioral role of the STN may depend upon the metabolic state of the animal (food deprived vs. fed ad libitum) and provide the first evidence demonstrating a role of opioid receptors of the STN in motivated behavior.
The subthalamic nucleus (STN) regulates movement, cognition, and motivation.
We tested STN inhibition or μ-opioid stimulation on food motivation in free-fed rats.
STN inhibition didn’t affect palatable feeding, progressive ratio, or DRL responding.
STN μ-opioid receptor stimulation increased food intake and impaired DRL performance.
Acknowledgments
This work was supported financially by the Wake Forest University Department of Psychology and by the National Institute of Drug Abuse (R15 DA030618). We would like to thank Dr. Matthew E. Andrzejewski for graciously providing the Med PC script for the operant behavioral paradigms. Emilia Brown and Brianna Lukasevics provided technical support for some aspects of these experiments. The examination of cannula placements and the taking of photomicrographs was supported by the Wake Forest University Microscopy Imaging Core facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baunez C, Gubellini P. Effects of GPi and STN inactivation on physiological, motor, cognitive and motivational processes in animal models of Parkinson's disease. Prog Brain Res. 2010;183:235–58. doi: 10.1016/S0079-6123(10)83012-2. [DOI] [PubMed] [Google Scholar]
- 2.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 4.Cheruel F, Dormont JF, Farin D. Activity of neurons of the subthalamic nucleus in relation to motor performance in the cat. Exp Brain Res. 1996;108:206–20. doi: 10.1007/BF00228095. [DOI] [PubMed] [Google Scholar]
- 5.Darbaky Y, Baunez C, Arecchi P, Legallet E, Apicella P. Reward-related neuronal activity in the subthalamic nucleus of the monkey. Neuroreport. 2005;16:1241–4. doi: 10.1097/00001756-200508010-00022. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol. 1992;67:1615–32. doi: 10.1152/jn.1992.67.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Teagarden MA, Rebec GV. Subthalamic and striatal neurons concurrently process motor, limbic, and associative information in rats performing an operant task. J Neurophysiol. 2007;97:2042–58. doi: 10.1152/jn.00368.2006. [DOI] [PubMed] [Google Scholar]
- 8.Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J Neurosci. 2002;22:562–8. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dujardin K, Defebvre L, Krystkowiak P, Blond S, Destee A. Influence of chronic bilateral stimulation of the subthalamic nucleus on cognitive function in Parkinson's disease. J Neurol. 2001;248:603–11. doi: 10.1007/s004150170139. [DOI] [PubMed] [Google Scholar]
- 10.Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, et al. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55:411–8. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- 11.Trepanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson's disease. Brain Cogn. 2000;42:324–47. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- 12.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson's disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Centonze D, Gubellini P, Rossi S, Picconi B, Pisani A, Bernardi G, et al. Subthalamic nucleus lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience. 2005;133:831–40. doi: 10.1016/j.neuroscience.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Baunez C, Nieoullon A, Amalric M. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J Neurosci. 1995;15:6531–41. doi: 10.1523/JNEUROSCI.15-10-06531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta A, Chesselet MF. Effect of GABA(A) receptor stimulation in the subthalamic nucleus on motor deficits induced by nigrostriatal lesions in the rat. Exp Neurol. 2005;193:110–7. doi: 10.1016/j.expneurol.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Phillips JM, Brown VJ. Reaction time performance following unilateral striatal dopamine depletion and lesions of the subthalamic nucleus in the rat. Eur J Neurosci. 1999;11:1003–10. [PubMed] [Google Scholar]
- 17.Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–56. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 18.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 19.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J Neurosci. 1997;17:6807–19. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res. 2002;43:111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 22.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–87. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 23.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 24.Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- 25.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Winter C, Lemke C, Sohr R, Meissner W, Harnack D, Juckel G, et al. High frequency stimulation of the subthalamic nucleus modulates neurotransmission in limbic brain regions of the rat. Exp Brain Res. 2008;185:497–507. doi: 10.1007/s00221-007-1171-1. [DOI] [PubMed] [Google Scholar]
- 27.Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur J Neurosci. 1997;9:2086–99. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 28.Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 1999;141:57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- 29.Bezzina G, Cheung TH, Body S, Deakin JF, Anderson IM, Bradshaw CM, et al. Quantitative analysis of the effect of lesions of the subthalamic nucleus on intertemporal choice: further evidence for enhancement of the incentive value of food reinforcers. Behav Pharmacol. 2009;20:437–46. doi: 10.1097/FBP.0b013e3283305e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uslaner JM, Dell'Orco JM, Pevzner A, Robinson TE. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology. 2008;33:2352–61. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- 31.Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–54. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- 32.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nat Neurosci. 2005;8:484–9. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 33.Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107:1196–200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki T, Kennedy JL, Nobrega JN. Autoradiographic mapping of mu opioid receptor changes in rat brain after long-term haloperidol treatment: relationship to the development of vacuous chewing movements. Psychopharmacology (Berl) 1996;128:97–104. doi: 10.1007/s002130050115. [DOI] [PubMed] [Google Scholar]
- 35.Slamberova R, Rimanoczy A, Riley MA, Schindler CJ, Vathy I. Mu-opioid receptors in seizure-controlling brain structures are altered by prenatal morphine exposure and by male and female gonadal steroids in adult rats. Brain Res Bull. 2002;58:391–400. doi: 10.1016/s0361-9230(02)00805-5. [DOI] [PubMed] [Google Scholar]
- 36.Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental area region of rats. Physiol Behav. 2002;76:107–16. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 38.Noel MB, Wise RA. Ventral tegmental injections of a selective mu or delta opioid enhance feeding in food-deprived rats. Brain Res. 1995;673:304–12. doi: 10.1016/0006-8993(94)01442-k. [DOI] [PubMed] [Google Scholar]
- 39.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–60. [PubMed] [Google Scholar]
- 40.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 43.Skelly MJ, Guy EG, Howlett AC, Pratt WE. CB1 receptors modulate the intake of a sweetened-fat diet in response to mu-opioid receptor stimulation of the nucleus accumbens. Pharmacol Biochem Behav. 2010;97:144–51. doi: 10.1016/j.pbb.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Will MJ, Pritchett CE, Parker KE, Sawani AM, Ma H, Lai AY. Behavioral characterization of amygdala involvement in mediating intra-accumbens opioid-driven feeding behavior. Behav Neurosci. 2009;123:781–93. doi: 10.1037/a0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 46.Dybdal D, Gale K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J Neurosci. 2000;20:6728–33. doi: 10.1523/JNEUROSCI.20-17-06728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 48.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 49.Bezzina G, Boon FS, Hampson CL, Cheung TH, Body S, Bradshaw CM, et al. Effect of quinolinic acid-induced lesions of the subthalamic nucleus on performance on a progressive-ratio schedule of reinforcement: a quantitative analysis. Behav Brain Res. 2008;195:223–30. doi: 10.1016/j.bbr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–37. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 51.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 2009;78:122–30. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Baunez C, Lardeux S. Frontal cortex-like functions of the subthalamic nucleus. Front Syst Neurosci. 2011;5:83. doi: 10.3389/fnsys.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–88. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 55.Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–32. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- 56.Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–65. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- 57.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–11. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 59.Dunnett SB, Iversen SD. Neurotoxic lesions of ventrolateral but not anteromedial neostriatum in rats impair differential reinforcement of low rates (DRL) performance. Behav Brain Res. 1982;6:213–26. doi: 10.1016/0166-4328(82)90024-9. [DOI] [PubMed] [Google Scholar]
- 60.Fletcher PJ, Chambers JW, Rizos Z, Chintoh AF. Effects of 5-HT depletion in the frontal cortex or nucleus accumbens on response inhibition measured in the 5-choice serial reaction time test and on a DRL schedule. Behav Brain Res. 2009;201:88–98. doi: 10.1016/j.bbr.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 61.Neill DB, Herndon JG., Jr Anatomical specificity within rat striatum for the dopaminergic modulation of DRL responding and activity. Brain Res. 1978;153:529–38. doi: 10.1016/0006-8993(78)90337-2. [DOI] [PubMed] [Google Scholar]
- 62.Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22:2605–16. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]