Abstract
Although the development of gene delivery systems via non-viral-mediated methods is advancing rapidly, it remains a challenge to deliver plasmids into hard-to-transfect cells, such as lymphoma/leukemia cells. To develop an efficient transfection method, we formulated a simple nanocomplex by incorporating poly β-amino ester (PBAE) polymers with plasmid DNAs containing a GFP reporter gene. The formed PBAE-plasmid nanocomplexes are approximately 200 nm in diameter and stable under physiological conditions, but become rapidly biodegradable when pH decreases < 7.0. Cultured lymphoma/leukemia cells were used for transfection assays and resultant gene delivery rates were determined by quantifying GFP expression. Exposure of cells to the nanocomplexes composed of fractioned PBAE (> 7 kDa) resulted in GFP expression in 3% of cells, similar to that mediated by the standard Lipofectamine method. However, with polybrene pre-treatment, the nanocomplex could achieve GFP expression in up to 32% of lymphoma/leukemia cells, an 8-fold increase over that mediated by Lipofectamine. These findings demonstrated a simple, efficient method for in vitro gene delivery into hard-to-transfect cells. The nanocomplexes are biodegradable and have minimal cytotoxicity, suggesting the potential use for in vivo gene delivery.
Keywords: β amino ester polymers, cell transfection, gene delivery, hard-to-transfect, leukemia/lymphoma, polybrene
Introduction
In vitro gene delivery (cell transfection) research has made significant advances in recent decades. The research can be categorized by gene delivery method, either viral- or non-viral-mediated. Although viral-mediated transfection is highly efficient, the integration of a viral gene into a host cell genome has raised serious safety concerns [1, 2]. Non-viral-mediated gene delivery is relatively safe and more easily used, and includes various chemical and physical systems to enhance gene delivery. Physical gene delivery can be assisted by needle injection[3], electroporation [4, 5], particle bombardment (gene gun) [6, 7], ultrasound[8], and hydrodynamic methods[9, 10]; however, these procedures are relatively complicated and resultant cell viability is dismal. Lipofectamine, a product of lipids, is one of the most common methods for cellular gene transfection [11]. It is very effective in adhesion cells and can also efficiently deliver small interference RNA into suspension cells. However, transfection rates of Lipofectamine with plasmid DNAs in the hard-to-transfect lymphoma/leukemia cells are disappointing [12–16]. As a biomaterial, peptides including Tat [17], antennapedia homedomain peptide [18], and transportan peptide [19] were tested for gene delivery into cells with some promising in vitro results.
Chemical cationic polymers have demonstrated high potential for gene transfection in both adhesion and suspension cells [20–22]. The chemical systems involve the use of synthetic or natural compounds as carriers to incorporate gene(s) of interest and form nano-sized complexes for gene delivery into cells [23–25].Among them, poly(L-lysine), polyethylenimine (PEI), polymethacrylate, carbohydrate-based polymers, linear poly(amido-amine), and biodegradable polymers are widely studied. For years, PEI was considered as the gold standard approach for cell transfection since the initial successful PEI-mediated DNA transfection was reported in 1995 [26]. Subsequently, PEI derivatives such as the synthesized Polyethylene glycol (PEG)-PEI cholesterol complex are developed and currently being investigated in clinical trials [27]. However, the tested polymers such as PEI are cytotoxic [28, 29], leading researchers to search for a non-toxic and biodegradable polymer carrier. Using high-throughput synthesis and screening techniques, Anderson et al. have recently created libraries of over 2000 structurally unique poly β-amino esters (PBAE) and used their polymers as carriers for cell gene transfection [30]. This class of polymers displays properties such as self-assembly (wherein the plasmid DNA condenses to form small nanoparticles), and have amine-terminated chains that promote cellular uptake [31, 32]. These polymeric nano-carriers transfect genes into cells via endocytosis and are shown to escape endocytes leading to high gene delivery efficiency in adhesion cells [32–34]. Studies have shown that the PBAE polymers composed of 5-amino-1-pentanol and 1,4-butanediol diacrylate could achieve high-yield transfection in adhesion cells and, therefore, allow for the subsequent study of cellular functions as a result of the introduced genes [34]. However, there are no simple and efficient methods available for transfection of large-sized plasmids into suspension lymphoma/leukemia cells, which are known as hard-to-transfect. In this study, we investigated the potential application of simply synthesized PBAE polymers to deliver plasmid genes into suspension lymphoma/leukemia cells.
Materials and Methods
Reagents and cells
Monomers of 5-amino-1- pentanol and 1, 4-butanediol diacrylate were purchased from Sigma-Aldrich (St Louis, MO), 25 mM sodium acetate buffer (pH 5.2) was prepared by diluting a 3M stock (Sigma-Aldrich). Plasmid pMax-GFP was obtained from Lonza (Basel, Switzerland). Karpas 299 cells (human anaplastic large cell lymphoma) were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Jurkat cells (human T-cell lymphoma/leukemia) and K562 (human myeloid leukemia) were obtained from ATCC (Manassas, VA). Cells were cultured in RPMI1640 medium (Altanta Biological, Lawrenceville, GA) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 100 U/mL penicillin and 100 μg/mL streptomycin (Fisher Scientific). The PEI polymer was purchased from Sigma-Aldrich (St Louis, MO).
Preparation and fraction of poly β-amino ester C32 polymers
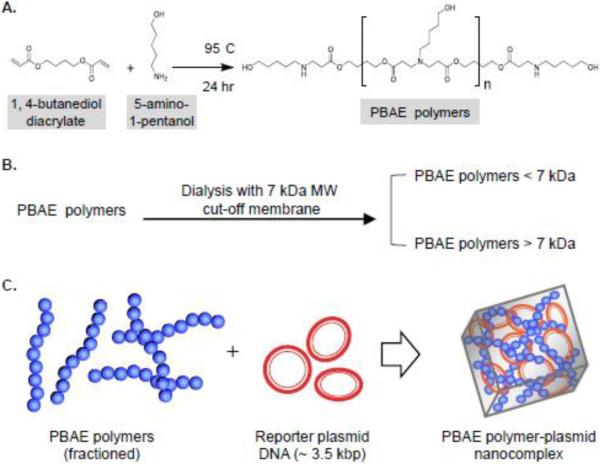
The PBAE polymers were synthesized according to the report by Anderson et al.[30]. Briefly, 0.342 g (3.20 mmol) of 5-amino-1-pentanol monomers and 0.526 g (2.65 mmol) of 1, 4-butanediol diacrylate monomers were added to a 25 mL flask at an amino/diacrylate ratio of 1.2:1.0, flashed with N2 and stirred at 95° C for 24 hr to generate amino-terminated polymer chains (Figure 1A). The average MW of PBAE polymers was reported ~14–20 KDa [35]. Subsequently, the formed PBAE polymers were fractioned into > 7 kDa and < 7 kDa (Figure 1B) by dissolving 200 mg of PBAE polymers in 4 mL of 80% ethanol and loading into pre-wet dialysis tubing (7 kDa MW cut-off, Thermo Scientific, Waltham, MA), followed by dialyzing in a 500 mL beaker with 400 mL 80% ethanol and stirring at room temperature for 2 hr. The dialysis solution was then replaced for overnight dialysis. The PBAE polymers > 7 kDa within the dialysis tubing were transferred into a round-bottom flask and the solvent was removed under reduced pressure. The PBAE polymers < 7 kDa in dialysis solution were collected and the solvent was removed. The fractioned PBAE polymers were dissolved in DMSO (Sigma-Aldrich) to 100 mg/mL and stored at 4° C until use.
Figure 1. Formulation of PBAE polymers and the PBAE polymer-plasmid nanocomplex.
A, The PBAE polymers were synthesized by a simple chemical reaction of 5-amino-1- pentanol and 1,4-butanediol diacrylate monomers at 95° C for 24 hr[30]. B, The synthesized PBAE polymers were fractioned into < 7 kDa and > 7 kDa polymers by dialysis using a 7 kDa MW cut-off membrane. C, The fractioned PBAE polymers were incorporated with the plasmid pMax-GFP (~3.5 kbp) containing a GFP reporter gene to formulate a PBAE polymer-plasmid nanocomplex.
Formulation and characterization of the nanocomplex for cell transfection
Plasmid vector pMax-GFP (Lonza, Basel, Switzerland), which has a MW of approximately 3500 bp and encodes green fluorescent protein (GFP), was used as a reporter of gene expression. To assemble the nanocomplex, 1μg of pMax-GFP was dissolved into 25 μl of 25 mM sodium acetate buffer at pH 5.2. The synthesized PBAE polymers (25 μl) were added into the sodium acetate buffer containing plasmid DNA (not reverse order) at different weight ratios as described, immediately followed by vortex mixing for 10 seconds. Nanocomplexes self-assembled in 10 minutes at room temperature (Figure 1C). For physical characterization, the PBAE-plasmid nanocomplexes composed of > 7 kDa PBAE polymers were diluted in RPMI1640 medium with pH 7.4 at room temperature and the size was kinetically measured using a Zetasizer nano detector (Malvern Instruments Ltd, Worcestershire, UK) at 0.5, 2, 4, 8, and 12 hr. In addition, changes in the nanocomplex size were also measured at pH 4, 5, 6, and 7. Shape and size of the nanocomplex were directly observed by scanning electron microscopy. In addition, polybrene nanocomplexes were also generated under the same conditions by adding polybrene polymers into the sodium acetate buffer (pH 5.2) containing plasmid DNA at different ratios as described in Figure 3D. At least three repeats were performed for each experiment condition with similar results.
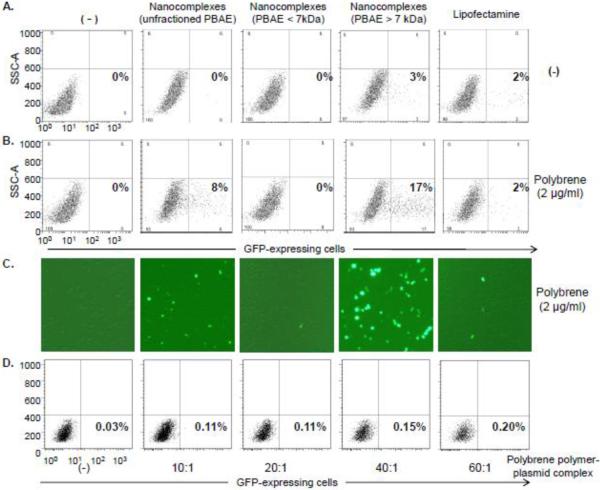
Figure 3. The PBAE polymer-plasmid nanocomplex-mediated cell transfection.
A, cultured lymphoma/leukemia Jurkat cells (5×106) were transfected by the nanocomplexes composed of pMax-GFP reporter genes and PBAE (unfractioned or fractioned polymers < 7 or > 7 kDa). In control groups, cells were transfected with Lipofectamine-pMax-GFP complex or pMax-GFP alone (−). After culture for 48 hr, GFP-expressing cells were quantified by flow cytometry (%). B, Cells were pre-treated with polybrene (2 ug/mL) for 5 min, followed by the same sets of transfection treatments as described in (A). Resultant cell transfection rates were determined by quantifying GFP-expressing cells (%). C, Fluorescent microscopy confirmation of the resultant GFP expression in the transfected cells. D, Exposure of Jurkat cells to the polybrene-plasmid complexes had little cell transfection effect.
Cell transfection, gene expression, and cytotoxicity assays
To evaluate the gene delivery capacity of the PBAE polymer-plasmid nanocomplexes in hard-to-transfect cells, fresh cultured human lymphoma and leukemia cells were suspended in RPMI1640 medium without serum (1×105/mL). To enhance transfection, the cells were pre-treated with polybrene at different concentrations (0–8 μg/mL) as described in Figure 4. Following pre-treatment for 5 min, the nanocomplexes were added into cell cultures. After incubation for 4 hr at 37° C, the culture supernatants were removed and replaced with 0.5 mL RPMI1640 medium containing 10% serum for continuous culture. Cells were then harvested 48 hr post transfection and GFP-expressing cells were quantified by flow cytometry and data were analyzed by using FlowJo 7.6 software. GFP-expressing cells were also confirmed by fluorescent microscopy. At least three repeats were performed for each experiment condition with similar results
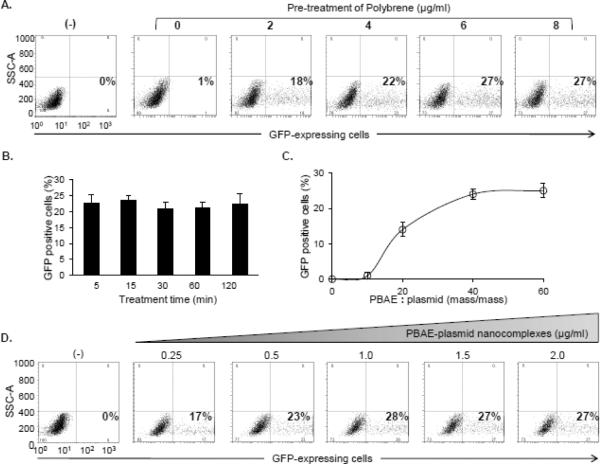
Figure 4. Optimization of the nanocomplex-mediated cell transfection.
A, Dose effect of polybrene. Jurkat cells (5×106) were pre-treated with polybrene for 5 min at different concentrations (0–8 μg/mL) and then exposed to the PBAE polymer (> 7 kDa)-plasmid nanocomplex. In the control experiment, reporter plasmid DNA alone (0.25 μg/mL) was used (−). After treatment for 48 hr, GFP-expressing cells were quantified by flow cytometry (%). B, Time-course of polybrene pre-treatment. Cells were pre-treated with 6 μg/mL polybrene for different times (5–120 min) and followed by exposure to the PBAE polymer-plasmid nanocomplex. Resultant GFP-expressing cells were quantified by flow cytometry (%). C, Optimal ratio of PBAE polymers and plasmid DNA reporters in the nanocomplex. Cells were pre-treated with polybrene (6 μg/mL) for 5 min and transfected by the nanocomplexes composed of PBAE > 7 kDa and pMax-GFP at different ratios (mass/mass) as indicated. Resultant GFP-expressing cells were quantified by flow cytometry (%). D, Optimal amount of reporter gene in cell transfection. Cells were pre-treated with polybrene (6 μg/mL) for 5 min and transfected by different doses of the nanocomplexes with the fixed 40:1 ratio of PBAE polymers (> 7 kDa) and pMax-GFP. The final amounts of reporter pMax-GFP were used from 0.25 to 2.0 μg/mL. Reporter plasmid DNA alone was used in control cells (−). Resultant GFP-expressing cells were quantified by flow cytometry (%).
To rule out the adverse effects on surface biomarker expression, the cells treated for 24 hr were stained with antibodies and surface expression of CD2, CD3, CD5, and CD45 was quantified by flow cytometry. In addition, for cytotoxicity assays, cells were treated with the PBAE polymers > 7 kDa and the PEI polymers at different concentrations (0–200 μg/mL) for 4 hr, then cultured in RPMI1640 medium with 10% serum for 48 hr. Viability of the treated cells was then evaluated with trypan blue staining[36]. Cell proliferation rate was monitored by MTT assay 48 hr post treatment as described previously [37]. At least three repeats were performed for each experiment condition with similar results
Results and Discussion
Formulation and characterization of the PBAE polymer-plasmid DNA nanocomplexes
As a carrier system, the PBAE polymers were synthesized (Figure 1A) and fractioned into polymers with MW > 7 or < 7 kDa by membrane tube dialysis (Figure 1B). The plasmid DNA, pMax-GFP (MW ~ 3.5 kbp), was used as a reporter system of gene expression. The nanocomplexes were formulated by incorporating the PBAE polymers with reporter plasmid DNAs (polymer:DNA ratio 40:1, mass/mass) through a non-covalent charge force at pH 5.2 as described under Materials and Methods (Figure 1C).
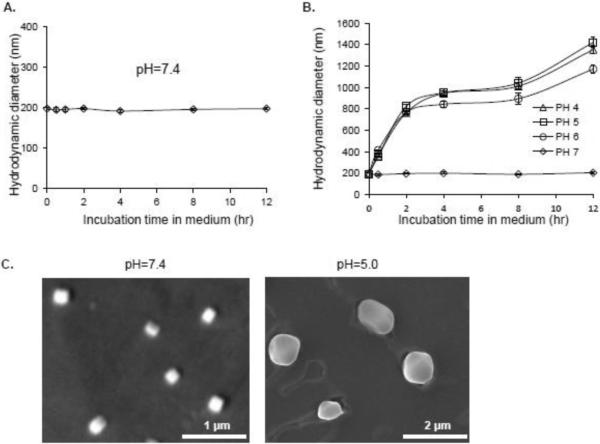
For physical characterization, the PBAE-plasmid nanocomplexes containing > 7 kDa PBAE were incubated in cell culture medium at 37° C and changes in size were kinetically monitored by dynamic light scattering (DLS) measurement. As shown in Figure 2A, the nanocomplexes were stable in size (~200 nm in diameter) at pH 7.4 over 12 hr, indicating that they could steadily carry and transport plasmid DNA under physiological pH (neutral pH). In addition to transportation, intracellular releasing of the carried plasmid DNA is also a critical factor for gene expression. It is known that following intracellular delivery, the nanocomplexes will be incorporated into cell endosomes/lysosomes, where a low pH condition exists. To understand the potential pH effect on biostability, the nanocomplexes were added into culture medium with pH 4.0, 5.0, 6.0, or 7.0 and incubated at room temperature for up to 12 hr. Resultant changes in the size of nanocomplexes were monitored by DLS measurement. Decreases in pH induced a rapid enlargement of the nanocomplexes (Figure 2B), indicating dissociation of the condensed PBAE-plasmids (nanocomplexes) as a result of the “proton sponge” phenomenon, which lead to the release of the transported plasmid DNA into the cytoplasm for gene expression. Transmission electron microscopy confirmed the formation of condensed PBAE-plasmid nanocomplexes at pH 7.4 with an average size of ~200 nm in diameter (Figure 2C), and dissociation of the nanocomplexes at pH 5.0 with an average size of 1200 nm.
Figure 2. Characterization of the PBAE polymer-plasmid nanocomplex.
A, The formed nanocomplexes composed of PBAE polymers > 7 kDa and plasmid DNA reporter were incubated in cell culture medium (pH 7.4) at 37° C and changes in size were kinetically monitored by DLS measurement. Under physiological pH conditions, the nanocomplex had a condensed size of ~200 nm in diameter and was stable for 12 hr. B, The PBAE polymer-plasmid nanocomplexes became unstable, rapidly dissociated, and enlarged under lower pH conditions (pH < 7). C, Transmission electron microscopy confirmed the condensed nanocomplexes at pH 7.4 with an average size of ~200 nm in diameter and dissociation of the nanocomplexes at pH 5.0 with an enlarged size ranging from 800 to 1200 nm.
The PBAE polymer nanocomplex had a high capacity to deliver plasmid DNA into the hard-to-transfect human lymphoma/leukemia cells
For cell transfection, the cultured Jurkat cells (human lymphoma/leukemia cell line) were treated for 4 hr with the nanocomplexes containing fractioned or unfractioned PBAE polymers and plasmid DNA reporters, or Lipofectamine containing plasmid DNA reporters as a standard cell transfection approach. The plasmid DNA alone was used as a negative control (−). After 48 hr culture, GFP-expressing cells were quantified by flow cytometry analysis (%). As shown in Figure 3A, the treatment by the nanocomplex composed of PBAE > 7 kDa and plasmid DNA reporter resulted in GFP expression in 3% of cells, similar to that observed by the Lipofectamine transfection method (2%). To enhance the transfection rate, Jurkat cells were pre-treated for 5 min with polybrene (2 μg/mL final concentration), which is routinely used to increase the efficiency of viral-mediated in vitro cell transduction. Interestingly, the polybrene pre-treatment significantly enhanced the cell transfection mediated by the nanocomplexes containing non-fractioned PBAE polymers (8%) and fractioned PBAE > 7 kDa (17%) (Figure 3B). However, the polybrene pre-treatment had no effect on cell transfection mediated by Lipofectamine. Resultant GFP expression in the transfected cells was also confirmed by fluorescent microscopy (Figure 3C). In addition, polybrene-plasmid DNA complexes were also generated under the same conditions with different ratios. However, exposure of Jurkat cells to the polybrene-plasmid complexes resulted in little or no increase in GFP-expressing cells (Figure 3D).
To determine the optimal cell transfection condition, Jurkat cells were pre-treated with different concentrations of polybrene for 5 min and transfected by the nanocomplexes containing a PBAE > 7 kDa and plasmid DNA reporter. Flow cytometry analysis revealed that a 27% cell transfection rate was reached with ≥ 6 μg/mL of polybrene pre-treatment (Figure 4A). In addition, a time-course study showed that 5 min pre-treatment of polybrene was enough to achieve the highest cell transfection rate (Figure 4B). Therefore, this optimal pre-treatment condition with 6 μg/mL polybrene for 5 min was used in the rest of the experiments. Moreover, the nanocomplexes were formulated by incorporating PBAE polymers > 7 kDa with plasmid DNA reporters at different ratios (Figure 4C). Flow cytometry showed that the nanocomplexes with a ratio of PBAE > 7 kDa to plasmid DNA at 40:1 resulted in the best cell transfection rate. Subsequently, the cells were exposed to the nanocomplexes containing different concentrations of reporter plasmid DNA from 0.25 to 2.0 μg/mL. As concentrations of plasmid DNA increased, the cell transfection rate increased and reached a maximum of 28% at 1 μg of plasmid DNA/mL (Figure 4D).
The PBAE polymers and the formed nanocomplexes were not cytotoxic
Changes in expression levels of cell surface biomarkers were initially examined to determine cytotoxicity of the cell treatments. Jurkat cells were treated with polybrene (6 μg/mL) alone, PBAE > 7 kDa (80 μg/mL) alone, the PBAE-plasmid nanocomplexes (80 μg/mL), or vehicle only in a control group (−). After each treatment for 24 hr, the cells were probed by antibodies specific for individual biomarkers and evaluated by flow cytometry. As shown in Figure 5A, the treatments of polybrene alone, PBAE polymer > 7 kDa alone or the nanocomplexes had no effect on the expression of cell surface biomarkers CD2, CD3, CD5, and CD45. In addition, the proliferation rate of cells with the same set of treatments was simultaneously monitored 48 hr post treatment. MTT assays revealed that exposure of cells to the nanocomplex or polybrene at their using concentrations had no effect on cell proliferation (Figure 5B). Studies have shown that PEI polymers are effective carriers for cell transfection and gene delivery, although they appear to be cytotoxic [28, 29]. For comparison, cultured Jurkat cells were exposed to PBAE or PEI polymers at different concentrations as indicated in Figure 5C. Cells were then harvested 48 hr post treatment and cell viability was examined with trypan blue staining. The synthesized PBAE polymers > 7 kDa had much less cytotoxicity in contrast to PEI polymers, which showed significant toxicity to Jurkat cells (Figure 5C).
Figure 5. The nanocomplex had little or no adverse-effect on lymphoma cells.
A, No effect on expression levels of cell surface biomarkers. Jurkat cells were treated with polybrene (6 μg/mL) alone, the fractioned PBAE polymer > 7 kDa alone, or combination treatments of polybrene and the nanocomplex as described in Figure 4. Control cells received no treatment (−). After treatment for 24 hr, cells were stained with antibodies for CD2, CD3, CD5, and CD45, and expression of these surface biomarkers was then quantified by flow cytometry. Non-stained cells were used as a background control (non-staining). B, No effect on cell proliferation. Proliferation rates of the treated cells in (A) were also evaluated by MTT assay 48 hr post treatment. C, PBAE polymers had minimal effect on cell viability. Jurkat cells were treated with different concentrations of PBAE polymers > 7 kDa or PEI polymers as indicated. After culture for 48 hr, cell viability was examined by trypan blue staining.
The PBAE polymer-plasmid nanocomplex-mediated cell transfection led to a high level of gene expression in human leukemia and lymphoma cells
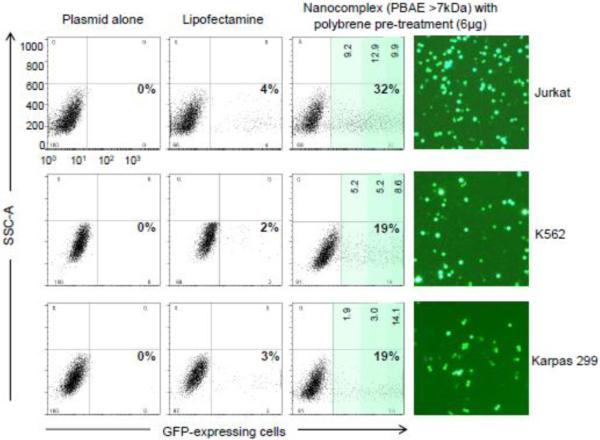
For further validation, the cell transfection rate of the nanocomplexes was compared to that mediated by Lipofectamine, a widely used and commercially available reagent for cell transfection. Cultured suspension cells including Jurkat, K562 (a human myeloid leukemia cell line), and Karpas 299 (a human anaplastic large cell lymphoma cell line) were tested. The pMax-GFP was used as a reporter and cells were pre-treated with polybrene to enhance cellular gene delivery. After transfection for 48 hr, GFP-expressing cells were quantified by flow cytometry analysis. Based on our experiences, the Lipofectamine-medicated transfection routinely resulted in GFP gene expression in 2–4 % (with or without polybrene pre-treatment) of the tested human lymphoma/leukemia cells. In contrast, exposure of cells to the nanocomplexes with polybrene pre-treatment achieved significantly high cell transfection and gene expression rates: 32% in Jurkat cells, 19% in K562 cells, and 19% in Karpas 299 cells; 6 to 9.5-fold of that mediated by Lipofectamine (Figure 6). Interestingly, although the total number of GFP-expressing cells were lower in K562 and Karpas 299 cells, the majority of the transfected cells expressed a high level of GFP, >102 higher than background cells (Figure 6, darker green zones). Cellular GFP expression in the transfected cells was also confirmed by fluorescent microscopy.
Figure 6. The nanocomplex-mediated gene delivery in different lymphoma/leukemia cells.
Cultured Jurkat leukemia/lymphoma cells, K562 myeloid leukemia cells, and Karpas 299 anaplastic large T-cell lymphoma cells were pre-treated with polybrene (6μg/mL) and then transfected with Lipofectamine containing the pMax-GFP reporter or the PBAE polymer (> 7 kDa)-plasmid nanocomplexes as described above. Plasmid pMax-GFP alone was used in control experiments (−). The total GFP-expressing cells were quantified by flow cytometry 48 hr post-transfection (%). The percentages of cells with different levels of GFP expression were also calculated (light green zone: <101; intermediate green: 101 to 102, and darker green: >102). Cellular GFP expression was also confirmed by fluorescent microscopy.
Conclusions
In summary, our study demonstrated a new approach for gene delivery by using a simple PBAE polymer-plasmid nanocomplex. This system showed several advantages over currently available non-viral-mediated cell transfection systems: i) as a carrier, the PBAE polymers can incorporate gene vectors such as plasmid DNA with a size of ~3.5 kbp and form a stable nanocomplex under physiological conditions (Figures 2A and 2C); ii) the formed nanocomplex has high potential to deliver plasmid DNA into the suspension lymphoma/leukemia cells, 6 to 8-fold more efficiently than that mediated by the standard Lipofectamine method under the same conditions (Figure 6); iii) in contrast to PEI, a well-studied cationic polymer for cell transfection, the PBAE polymers showed mild toxicity to the tested human cells (Figure 4); iv) more interestingly, the PBAE-plasmid nanocomplexes became unstable when environmental pH was lower than 7 (Figures 5B and 5C), which facilitated the unloading and delivery of the carried plasmid DNA into the cytoplasm of the transfected cells (Figure 2C); and v) the dissociated PBAE polymers are biodegradable, thus, more suitable for in vivo use. In addition, this study also showed the first evidence that pre-treatment of cells with polybrene significantly increases transfection efficiency of the PBAE polymer nanocomplexes (Figures 3 and 6). Although the detailed mechanism is unknown, the polybrene pre-treatment may neutralize the negative charge on the cell surface and enhance subsequent interactions of the PBAE nanocomplexes and cells.
Notably, due to its high transfection capacity of suspension cells, the PBAE nanocomplex might also be useful in enhancing gene delivery in other hard-to-transfect cells, including stem cells and suspension hematopoietic cells. In addition, since the PBAE polymer nanocomplexes showed less cytotoxicity, they may be used for ex vivo manipulation of cellular functions by introducing genes of interest into cells. The biodegradable property makes PBAE polymers suitable for in vivo use. Although the transfection rate of the PBAE polymer nanocomplex alone in lymphoma/leukemia cells is relatively low without polybrene pre-treatment, it is reasonable to believe that addition of specific ligand(s), through covalent or non-covalent bonds, into the PBAE polymer nanocomplexes will significantly improve their cell transfection potential and enable targeted gene delivery in vivo.
Acknowledgments
This study was supported by National Cancer Institute grant RO1CA151955 and the Collaborative Research Fund from the Virginia and L.E. Simmons Family Foundation (to Dr. Youli Zu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lundstrom K. Latest development in viral vectors for gene therapy. Trends in biotechnology. 2003;21(3):117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- [2].Woods NB, Muessig A, Schmidt M, Flygare J, Olsson K, Salmon P, Trono D, von Kalle C, Karlsson S. Lentiviral vector transduction of NOD/SCID repopulating cells results in multiple vector integrations per transduced cell: risk of insertional mutagenesis. Blood. 2003;101(4):1284–1289. doi: 10.1182/blood-2002-07-2238. [DOI] [PubMed] [Google Scholar]
- [3].Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science (New York, N.Y. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- [4].Heller LC, Ugen K, Heller R. Electroporation for targeted gene transfer. Expert opinion on drug delivery. 2005;2(2):255–268. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- [5].Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO journal. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang NS, Sun WH. Gene gun and other non-viral approaches for cancer gene therapy. Nature medicine. 1995;1(5):481–483. doi: 10.1038/nm0595-481. [DOI] [PubMed] [Google Scholar]
- [8].Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene therapy. 2000;7(23):2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- [9].Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene therapy. 1999;6(7):1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- [10].Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Human gene therapy. 1999;10(10):1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- [11].Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guven H, Konstantinidis KV, Alici E, Aints A, Abedi-Valugerdi M, Christensson B, Ljunggren HG, Dilber MS. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Experimental hematology. 2005;33(11):1320–1328. doi: 10.1016/j.exphem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [13].Huang H, Pannetier C, Hu-Li J, Paul WE. Transient transfection of primary T helper cells by particle-mediated gene transfer. Journal of immunological methods. 1998;215(1–2):173–177. doi: 10.1016/s0022-1759(98)00088-x. [DOI] [PubMed] [Google Scholar]
- [14].Baum C, Forster P, Hegewisch-Becker S, Harbers K. An optimized electroporation protocol applicable to a wide range of cell lines. BioTechniques. 1994;17(6):1058–1062. [PubMed] [Google Scholar]
- [15].Migliaccio AR, Bengra C, Ling J, Pi W, Li C, Zeng S, Keskintepe M, Whitney B, Sanchez M, Migliaccio G, Tuan D. Stable and unstable transgene integration sites in the human genome: extinction of the Green Fluorescent Protein transgene in K562 cells. Gene. 2000;256(1–2):197–214. doi: 10.1016/s0378-1119(00)00353-x. [DOI] [PubMed] [Google Scholar]
- [16].Brielmeier M, Bechet JM, Falk MH, Pawlita M, Polack A, Bornkamm GW. Improving stable transfection efficiency: antioxidants dramatically improve the outgrowth of clones under dominant marker selection. Nucleic acids research. 1998;26(9):2082–2085. doi: 10.1093/nar/26.9.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rajagopalan R, Xavier J, Rangaraj N, Rao NM, Gopal V. Recombinant fusion proteins TAT-Mu, Mu and Mu-Mu mediate efficient non-viral gene delivery. The journal of gene medicine. 2007;9(4):275–286. doi: 10.1002/jgm.1014. [DOI] [PubMed] [Google Scholar]
- [18].Huang R, Yang W, Jiang C, Pei Y. Gene delivery into brain capillary endothelial cells using Antp-modified DNA-loaded nanoparticles. Chemical & pharmaceutical bulletin. 2006;54(9):1254–1258. doi: 10.1248/cpb.54.1254. [DOI] [PubMed] [Google Scholar]
- [19].Kilk K, El-Andaloussi S, Jarver P, Meikas A, Valkna A, Bartfai T, Kogerman P, Metsis M, Langel U. Evaluation of transportan 10 in PEI mediated plasmid delivery assay. J Control Release. 2005;103(2):511–523. doi: 10.1016/j.jconrel.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [20].Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene therapy. 1999;6(8):1380–1388. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- [21].Oh YK, Suh D, Kim JM, Choi HG, Shin K, Ko JJ. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene therapy. 2002;9(23):1627–1632. doi: 10.1038/sj.gt.3301735. [DOI] [PubMed] [Google Scholar]
- [22].Swami A, Goyal R, Tripathi SK, Singh N, Katiyar N, Mishra AK, Gupta KC. Effect of homobifunctional crosslinkers on nucleic acids delivery ability of PEI nanoparticles. International journal of pharmaceutics. 2009;374(1–2):125–138. doi: 10.1016/j.ijpharm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [23].Schenborn ET, Goiffon V. DEAE-dextran transfection of mammalian cultured cells. Methods in molecular biology (Clifton, N.J. 2000;130:147–153. doi: 10.1385/1-59259-686-x:147. [DOI] [PubMed] [Google Scholar]
- [24].Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Current opinion in neurobiology. 2002;12(5):566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- [25].Mehier-Humbert S, Guy RH. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Advanced drug delivery reviews. 2005;57(5):733–753. doi: 10.1016/j.addr.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [26].Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khalil H, Chen T, Riffon R, Wang R, Wang Z. Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy. 2008;52(5):1635–1641. doi: 10.1128/AAC.01071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Florea BI, Meaney C, Junginger HE, Borchard G. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS pharmSci. 2002;4(3):E12. doi: 10.1208/ps040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu P, Li SY, Li Q, Ren J, Van Kirk EA, Murdoch WJ, Radosz M, Shen Y. Biodegradable cationic polyester as an efficient carrier for gene delivery to neonatal cardiomyocytes. Biotechnology and bioengineering. 2006;95(5):893–903. doi: 10.1002/bit.21036. [DOI] [PubMed] [Google Scholar]
- [30].Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angewandte Chemie (International ed. 2003;42(27):3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- [31].Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A polymer library approach to suicide gene therapy for cancer. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zugates GT, Peng W, Zumbuehl A, Jhunjhunwala S, Huang YH, Langer R, Sawicki JA, Anderson DG. Rapid optimization of gene delivery by parallel end-modification of poly(beta-amino ester)s. Mol Ther. 2007;15(7):1306–1312. doi: 10.1038/mt.sj.6300132. [DOI] [PubMed] [Google Scholar]
- [33].Green JJ, Langer R, Anderson DG. A Combinatorial Polymer Library Approach Yields Insight into Nonviral Gene Delivery. Accounts of chemical research. 2008;4(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zugates GT, Tedford NC, Zumbuehl A, Jhunjhunwala S, Kang CS, Griffith LG, Lauffenburger DA, Langer R, Anderson DG. Gene delivery properties of end-modified poly(beta-amino ester)s. Bioconjugate chemistry. 2007;18(6):1887–1896. doi: 10.1021/bc7002082. [DOI] [PubMed] [Google Scholar]
- [35].Sunshine BJ, Green JJ, Mahon KP, Yang F, Eltoukhy AA, Nguyen DN, Langer R, Anderson DG. Small-Molecule End-Groups of Linear Polymer Determine Cell-Type Gene-Delivery Efficacy. Adv. Mater. 2009;21:4947–4951. doi: 10.1002/adma.200901718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao N, Bagaria HG, Wong MS, Zu Y. A nanocomplex that is both tumor cell-selective and cancer gene-specific for anaplastic large cell lymphoma. Journal of nanobiotechnology. 2011;9(1):2. doi: 10.1186/1477-3155-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao N, Fogg JM, Zechiedrich L, Zu Y. Transfection of shRNA-encoding Minivector DNA of a few hundred base pairs to regulate gene expression in lymphoma cells. Gene therapy. 2010;18(3):220–224. doi: 10.1038/gt.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]