Abstract
Early-onset generalized torsion dystonia (dystonia 1) is an inherited movement disorder caused by mutations in DYT1 (TOR1A), which codes for torsinA. Most patients have a 3-base pair deletion (ΔGAG) in one allele of DYT1, corresponding to a loss of a glutamic acid residue (ΔE) in the C-terminal region of the protein. Functional alterations in basal ganglia circuits and the cerebellum have been reported in dystonia. Pharmacological manipulations or mutations in genes that result in functional alterations of the cerebellum have been reported to have dystonic symptoms and have been used as phenotypic rodent models. Additionally, structural lesions in the abnormal cerebellar circuits, such as cerebellectomy, have therapeutic effects in these models. A previous study has shown that the Dyt1 ΔGAG heterozygous knock-in (KI) mice exhibit motor deficits in the beam-walking test. Both Dyt1 ΔGAG heterozygous knock-in (KI) and Dyt1 Purkinje cell-specific knockout (Dyt1 pKO) mice exhibit dendritic alterations of cerebellar Purkinje cells. Here, Dyt1 pKO mice exhibited significantly less slip numbers in the beam-walking test, suggesting better motor performance than control littermates, and normal gait. Furthermore, Dyt1 ΔGAG KI/Dyt1 pKO double mutant mice exhibited significantly lower numbers of slips than Dyt1 ΔGAG heterozygous KI mice, suggesting Purkinje-cell specific knockout of Dyt1 wild-type (WT) allele in Dyt1 ΔGAG heterozygous KI mice rescued the motor deficits. The results suggest that molecular lesions of torsinA in Purkinje cells by gene therapy or intervening in the signaling pathway downstream of the cerebellar Purkinje cells may rescue motor symptoms in dystonia 1.
Keywords: cerebellum, conditional knock-out mouse, dystonia, DYT1, Purkinje cell, torsinA
1. Introduction
Early-onset generalized torsion dystonia (dystonia 1; OMIM 128100) is an inherited hyperkinetic movement disorder caused by mutations in DYT1 (TOR1A), which codes for torsinA [1]. TorsinA is a member of AAA+ family of ATPases that is widely expressed in the brain and throughout body [1–3], and may be involved in trafficking of polytopic membrane proteins and protein processing in the secretory pathway [4, 5]. This disease has an approximately 30% penetrance [6] and phenotypic variability [7]. Most of the patients have a 3 base-pair deletion (ΔGAG) in DYT1, corresponding to a loss of a glutamic acid residue in the C-terminal region of torsinA. However, an Arg288Gln missense mutation was found in one patient family [8], and an 18 bp-deletion was also reported in patients diagnosed as possible dystonia from another family [9–11]. Furthermore, a frame-shift mutation caused by a 4 bp-deletion was found in a patient diagnosed as a possible myoclonus-dystonia from another family [12]. No homozygous DYT1 mutation carrier has been reported in humans. Similarly, both Dyt1 ΔGAG homozygous KI mice and Dyt1 knockout (KO) mice do not survive after birth, suggesting homozygous DYT1 mutation carriers may exhibit neonatal lethality [13–15]. Onset of dystonia 1 usually occurs between 5 to 28 years of age [16]. Symptoms typically begin in a lower limb and progress up the body over years, whereas in later-onset dystonia cases symptoms are usually limited to upper-body parts.
Dysfunction of cerebellum contributes to the pathogenesis in dystonia patients. Trauma involving the cerebellum and cerebellar atrophy cause dystonia in humans [17, 18]. Structural grey matter alterations in the cerebellum have been observed in upper limb dystonia [19], cervical dystonia [20], and focal dystonia [21]. Abnormal activities of the cerebellum in DYT1 mutation carriers and alterations in the olivo-cerebellar pathway have been reported in primary focal dystonia [22]. Microinjection of kainic acid at low doses into the cerebellar vermis of mice can elicit dystonic postures of the trunk and limbs [23]. Additionally, infusion of ouabain, a sodium pump blocker, into the cerebellum induces dystonia-like symptoms in a mouse model of rapid-onset dystonia parkinsonism [24]. Dysfunction of the cerebellum has also been reported in other phenotypic dystonia rodent models produced by spontaneous mutations. A genetically dystonic (dt) rat was isolated from a spontaneous mutant colony with dystonic symptoms [25]. The dt rat has a mutation in Atcay coding for Caytaxin [26], which plays a critical role in the molecular response of Purkinje cells to climbing fiber input [27]. Additionally, tottering (tg) mice exhibit ataxia and paroxysmal dystonia, which is caused by a recessive mutation in a calcium channel α1A subunit gene predominantly expressed in the cerebellar granule cell layer, cell bodies in the deep cerebellar nuclei, and cerebellar Purkinje cells [28].
While pharmacological manipulations and the mutations described above cause dysfunction of the cerebellum and produce dystonic symptoms in rodents, further structural lesions in the already abnormal cerebellum or cerebellar circuits have been shown to improve or even eliminate the dystonic symptoms. Transgenic mice lacking cerebellar Purkinje cells exhibit less dystonia after kainic acid microinjection, suggesting the loss of cerebellar Purkinje cell’s function suppresses the induction of dystonia [23]. Surgical removal of the cerebellum (cerebellectomy) eliminates the motor symptoms and rescues the dt rats from juvenile lethality [29]. Consistent with the effect of cerebellectomy, electronic lesions of the dorsal portions of the lateral vestibular nuclei, which receive input from cerebellar Purkinje cells, also produce motor improvement in the dt rats [30]. Dystonic episode and abnormal brain activation in tottering (tg) mice were also eliminated by cerebellectomy or crossing with cerebellar Purkinje cell-specific degenerative (pcd) mutant [31, 32].
Beam-walking is a classical behavioral test to examine motor coordination and balance in rodents [33]. Although genetic dystonia mouse models, which mimic mutations in primary dystonia patients, do not exhibit overt abnormal postures, they exhibit motor deficits in the beam-walking test [13, 15, 34–40]. Our recent studies argue for the beam-walking test as a test of motor control abnormalities that could be due to the dystonic symptoms in the genetic dystonia mouse models. Dyt1 ΔGAG heterozygous KI male mice exhibit increased slip numbers of the hindpaws in the beam-walking test [13]. This beam-walking deficit in Dyt1 ΔGAG heterozygous KI male mice can be rescued with trihexyphenidyl, an anticholinergic drug, which is commonly used to treat dystonic symptoms in DYT1 patients [39]. Additionally, in rapid-onset dystonia-parkinsonism (dystonia 12; OMIM 128235) patients, dystonic symptoms are triggered by stress [41]. Consistent with patients, motor deficits in beam-walking test are triggered by stress in a genetic mouse model of dystonia 12 [38]. Furthermore, since dystonic symptoms commonly start from the legs in dystonia 1 patients, the beam-walking test may be one of the most appropriate behavior tests to detect the early motor symptoms in dystonia 1 mouse models.
We recently reported the generation of Dyt1 pKO mice [42] using cre-loxP technology [43] applied to mouse gene recombination [44] using Dyt1 loxP mice [15] and Pcp2-cre mice [45], which causes specific knockout of Dyt1 in cerebellar Purkinje cells. Dyt1 pKO mice exhibit shorter primary large dendrites and reduction of spine numbers in the quaternary dendrite branches of the cerebellar Purkinje cells, which are similar to those of Dyt1 ΔGAG heterozygous KI mice [42]. Since torsinA is highly expressed in cerebellar Purkinje cells, knocking out Dyt1 specifically in cerebellar Purkinje cells may substantially influence the output signals from the cerebellum and affect motor performance. In the present study, motor behavioral tests were performed in Dyt1 pKO mice and their littermates. Based on the results of the behavior tests, Dyt1 ΔGAG KI/pKO double mutant mice were produced and their motor performance was evaluated to examine the effect of the cerebellar Purkinje cell-specific knocking-out of Dyt1 WT allele of Dyt1 ΔGAG heterozygous KI mice.
2. Materials and methods
All experiments were carried out by investigators blind to the genotypes and in compliance with the USPHS Guide for Care and Use of Laboratory Animals and approved by IACUCs at the University of Illinois at Urbana-Champaign (UIUC) and the University of Alabama at Birmingham (UAB). Mice were housed under a 12 hour light and 12 hour dark cycle with access to food and water ad libitum.
2.1. Animals
2.1.1. Generation of cerebellar Purkinje-cell specific Dyt1 conditional knockout mice
To selectively inactivate Dyt1 in cerebellar Purkinje cells, we used Pcp2-cre mice (Jackson Laboratory stock no. 004146; B6.129-Tg(Pcp2-cre)2Mpin/J) that express Cre recombinase specifically in cerebellar Purkinje cells [45] and Dyt1 loxP mice (D3 129/Sv J, C57BL/6 mixed background) that we previously generated [15]. Pcp2-cre Dyt1 loxP double heterozygous mice were prepared by crossing Dyt1 loxP mice and Pcp2-cre mice. Dyt1 pKO mice were prepared by crossing Pcp2-cre Dyt1 loxP double heterozygous mice and Dyt1 loxP mice. Cerebellar Purkinje cell-specific reduction of torsinA mRNA was confirmed by in situ hybridization as described earlier [42]. Genotyping for Dyt1 pKO and control littermate (CT) mice was performed by multiplex PCR using F (5’-ATTCAAAAATGTTGTCATAGCCAGG-3’) and T (5’-CTACAGTGACCTGAATCATGTGGC-3’) primer sets for Dyt1 loxP [15], creA (5’-ATCTCCGGTATTGAAACTCCAGCGC-3’) and cre6 (5’-CACTCATGGAAAATAGCGATC-3’) primer sets for cre [46], and the tail DNA. A group of Dyt1 pKO and CT mice was used for behavioral semi-quantitative assessments of motor disorders, accelerated rotarod test, pawprint gait analysis and beam-walking test in this order. Dyt1 pKO and CT mice were also used for transmission electron microscopy analysis.
2.1.2. Generation of Dyt1 ΔGAG KI/pKO double mutant mice
Dyt1 ΔGAG heterozygous KI mice (D3 129/Sv J, C57BL/6, BALB/c mixed background) were prepared and genotyped as previously described [13, 47]. Dyt1 ΔGAG heterozygous KI female mice were crossed with Dyt1 pKO male mice to generate Dyt1 ΔGAG KI/pKO double mutant mice. Genotyping for Dyt1 ΔGAG KI/pKO double mutant mice, Dyt1 pKO mice and Dyt1 loxP heterozygous mice was performed by multiplex PCR using F and T primer sets for Dyt1 loxP [15], creA and cre6 primer sets for cre [46], and Tcko1 (5’-CGGCTGAGCTATGCAGAACTA-3’) and Tcko2 (5’-CCATAGCTGGACCTGCAATTAAG-3’) primer sets for Dyt1 ΔGAG KI locus [13]. Dyt1 ΔGAG KI/pKO double mutant mice were used for the beam-walking test.
2.1.3. Generation of Dyt1 ΔGAG KI/KO double mutant mice
Dyt1 heterozygous KO mice (D3 129/Sv J, C57BL/6, BALB/c mixed background) were generated as described earlier [15]. Dyt1 ΔGAG KI/KO double mutant mice and their littermates were generated by crossing Dyt1 ΔGAG heterozygous KI mice and Dyt1 heterozygous KO mice. Genotyping was performed by PCR using a set of F and Tcko2 primers for Dyt1 KO locus and a set of Tcko1 and Tcko2 primers for Dyt1 ΔGAG KI locus as described above. Tail DNAs from neonatal day zero mice were used as templates. Dyt1 ΔGAG KI /KO mice were used for Western blot analysis of torsinA.
2.2. Transmission electron microscopy
Brain sections were prepared for transmission electron microscopy analysis as described earlier [36]. Adult Dyt1 pKO mice and CT mice (n = 3 each, 2 to 4 months of age) were perfused with chilled 0.1M phosphate-buffered saline (pH7.4) followed by Karnovsky's Fixative in phosphate buffered 2% glutaraldehyde and 2.5% paraformaldehyde. The brains were dissected out and left in Karnovsky’s Fixative overnight. The tissue was then trimmed and washed in cacodylate buffer with no further additives. Microwave fixation was used with the secondary 2% osmium tetroxide fixative, followed by the addition of 3% potassium ferricyanide for 30 minutes. After washing with water, saturated uranyl acetate was added for en bloc staining. The tissue was dehydrated in a series of increasing concentrations of ethanol starting at 50%. Acetonitrile was used as the transition fluid between ethanol and epoxy. Infiltration series was done with an epoxy mixture using the Epon substitute Lx112. The resulting blocks were polymerized at 90°C overnight, trimmed with a razor blade, and ultrathin sectioned with diamond knives. Sections were then stained with uranyl acetate and lead citrate, and photographed with a Hitachi H600 transmission electron microscope. The nuclear envelopes and other associated cerebellar structures in the cerebellum were examined.
2.3. Behavioral analysis
2.3.1. Behavioral semi-quantitative assessments of motor disorders
Behavioral semi-quantitative assessments of motor disorders were performed as described earlier [13, 48]. A group consisting of 18 Dyt1 pKO mice (11 males and 7 females) and 19 CT mice (11 males and 8 females) at 145– 219 days old were placed individually on the table and hindpaw clasping, hindpaw dystonia, truncal dystonia and balance adjustments to a postural challenge were examined. Hindpaw clasping was assessed as hindpaw movements for postural adjustment and attempt to straighten up while the mouse was suspended by the mid-tail. The hindpaw dystonia was assessed as the increased spacing between the limbs, poor limb coordination, crouching posture and impairment of gait. Truncal dystonia was assessed as the flexed posture. Postural challenge was performed by flipping the mouse onto its back and the ease of righting was noted.
2.3.2. Accelerated rotarod test
Motor performance was assessed with Economex accelerating rotarod (Columbus Instruments) as described earlier [13]. The apparatus started at an initial speed of 4 rpm. Rod speed was gradually accelerated at a rate of 0.2 rpm/s. The latency to fall was measured with a cutoff time of 2 minutes. The Dyt1 pKO mice and CT mice at 159– 233 days old were tested for three trials on each day for 2 days. The trials within the same day were performed approximately at 1 hour intervals.
2.3.3. Pawprint gait analysis
Pawprint gait analysis was performed as described earlier [13, 33]. A runway with a dark goal box at the end was lined with a sheet of white paper. Fore- and hindpaws of the Dyt1 pKO mice and CT mice at 164– 238 days old were painted with water soluble non-toxic paint of different colors. Mice were allowed to walk across the runway and into the goal box. One set of prints was collected for each animal after it walked continuously across the runway. Of each set, the four center pairs of hind- and forepaw prints were analyzed for stride, base, and overlap of the paws on each side.
2.3.4. Beam-walking test
The Dyt1 pKO mice and CT mice at 171–245 days old were used for the beam-walking test to evaluate their motor performance caused by loss of torsinA function in the cerebellar Purkinje cells. Another group consisted of 7 Dyt1 loxP heterozygous mice (6 males and 1 females), 11 Dyt1 ΔGAG KI/pKO double mutant mice (8 males and 3 females) and 15 Dyt1 ΔGAG heterozygous KI mice (with an additional Dyt1 loxP allele; 8 males and 7 females) at 161–175 days old was used to evaluate the effect of cerebellar Purkinje-cell specific knocking-out of Dyt1 in WT allele of Dyt1 ΔGAG heterozygous KI mice. Dyt1 loxP heterozygous littermate mice were used as CT mice in this group. The beam-walking test was performed within the last 8 hours of light period after acclimation to a sound-attenuated testing room for 1 hour as described earlier [13, 34, 35]. The mice were trained to transverse a medium square beam (14 mm wide) in three consecutive trials each day for 2 days and tested twice each on the medium square beam and a medium round beam (17mm diameter) on the third day. The mice were then tested twice each on a small round beam (10 mm diameter) and a small square beam (7 mm wide) on the fourth day. Their hind paw slips on each side while walking the 80 cm beams were counted.
2.4. Western blot for torsinA
TorsinA level in mouse brains was quantified by Western blot analysis as described earlier [36]. The brains were dissected from WT littermates (n=3), Dyt1 ΔGAG heterozygous KI mice (n=4), and Dyt1 ΔGAG KI/KO double mutant mice (n=4) at postnatal day zero and quickly frozen in liquid nitrogen. The brains were homogenized in ice-cold lysis buffer [50 mM Tris·Cl (pH7.4), 175 mM NaCl, 5 mM EDTA·2Na, Complete Mini protease inhibitor cocktail (Roche); 100 mg of brain/ml of lysis buffer] and sonicated for 10 sec. One ninth volume of ice-cold 10% TritonX-100 in the lysis buffer was added to the homogenates and the homogenates were incubated for 30 min on ice. The homogenates were centrifuged at 10,000 × g for 15 min at 4°C and then the supernatants were obtained. The protein concentration of each supernatant was measured by Bradford assay with bovine serum albumin as standards [49]. The samples were mixed with loading buffer for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and boiled for 5 min, incubated on ice for 1 min, and then centrifuged for 5 min to obtain the supernatant. Forty µg of the samples were loaded on SDS-PAGE with a Precision Plus Protein Standards All Blue (Bio-Rad) as a molecular mass marker. The separated proteins on the gel were transferred to a PROTRAN nitrocellulose transfer membrane (Whatman). The membrane was blocked in 5% milk (Bio-Rad) in TBS-T buffer [20 mM Tris·Cl (pH7.6), 137 mM NaCl, 0.1% (v/v) Tween 20] and incubated overnight at 4 °C with rabbit polyclonal torsinA antibody (Abcam; ab34540) in the blocking buffer. The membrane was washed in TBS-T buffer and incubated with bovine anti-rabbit IgG-horseradish peroxidase (HRP; Santa Cruz; sc-2370) in the blocking buffer at room temperature for 1hr, and then washed. The band was detected by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). The signal was captured by Alpha Innotech FluorChem Q MultiImage III and the density of each band was quantified with UN-SCAN-IT gel software (Silk Scientific). Restore Western Blot Stripping buffer (Thermo Scientific) was used for stripping torsinA antibody off the membrane to detect glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. After stripping the membrane of the torsinA antibody, the membrane was washed in TBS-T buffer and re-blocked in the blocking buffer. Levels of GAPDH were detected with HRP-conjugated GAPDH antibody (Santa cruz; sc-25778 HRP). Western blot analysis was performed in duplicate.
2.5. Statistics
Data in pawprint test were analyzed by analysis of variance (ANOVA) mixed model in SAS/STAT Analyst software (Version 9.1.3; SAS Institute Inc. NC) as described earlier [13]. Latency to fall in the accelerated rotarod test and slips numbers in beam-walking test were analyzed by logistic regression (GENMOD) with negative binominal distribution using GEE model in the software [13, 15, 34]. Sex, age, and body weight were input as variables. Data in the accelerated rotarod and beam-walking tests were analyzed after natural log transformation to obtain a normal distribution. The density of torsinA band was standardized to that of GAPDH band in Western blot analysis. The standardized pixel ratios were analyzed by Student’s t-test. The data in WT littermates were normalized to 100%. Significance was assigned at p < 0.05.
3. Results
3.1. No significant alteration of the nuclear envelopes in Dyt1 pKO mice
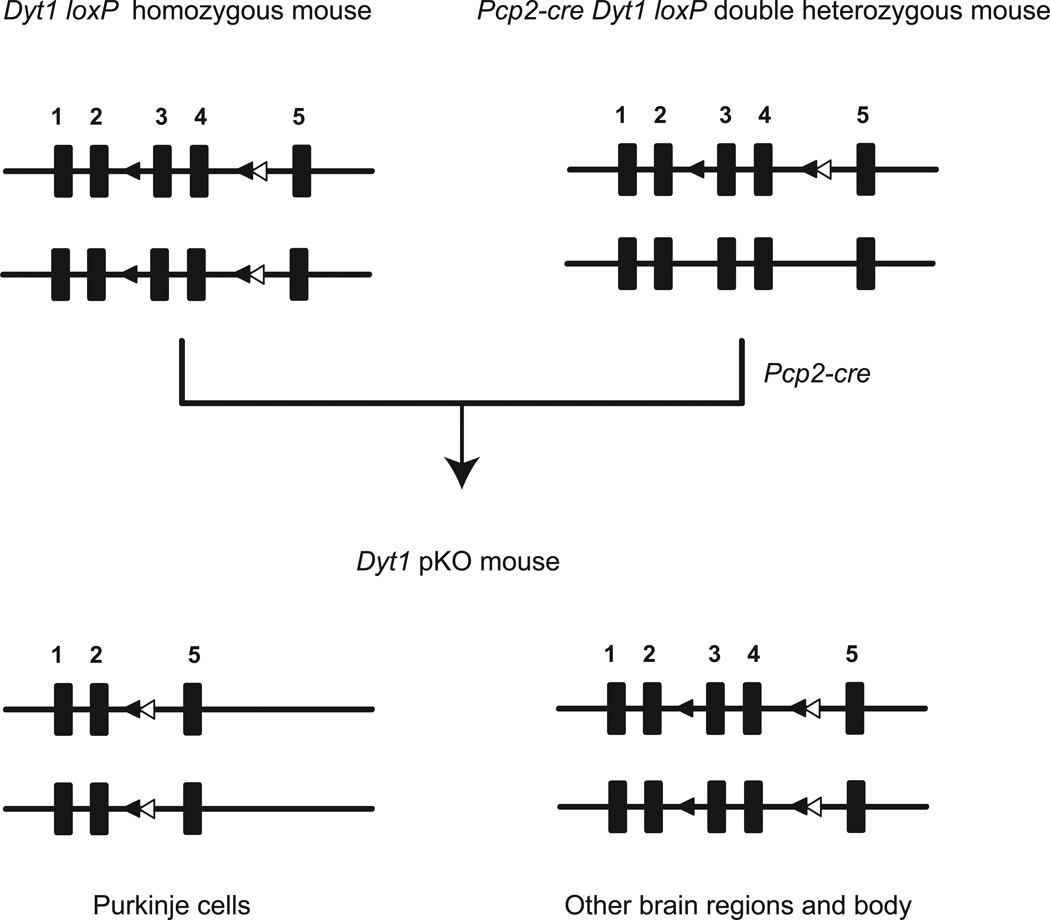
Pcp2-cre Dyt1 loxP double heterozygous mice were produced by crossing Dyt1 loxP mice and Pcp2-cre mice. To selectively inactivate Dyt1 in cerebellar Purkinje cells, Dyt1 pKO mice were produced by crossing Dyt1 loxP mice and Pcp2-cre Dyt1 loxP double heterozygous mice (Fig. 1). Dyt1 pKO mice were born according to Mendelian ratio and developed to adult. In Dyt1 pKO mice, exons 3 and 4 are deleted in cerebellar Purkinje cells because cre is expressed specifically in cerebellar Purkinje cells and the recombination occurs in the cells, while these exons are intact in other brain regions and body.
Fig. 1.
Generation of Dyt1 pKO mice. Pcp2-cre Dyt1 loxP double heterozygous mice were prepared by crossing Dyt1 loxP mice and Pcp2-cre mice. Dyt1 pKO mice were prepared by crossing Dyt1 loxP homozygous mice and Pcp2-cre Dyt1 loxP double heterozygous mice. Black rectangles are exons of Dyt1 and the exon numbers are shown above the rectangles. Black triangles are loxP sites flanking exons 3 and 4. White triangles are FRT sites that remained after removing Neo cassette. In Dyt1 pKO mice, exons 3 and 4 are deleted in cerebellar Purkinje cells because cre is expressed specifically in cerebellar Purkinje cells and the recombination occurs in the cells, while these exons are intact in other brain regions and the rest of the body.
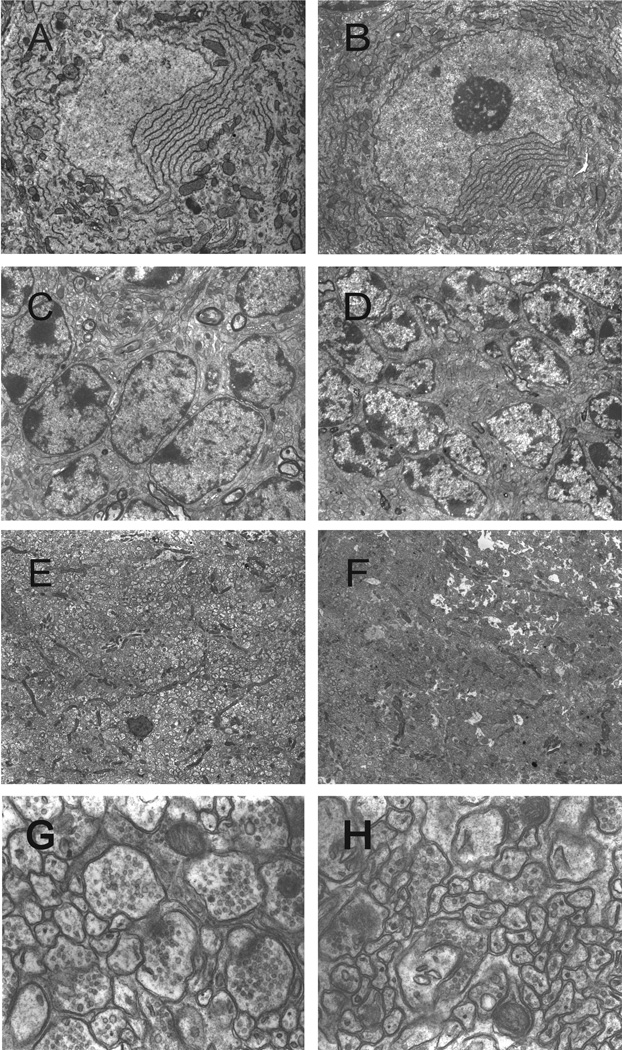
We examined the morphology of the cerebellar Purkinje cells and other associated cerebellar structures in Dyt1 pKO mice with a transmission electron microscope (Fig. 2A–H). Dyt1 pKO mice showed intact nuclear envelopes in cerebellar Purkinje cells (Fig. 2B). Furthermore, the cytoplasmic structures and the contents showed no significant abnormalities (5,000 ×; Fig. 2A, B). Similarly, the granule cells showed no gross alteration in nuclear envelope structures (4,000 ×; Fig. 2C, D). In the molecular layer at the low magnification (2,500 ×; Fig. 2E, F), both axons and dendrites did not show gross alteration of diameters and packing densities. Additionally, at higher magnification (30,000 ×; Fig. 2G, H), boutons and postsynaptic densities were clearly visible, suggesting synapse formation in the molecular layer has no gross alteration in Dyt1 pKO mice.
Fig. 2.
Ultrastructure of cerebellar cortex of a CT mouse (left panels) and a Dyt1 pKO mouse (right panels). Both mice showed normal nuclear structures of cerebellar Purkinje cells (A, B), granule cells (C, D), molecular layers (E, F), and synapses in molecular layers (G, H). Magnifications: A and B: 5,000×; C and D: 4,000×; E and F: 2,500×; G and H: 30,000×.
3.2. No overt abnormal postures in Dyt1 pKO mice
When suspended by their tail, both Dyt1 pKO and CT mice had normal splaying of their hindpaws. Dyt1 pKO mice had no observable hindpaw extension or truncal arching compared to CT mice. All mice exhibited strong righting reflexes when tipped on their side. The results suggest that Dyt1 pKO mice had no overt abnormal postures, similar to Dyt1 ΔGAG heterozygous KI mice [13], Dyt1 knock-down mice [35], cerebral cortex-specific Dyt1 conditional KO mice [15], and striatum-specific Dyt1 conditional KO mice [50].
3.3. Improved motor performance in Dyt1 pKO mice
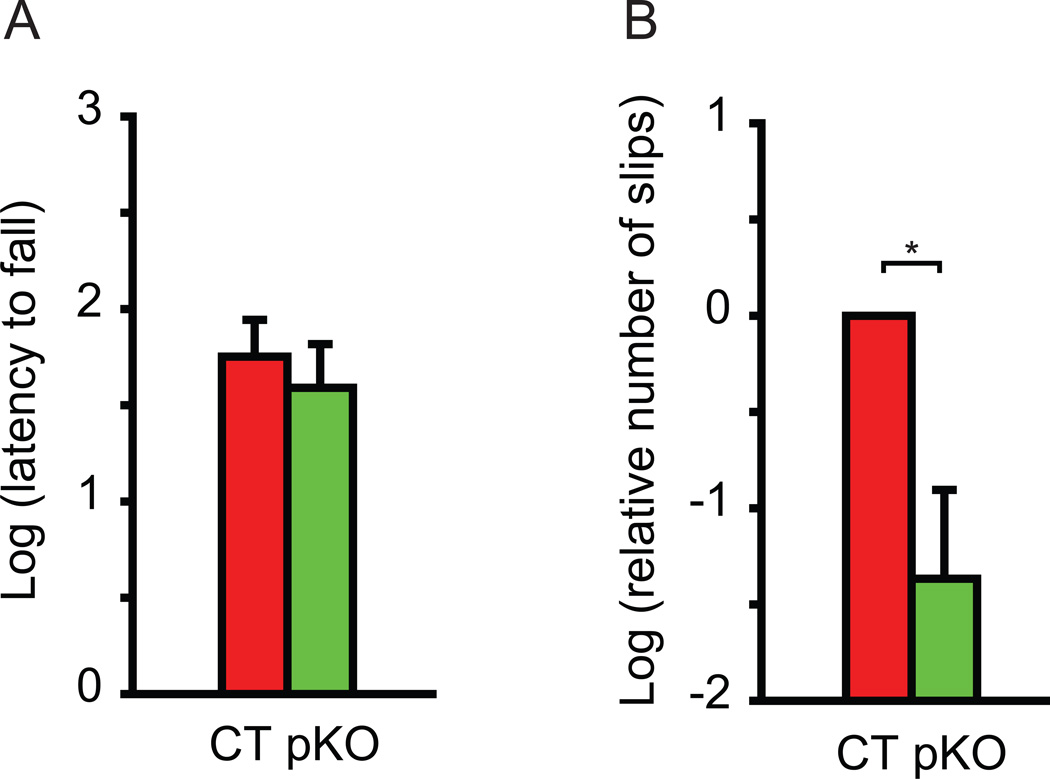
Motor performance was assessed by the accelerated rotarod and beam-walking tests. Each mouse was put on the accelerated rotarod and the latency to fall was measured. Since mice can hold onto the rotarod with four paws, the latency to fall is an indicator of total motor performance, with shorter latency indicating motor deficits. Dyt1 pKO mice did not show significant difference in latency to fall (Fig. 3A; p = 0.4861), suggesting no total motor performance deficits, consistent with findings reported earlier in Dyt1 ΔGAG heterozygous KI mice [13], Dyt1 knock-down mice [35], cerebral cortex-specific Dyt1 conditional KO mice [15], and striatum-specific Dyt1 conditional KO mice [50]. We further analyzed the motor coordination and balance by the beam-walking test. Dyt1 pKO mice showed 84% less slip numbers in the beam-walking test compared to CT mice (Fig. 3B; p = 0.012), suggesting improved motor performance.
Fig. 3.
Motor performance in Dyt1 pKO mice and CT mice. (A) Latency to fall in accelerated rotarod test. Dyt1 pKO mice did not exhibit significant difference in latency to fall, suggesting no motor deficits with four paws. The latency to fall was transformed in natural log to obtain a normal distribution. (B) Beam-walking performances in Dyt1 pKO mice and CT mice. Dyt1 pKO mice showed significantly decreased numbers of slips in beam-walking test, suggesting improved motor performance. The slips numbers were transformed in natural log to obtain a normal distribution and normalized to CT mice. Vertical bars represent means ± standard errors (SE). *p < 0.05.
3.4. Normal gait performance in Dyt1 pKO mice
Gait performance was assessed by pawprint analysis. Dyt1 pKO mice did not show any significant difference in stride length, base length, or distance of overlap, suggesting Dyt1 pKO mice exhibited normal gait performance (Table 1).
Table 1.
Normal gait performance in Dyt1 pKO mice.
| Pawprint | CT | Dyt1 pKO | p |
|---|---|---|---|
| Stride length | 74.5 ± 1.1 | 75.2 ± 1.5 | 0.71 |
| Base length | 20.5 ± 0.5 | 20.4 ± 0.6 | 0.87 |
| Overlap | 9.3 ± 0.5 | 9.2 ± 0.7 | 0.98 |
Stride length, base length and distance of overlap are shown as means ± standard errors (mm). CT: control littermate mice; Dyt1 pKO: cerebellar Purkinje cell-specific Dyt1 conditional knockout mice.
3.5. Generation of Dyt1 ΔGAG KI/pKO double mutant mice
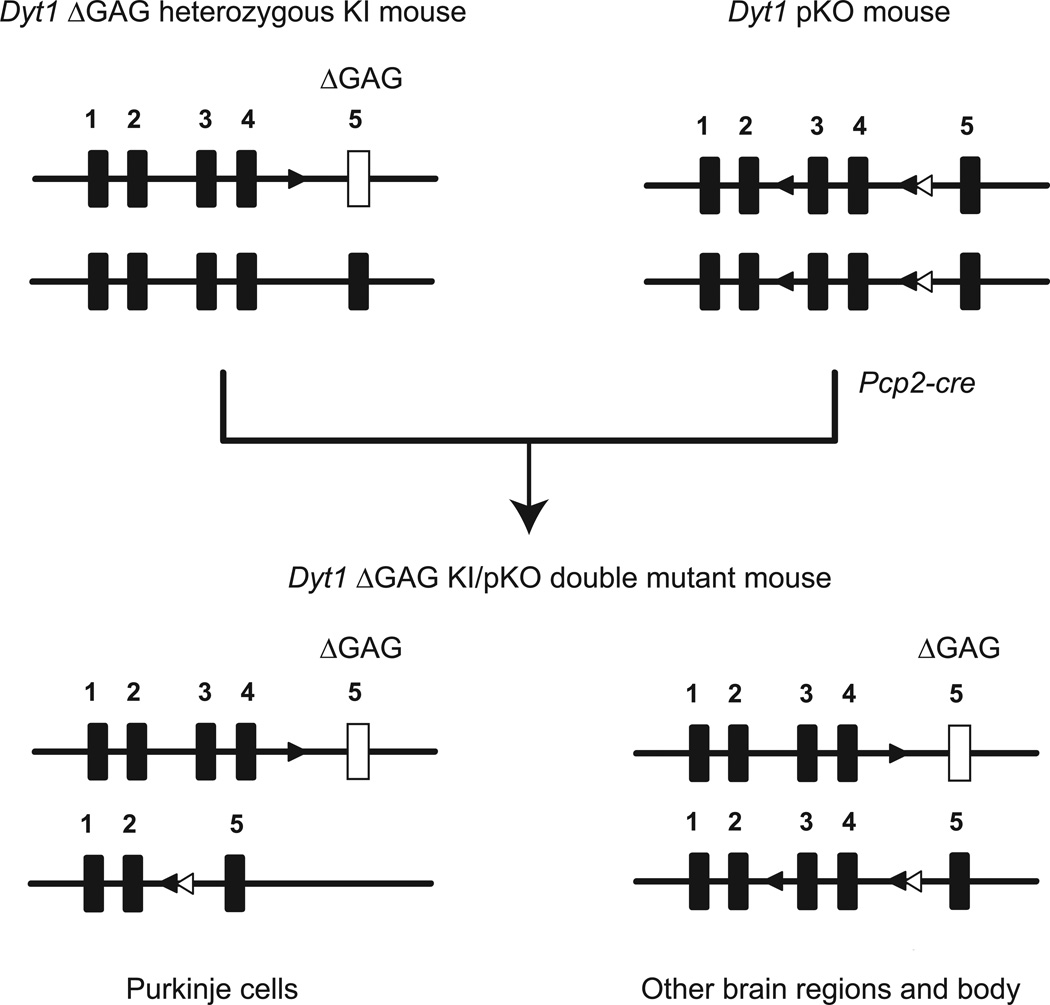
Based on the enhanced performance of Dyt1 pKO mice in beam-walking test, we generated Dyt1 ΔGAG KI/pKO double mutant mice to explore whether cerebellar Purkinje-cell specific knocking-out of Dyt1 WT allele in Dyt1 ΔGAG heterozygous KI mice could rescue the motor deficits. The double mutant mice were generated by crossing Dyt1 ΔGAG heterozygous KI female mice with Dyt1 pKO male mice (Fig. 4). Dyt1 ΔGAG KI/pKO double mutant mice were born according to Mendelian ratio and developed to adulthood. In Dyt1 ΔGAG KI/pKO double mutant mice, exons 3 and 4 of Dyt1 loxP allele are deleted in cerebellar Purkinje cells because Pcp2-cre-mediated recombination specifically occurred in cerebellar Purkinje cells, while these exons are intact in other brain regions and body. However, the Dyt1 ΔGAG KI mutation remains unchanged in all brain and body regions in Dyt1 ΔGAG KI/pKO double mutant mice.
Fig. 4.
Generation of Dyt1 ΔGAG KI/pKO double mutant mice. Dyt1 ΔGAG heterozygous KI mice were crossed with Dyt1 pKO mice to produce Dyt1 ΔGAG KI/pKO double mutant mice. Black rectangles are exons of Dyt1. White rectangles are Dyt1 exon 5 with ΔGAG KI mutation. Exon numbers are shown above the rectangles. Black triangles are loxP sites flanking exons 3 and 4. White triangles are FRT sites that remained after removing Neo cassette. In Dyt1 ΔGAG KI/pKO double mutant mice, exons 3 and 4 in Dyt1 loxP allele are deleted in cerebellar Purkinje cells because Pcp2-cre expresses specifically in cerebellar Purkinje cells and the recombination occurs in the cells, while these exons are intact in other brain regions and the rest of the body. The Dyt1 ΔGAG mutation remains in all regions. For simplicity, the gene map of the CT mice is not shown.
3.6. Improved motor performance in Dyt1 ΔGAG heterozygous KI mice by the cerebellar Purkinje-cell specific Dyt1 conditional knocking-out
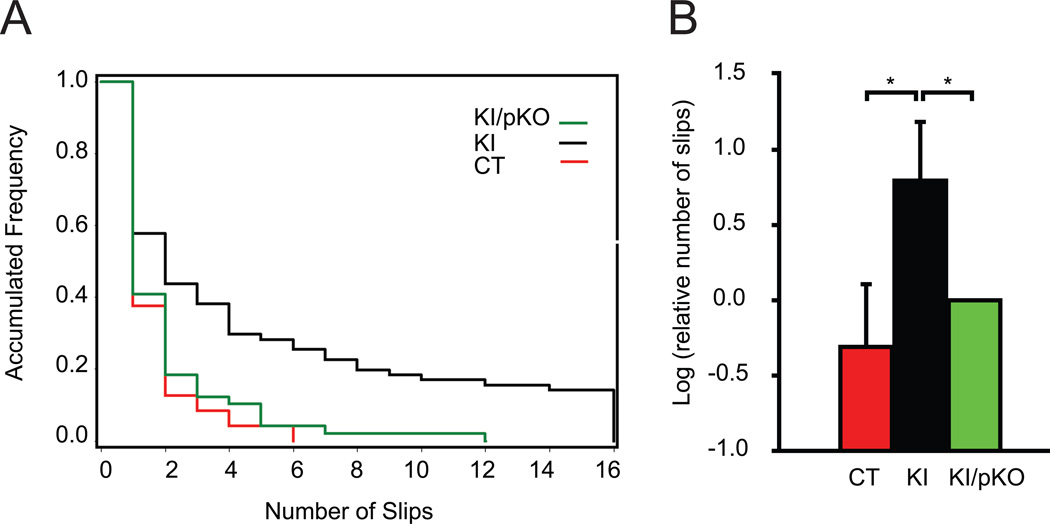
We examined whether knocking-out of WT allele of Dyt1 in Dyt1 ΔGAG heterozygous KI mice can rescue the motor deficits in beam-walking test. Raw data from the 4 beams in the beam-walking test were pooled and plotted (Fig. 5A). Only 30% of Dyt1 ΔGAG heterozygous KI mice exhibited more slips than the maximum slips found in CT mice, suggesting a low penetrance of motor deficits in Dyt1 ΔGAG heterozygous KI mice. On the other hand, most of Dyt1 ΔGAG KI/pKO double mutant mice exhibited lower slips numbers than Dyt1 ΔGAG heterozygous KI mice and showed similar frequency to CT mice. Statistical analysis showed that Dyt1 ΔGAG heterozygous KI mice showed significantly more slips than CT mice (p = 0.03) or Dyt1 ΔGAG KI/pKO double mutant mice (p = 0.04) in beam-walking test (Fig.5B), while there is no significant difference in slips numbers between CT mice and Dyt1 ΔGAG KI/pKO double mutant mice (p = 0.53). The results suggest that there is an improved motor performance in Dyt1 ΔGAG heterozygous KI mice by additionally knocking-out the WT Dyt1 specifically in cerebellar Purkinje cells.
Fig. 5.
Beam-walking performance in CT mice, Dyt1 ΔGAG heterozygous KI mice, and Dyt1 ΔGAG KI/pKO double mutant mice. (A) Accumulated frequency of control and mutant mice, and their numbers of slips. Raw data from the 4 beams were pooled and plotted. (B) Dyt1 ΔGAG heterozygous KI mice showed significantly more slips than CT mice or Dyt1 ΔGAG KI/pKO double mutant mice in beam-walking test, while there is no significant difference in slips numbers between CT mice and Dyt1 ΔGAG KI/pKO double mutant mice. The slips data were transformed in natural log to obtain a normal distribution and normalized to Dyt1 ΔGAG KI/pKO double mutant mice. Dyt1 loxP heterozygous littermate mice were used as CT mice. Vertical bars represent means ± SE. *p < 0.05.
3.7. Significant reduction of torsinA in Dyt1 ΔGAG KI/KO double mutant mice
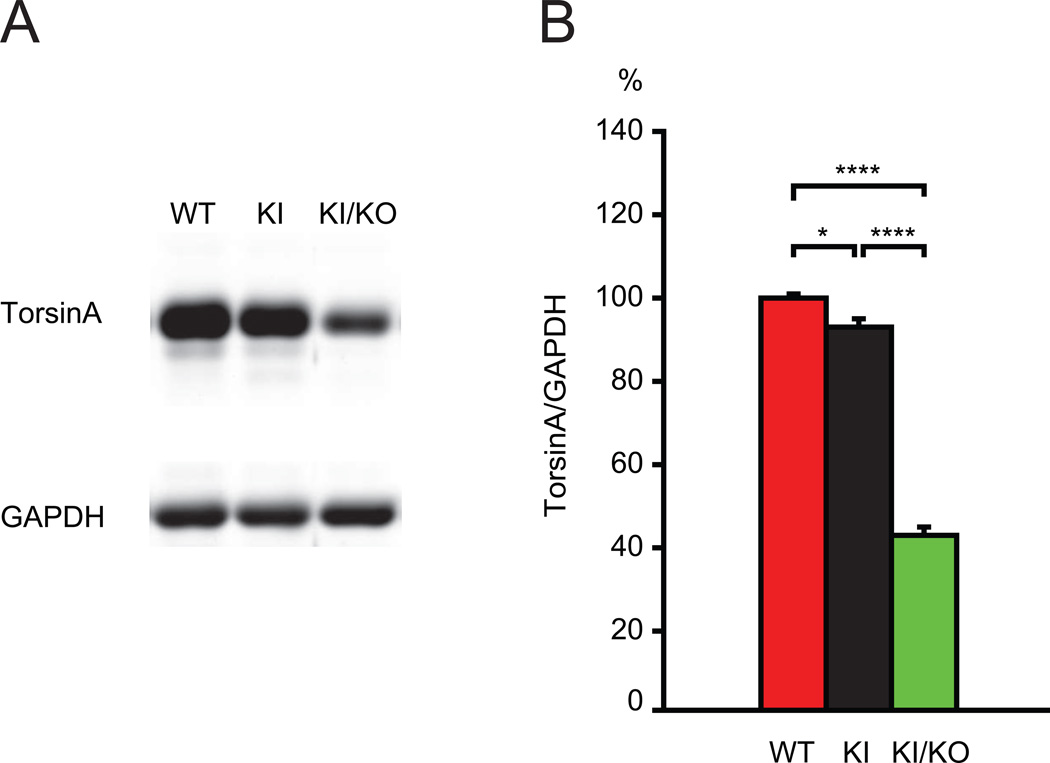
We previously have shown that Dyt1 pKO mice have a significant, diminished expression of torsinA mRNA in cerebellar Purkinje cell by in situ hybridization [42]. Therefore, Dyt1 ΔGAG heterozygous KI mice with an additional cerebellar Purkinje-cell specific conditional knock-out of the Dyt1 WT allele should cause similar reduction in torsinA level in Purkinje cells, while other brain regions and the rest of the body are only affected by Dyt1 ΔGAG heterozygous KI mutation. Due to the difficulty of distinguishing slight difference of torsinA level in the brain by immunohistochemistry, we used Western blot analysis of double mutant mice with Dyt1 ΔGAG KI and Dyt1 KO mutations to examine the effect of an additional KO mutation of the WT allele of Dyt1 in Dyt1 ΔGAG heterozygous KI mice on torsinA level in the brain. As expected, the double mutant mice exhibited neonatal lethality similar to Dyt1 ΔGAG homozygous KI and Dyt1 KO mice. Therefore we used the brains from postnatal day zero mice for quantification of torsinA. Western blot analysis showed significantly reduced torsinA level in Dyt1 ΔGAG heterozygous KI mice in comparison to WT mice (p = 0.024; Fig. 6A, B). Moreover, torsinA level was significantly reduced in the double mutant mice with Dyt1 ΔGAG and Dyt1 KO mutations in comparison to Dyt1 ΔGAG heterozygous KI mice (p = 0.000003) and WT mice (p = 0.000007). The result suggests that Dyt1 ΔGAG heterozygous KI mutation causes reduction of torsinA and an additional KO of the other allele causes further reduction of torsinA in the brains as predicted. Significant reduction of torsinA function in cerebellar Purkinje cells may underlie the mechanism of better motor performance in Dyt1 ΔGAG KI/pKO double mutant mice and Dyt1 pKO mice.
Fig. 6.
Western blot for torsinA. (A) The representative bands for torsinA and GAPDH in WT littermates (WT), Dyt1 ΔGAG heterozygous KI mice (KI), and Dyt1 ΔGAG KI/KO double mutant mice (KI/KO). (B) The quantified torsinA levels in the brains of three lines. TorsinA level in Dyt1 ΔGAG heterozygous KI mice was significantly reduced in comparison to WT mice. Moreover, torsinA level was significantly reduced in the double mutant mice with Dyt1 ΔGAG KI and Dyt1 KO mutations in comparison to Dyt1 ΔGAG heterozygous KI mice and WT mice. The density of torsinA band was standardized to that of GAPDH band. The data in WT littermates were normalized to 100%. WT (n=3), 100 ± 1%; KI (n=4), 93 ± 2 %; KI/KO (n=4), 43 ± 2%. Vertical bars represent means ± SE. *p < 0.05, ****p < 0.0001.
4. Discussion
Dyt1 is inactivated specifically in the cerebellar Purkinje cells in Dyt1 pKO mice, while other brain regions and the rest of the body express a normal level of torsinA. Since cerebellar Purkinje cells integrate incoming signals and produce inhibitory output signals to the deep cerebellar nuclei, Dyt1 pKO mice may produce altered output signals from the cerebellum. In the present study, Dyt1 pKO mice exhibited enhanced motor coordination in the beam-walking test, while they showed normal gait, ultrastructure of nuclear envelopes in Purkinje cells, and synapse formation in molecular layers. We applied this enhanced motor coordination effect in an attempt to rescue the motor deficits of Dyt1 ΔGAG heterozygous KI mice. Dyt1 ΔGAG heterozygous KI mice express both WT and mutant Dyt1 throughout the body and exhibit motor deficits in beam-walking test [13]. Dyt1 ΔGAG KI/pKO double mutant mice exhibited decreased numbers of slips comparing to Dyt1 ΔGAG heterozygous KI mice, suggesting that the cerebellar Purkinje-cell specific inactivation of Dyt1 WT allele of Dyt1 ΔGAG heterozygous KI mice rescued the motor deficits. The results suggest that molecular lesions of torsinA in cerebellar Purkinje cells could achieve beneficial effect. Gene therapy to inactivate torsinA specifically in cerebellar Purkinje cells or intervening to the output signals from cerebellar Purkinje cells may rescue motor symptoms in dystonia 1.
The mechanism of better motor performance of Dyt1 pKO mice in comparison to WT is not known. Since Dyt1 pKO mice exhibit loss of torsinA specifically in cerebellar Purkinje cells, the better motor performance should be caused by altered signals from cerebellar Purkinje cells in Dyt1 pKO mice. Cerebellar Purkinje cells send major inhibitory signals from the cerebellum to the deep cerebellar nuclei [51]. There are direct connections from deep cerebellar nuclei to the striatum by a di-synaptic pathway and to globus pallidus externa by a tri-synaptic pathway [52]. Therefore, functional alterations of cerebellar Purkinje cells may substantially influence the basal ganglia circuits and affect motor performance. It should be noted that dystonia 1 patients exhibit higher semantic fluency performance [53], and Dyt1 ΔGAG heterozygous KI mice exhibit enhancement of cued fear memory [47], suggesting that altered torsinA function may contribute to better memory formation in the brain for some tasks. Since cerebellar Purkinje cells contribute to motor learning, the loss of torsinA may contribute to enhanced memory formation of motor coordination and balance, and exhibit better motor performance.
In phenotypic dystonia rodent models, the first insult caused either by gene mutations [25, 28] or pharmacological manipulations [23, 24] result in dysfunction of the cerebellum and subsequent expression of dystonic symptoms. The second insult to the abnormal cerebellum, such as cerebellectomy [29, 32], introducing a cerebellar Purkinje cell-specific degeneration mutation [31], or lesion in the pathway downstream of the cerebellar Purkinje cells [30], shut off or attenuate the abnormal output signals and eliminate their dystonic symptoms. Since torsinA is highly expressed in the cerebellum [42, 54] and Dyt1 ΔGAG heterozygous KI male mice exhibit motor deficits [13], Dyt1 ΔGAG KI mutation may correspond to the first insult to the cerebellum in the phenotypic dystonia rodent models. Cerebellar Purkinje cell-specific Dyt1 knocking-out of the WT allele of the Dyt1 ΔGAG heterozygous KI mice may represent the second insult to the cerebellum and have a therapeutic effect. The results suggest that dysfunction of the cerebellum may contribute to the motor deficits in Dyt1 ΔGAG heterozygous KI mice and the further intervention to the cerebellar Purkinje cells has therapeutic effects. Although the globus pallidus has been used as a target in deep brain stimulation therapy for dystonia 1 patients with great success, some do not respond well [55]. Targeting the signaling pathways downstream of the cerebellar Purkinje cells or deep cerebellar nuclei, which receive signals from cerebellar Purkinje cells, may have promise in deep brain stimulation for dystonia 1 patients.
Since Dyt1 ΔGAG KI/pKO double mutant mouse is an artificial genetic model and has Purkinje cell-specific knockout of WT Dyt1 allele from early cerebellar development, this method itself is not practical for dystonia 1 patients. However, the present results suggest a possible gene therapy strategy by inactivating torsinA specifically in cerebellar Purkinje cells to rescue the motor deficits in dystonia 1. Gene therapy using intracerebellar delivery of AAV or lentiviral vector inactivating torsinA specifically in cerebellar Purkinje cells may be employed. AAV vector has been used in clinical trials and animal models of neurological disorders [56]. Recently, AAV-based shRNA was used to suppress the polyglutamine-induced neurodegeneration in a mouse model of spinocerebellar ataxia type 1(SCA1) [57]. The shRNA against human SCA1 sequence successfully down-regulated the expression of human ataxin-1-Q82 transgene without any effect on the endogenous mouse Sca1 and improved motor coordination. The same group also succeeded in achieving improvement in motor performance and neuropathological phenotypes in a Huntington’s disease mouse model using the similar strategy [58].
Dystonic symptoms in dystonia 1 seem to associate with dysfunction of the basal ganglia circuits [39, 50, 59]. Both striatum-specific Dyt1 conditional knockout (Dyt1 sKO) and cerebral cortex-specific Dyt1 conditional knockout (Dyt1 cKO) mice exhibit motor deficits in beam-walking deficits, suggesting that loss of torsinA function in both striatum and cerebral cortex contribute to the pathogenesis of dystonia 1 [15, 50]. Moreover, Dyt1 ΔGAG heterozygous KI male mice exhibit motor deficits and reduced corticostriatal LTD, which are restored with trihexyphenidyl [39], suggesting that the dysfunction of corticostriatal circuits produces abnormal signals in the basal ganglia circuits and induces motor deficits. Since lesion of cerebellum has been shown to modify corticostriatal LTD [60], it is tempting to speculate that the altered signals from additional knocking-out of Dyt1WT allele of Dyt1 ΔGAG heterozygous KI mice may rescue the corticostriatal LTD deficit and improve the motor performance. Future electrophysiological study to record the output signals from cerebellar Purkinje cells and corticostriatal LTD in Dyt1 ΔGAG KI/pKO double mutant mice will elucidate the detailed mechanism of cellular network alterations between dystonic status in Dyt1 ΔGAG heterozygous KI mice and the rescued status.
Abnormal nuclear envelopes have been reported in transfected cells over-expressing the mutant forms of torsinA [61–63], Dyt1 KO mice and Dyt1 ΔGAG homozygous KI mice [14]. Neuron-specific nuclear envelope abnormality in Dyt1 ΔGAG homozygous KI mice may be caused by malfunction of torsinA with incomplete compensation by torsinB, which is weakly expressed in neurons [64]. However, both transgenic mice overexpressing human WT torsinA and mutant torsinA using murine prion promoter also exhibit abnormal nuclear envelopes and abnormal motor performance [65], suggesting that the abnormal nuclear envelope is not a phenotype specifically caused by mutant torsinA, but may be that caused by disruption of torsinA pathway in vivo. Because abnormal envelopes in vivo are detected in Dyt1 ΔGAG homozygous KI mice and Dyt1 KO mice, that exhibit neonatal lethality, the abnormal nuclear envelope may be an indicator of neuronal cell death in these dying mice. Abnormal nuclear envelopes have not been reported in Dyt1 ΔGAG heterozygous KI mouse or DYT1 dystonia patient brains, suggesting nuclear envelope abnormality itself does not contribute to dystonia. Moreover abnormal nuclear envelopes were not found in both Dyt1 sKO and cKO mice, suggesting that nuclear envelope abnormality may not play any role in motor impairment in these mice. Consistent to the previous findings, Dyt1 pKO mice showed intact nuclear membranes in cerebellar Purkinje cells, suggesting that there is no relation between motor performance and the ultrastructure of nuclear envelope in Dyt1 pKO mice. Moreover the present qualitative analysis of slices in small scale suggested that Dyt1 pKO mice did not exhibit gross alteration in ultrastructure of cytoplasmic structures, axons or dendrites, while Dyt1 pKO mice may seem to have some small boutons with decreased amount of synaptic vesicles in comparison to CT mice in Fig. 2. Further large scale quantitative analysis of detailed ultrastructural parameters such as the diameter of boutons, the density of synaptic vesicles in boutons, the thickness and width of post-synaptic densities, remains as an important future study to determine the cellular and synaptic substrates of the observed behavioral phonotypes.
Both Dyt1 ΔGAG heterozygous KI and Dyt1 pKO mice exhibit similar morphological alterations of cerebellar Purkinje cells, namely shorter primary large dendrites and reduction of spine numbers in the quaternary dendrite branches [42]. If the morphological alterations of cerebellar Purkinje cells in Dyt1 ΔGAG heterozygous KI mice associate with their motor deficits, Dyt1 pKO mice should have shown similar motor deficits. However, Dyt1 pKO mice exhibited enhanced coordination in motor performance, suggesting that the morphological alterations in Dyt1 ΔGAG heterozygous KI mice may not associate with motor deficits. Recent studies suggest that the mutant forms of torsinA are quickly degraded by both proteasome and macroautophagy-lysosome pathways in transfected cells, while WT torsinA is stable and degraded primarily through the macroautophagy-lysosome pathway [66, 67]. Consistent with these in vitro data, torsinA is reduced in Dyt1 ΔGAG heterozygous KI mouse brains [14, 36, 37]. Therefore, the morphological alterations of cerebellar Purkinje cells in Dyt1 ΔGAG heterozygous KI mice may be simply caused by reduction of torsinA in cerebellar Purkinje cells. Since the reduced spine number may be an indicator of reduced input signals from parallel fibers to cerebellar Purkinje cells, both Dyt1 ΔGAG mutation and Dyt1 Purkinje-cell specific knockout may exhibit similar functional alteration of cerebellar Purkinje cells and attenuate abnormal signals from the cerebellum. Although the magnitude of the therapeutic effect caused by Dyt1 ΔGAG mutation in the cerebellar Purkinje cells may not be enough to eliminate overall motor deficits, complete knockout of both Dyt1 alleles in cerebellar Purkinje cells in Dyt1 pKO mice and additional knockout of Dyt1 WT allele of Dyt1 ΔGAG heterozygous KI mice may be enough to induce profound functional alteration of cerebellar Purkinje cells and exhibit the enhanced coordination and therapeutic effect to recovery from the motor deficits in beam-walking test, respectively.
Primary dystonia is classified into more than 20 types, although less than half of them have known gene mutations [16, 68]. The relationship between the mutant proteins and their common etiology is not well understood although there are several reports suggesting functional interactions of these genes [69–72]. For example, myoclonus-dystonia (M-D; dystonia 11; OMIM 159900) is caused by mutations in SGCE coding for ε-sarcoglycan. Two dystonia patients from a single family with double mutations in DYT1 and SGCE exhibit more severe symptoms [10, 11]. Dyt1 Sgce double mutant mice exhibit earlier onset of motor deficits than each single mutant mice in the beam-walking test, while functional deficits of torsinA and ε-sarcoglycan may independently cause motor deficits [36]. Both striatum-specific Dyt1 conditional knockout (Dyt1 sKO) [50] and striatum-specific Sgce conditional knockout (Sgce sKO) mice [73] exhibit motor deficits in beam-walking deficit, suggesting common dysfunction of the striatum in these dystonias. On the other hand, the cerebellar Purkinje cell-specific Sgce conditional knockout (Sgce pKO) mice exhibit only motor leaning deficits and reduced stereotypic behaviors [40]. Conversely, Dyt1 pKO mice exhibited enhanced coordination and rescue effect as presented in this study. Therefore, the striatum may be commonly affected in both dystonia 1 and dystonia 11, whereas the contributions of the cerebellar Purkinje cells to the pathogenesis are different between them. Like phenotypic variations in primary dystonia patients, cerebellar contributions to pathophysiological mechanisms in different dystonias are diverse. Genotypic animal models specific for each primary dystonia, especially those with cell type-specific and brain region-specific features offer potential to elucidate the common and specific mechanisms, and to develop appropriate therapeutics suitable to each primary dystonia.
Highlights.
Enhanced motor coordination in Purkinje cell-specific Dyt1 conditional knockout mice
Motor deficits rescue in Dyt1 knock-in mice by Purkinje cells-specific Dyt1 knockout
Purkinje cells-specific knockout of Dyt1 as potential gene therapy for DYT1 dystonia
Intervening in the output signaling from the Purkinje cells as a possible therapy
Acknowledgements
We thank Lisa Foster, Andrea McCullough and their staff for animal care; and Lou Ann Miller, Miki Jinno, Veena Ganesh, and Mark P. DeAndrade for their technical assistance. This work was supported by Tyler’s Hope for a Dystonia Cure, Inc. (UF), National Institutes of Health grants (NS37409, NS47466, NS47692, NS54246, NS57098, NS65273, NS72876, and NS74423) and startup funds from the Lucille P. Markey Charitable Trust (UIUC) and Department of Neurology (UAB).
Abbreviations
- CT mouse
control littermate mouse
- DYT1 (TOR1A)
torsinA gene in human
- Dyt1 (Tor1a)
torsinA gene in mouse
- Dyt1 cKO mouse
cerebral cortex-specific Dyt1 conditional knockout mouse
- Dyt1 pKO mouse
cerebellar Purkinje cell-specific Dyt1 conditional knockout mouse
- Dyt1 sKO mouse
striatum-specific Dyt1 conditional knockout mouse
- KI mouse
knock-in mouse
- KO mouse
knockout mouse
- Sgce pKO mouse
cerebellar Purkinje cell-specific Sgce conditional knockout mouse
- Sgce sKO mouse
striatum-specific Sgce conditional knockout mouse
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 2.Augood SJ, Penney JB, Jr, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, et al. Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann Neurol. 1998;43:669–673. doi: 10.1002/ana.410430518. [DOI] [PubMed] [Google Scholar]
- 3.Augood SJ, Martin DM, Ozelius LJ, Breakefield XO, Penney JB, Jr, Standaert DG. Distribution of the mRNAs encoding torsinA and torsinB in the normal adult human brain. Ann Neurol. 1999;46:761–769. [PubMed] [Google Scholar]
- 4.Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci U S A. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewett JW, Tannous B, Niland BP, Nery FC, Zeng J, Li Y, et al. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proc Natl Acad Sci U S A. 2007;104:7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risch N, de Leon D, Ozelius L, Kramer P, Almasy L, Singer B, et al. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995;9:152–159. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- 7.Opal P, Tintner R, Jankovic J, Leung J, Breakefield XO, Friedman J, et al. Intrafamilial phenotypic variability of the DYT1 dystonia: from asymptomatic TOR1A gene carrier status to dystonic storm. Mov Disord. 2002;17:339–345. doi: 10.1002/mds.10096. [DOI] [PubMed] [Google Scholar]
- 8.Zirn B, Grundmann K, Huppke P, Puthenparampil J, Wolburg H, Riess O, et al. Novel TOR1A mutation p.Arg288Gln in early-onset dystonia (DYT1) J Neurol Neurosurg Psychiatry. 2008;79:1327–1330. doi: 10.1136/jnnp.2008.148270. [DOI] [PubMed] [Google Scholar]
- 9.Leung JC, Klein C, Friedman J, Vieregge P, Jacobs H, Doheny D, et al. Novel mutation in the TOR1A (DYT1) gene in atypical early onset dystonia and polymorphisms in dystonia and early onset parkinsonism. Neurogenetics. 2001;3:133–143. doi: 10.1007/s100480100111. [DOI] [PubMed] [Google Scholar]
- 10.Klein C, Liu L, Doheny D, Kock N, Muller B, de Carvalho Aguiar P, et al. Epsilon-sarcoglycan mutations found in combination with other dystonia gene mutations. Ann Neurol. 2002;52:675–679. doi: 10.1002/ana.10358. [DOI] [PubMed] [Google Scholar]
- 11.Doheny D, Danisi F, Smith C, Morrison C, Velickovic M, De Leon D, et al. Clinical findings of a myoclonus-dystonia family with two distinct mutations. Neurology. 2002;59:1244–1246. doi: 10.1212/wnl.59.8.1244. [DOI] [PubMed] [Google Scholar]
- 12.Ritz K, Gerrits MC, Foncke EM, van Ruissen F, van der Linden C, Vergouwen MD, Bloem BR, Vandenberghe W, Crols R, Speelman JD, Baas F, Tijssen MA. Myoclonus-dystonia: clinical and genetic evaluation of a large cohort. J Neurol Neurosurg Psychiatry. 2009;80:653–658. doi: 10.1136/jnnp.2008.162099. [DOI] [PubMed] [Google Scholar]
- 13.Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, et al. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Yokoi F, Dang MT, Mitsui S, Li J, Li Y. Motor deficits and hyperactivity in cerebral cortex-specific Dyt1 conditional knockout mice. J Biochem. 2008;143:39–47. doi: 10.1093/jb/mvm191. [DOI] [PubMed] [Google Scholar]
- 16.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 17.Rumbach L, Barth P, Costaz A, Mas J. Hemidystonia consequent upon ipsilateral vertebral artery occlusion and cerebellar infarction. Mov Disord. 1995;10:522–525. doi: 10.1002/mds.870100424. [DOI] [PubMed] [Google Scholar]
- 18.Le Ber I, Clot F, Vercueil L, Camuzat A, Viemont M, Benamar N, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- 19.Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 20.Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. "Motor circuit" gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- 21.Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- 22.Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorden JF, McKeon TW, Baker HJ, Cox N, Walkley SU. Characterization of the rat mutant dystonic (dt): a new animal model of dystonia musculorum deformans. J Neurosci. 1984;4:1925–1932. doi: 10.1523/JNEUROSCI.04-08-01925.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao J, Ledoux MS. Caytaxin deficiency causes generalized dystonia in rats. Brain Res Mol Brain Res. 2005;141:181–192. doi: 10.1016/j.molbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Gong S, Ledoux MS. Caytaxin deficiency disrupts signaling pathways in cerebellar cortex. Neuroscience. 2007;144:439–461. doi: 10.1016/j.neuroscience.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 29.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120:302–310. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux MS, Lorden JF, Meinzen-Derr J. Selective elimination of cerebellar output in the genetically dystonic rat. Brain Res. 1995;697:91–103. doi: 10.1016/0006-8993(95)00792-o. [DOI] [PubMed] [Google Scholar]
- 31.Campbell DB, North JB, Hess EJ. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- 32.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter RJ, Morton AJ, Dunnett SB. Motor Coordination and Balance in Rodents. In: Crawley J, editor. Current Protocols in Neuroscience. John Wiley & Sons, Inc.; 2001. 8.12.1-8..4. [DOI] [PubMed] [Google Scholar]
- 34.Yokoi F, Dang MT, Li J, Li Y. Myoclonus, motor deficits, alterations in emotional responses and monoamine metabolism in epsilon-sarcoglycan deficient mice. J Biochem. 2006;140:141–146. doi: 10.1093/jb/mvj138. [DOI] [PubMed] [Google Scholar]
- 35.Dang MT, Yokoi F, Pence MA, Li Y. Motor deficits and hyperactivity in Dyt1 knockdown mice. Neurosci Res. 2006;56:470–474. doi: 10.1016/j.neures.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoi F, Yang G, Li J, Deandrade MP, Zhou T, Li Y. Earlier onset of motor deficits in mice with double mutations in Dyt1 and Sgce. J Biochem. 2010;148:459–466. doi: 10.1093/jb/mvq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao S, Hewett JW, Yokoi F, Lu J, Buckley AC, Burdette AJ, et al. Chemical enhancement of torsinA function in cell and animal models of torsion dystonia. Dis Model Mech. 2010;3:386–396. doi: 10.1242/dmm.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeAndrade MP, Yokoi F, van Groen T, Lingrel JB, Li Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behav Brain Res. 2011;216:659–665. doi: 10.1016/j.bbr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang MT, Yokoi F, Cheetham CC, Lu J, Vo V, Lovinger DM, et al. An anticholinergic reverses motor control and corticostriatal LTD deficits in Dyt1 DeltaGAG knock-in mice. Behav Brain Res. 2012;226:465–472. doi: 10.1016/j.bbr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoi F, Dang MT, Yang G, Li J, Doroodchi A, Zhou T, et al. Abnormal nuclear envelope in the cerebellar Purkinje cells and impaired motor learning in DYT11 myoclonus-dystonia mouse models. Behav Brain Res. 2012;227:12–20. doi: 10.1016/j.bbr.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, et al. Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Yokoi F, Jin YH, Deandrade MP, Hashimoto K, Standaert DG, et al. Altered Dendritic Morphology of Purkinje cells in Dyt1 DeltaGAG Knock-In and Purkinje Cell-Specific Dyt1 Conditional Knockout Mice. PLoS One. 2011;6:e18357. doi: 10.1371/journal.pone.0018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- 46.Campos VE, Du M, Li Y. Increased seizure susceptibility and cortical malformation in beta-catenin mutant mice. Biochem Biophys Res Commun. 2004;320:606–614. doi: 10.1016/j.bbrc.2004.05.204. [DOI] [PubMed] [Google Scholar]
- 47.Yokoi F, Dang MT, Miller CA, Marshall AG, Campbell SL, Sweatt JD, et al. Increased c-fos expression in the central nucleus of the amygdala and enhancement of cued fear memory in Dyt1 DeltaGAG knock-in mice. Neurosci Res. 2009;65:228–235. doi: 10.1016/j.neures.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 49.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 50.Yokoi F, Dang MT, Li J, Standaert DG, Li Y. Motor Deficits and Decreased Striatal Dopamine Receptor 2 Binding Activity in the Striatum-Specific Dyt1 Conditional Knockout Mice. PLoS One. 2011;6:e24539. doi: 10.1371/journal.pone.0024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 53.Balas M, Peretz C, Badarny S, Scott RB, Giladi N. Neuropsychological profile of DYT1 dystonia. Mov Disord. 2006;21:2073–2077. doi: 10.1002/mds.21070. [DOI] [PubMed] [Google Scholar]
- 54.Konakova M, Pulst SM. Immunocytochemical characterization of torsin proteins in mouse brain. Brain Res. 2001;922:1–8. doi: 10.1016/s0006-8993(01)03014-1. [DOI] [PubMed] [Google Scholar]
- 55.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–1389. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- 56.Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6:482–490. [PubMed] [Google Scholar]
- 57.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 58.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Napolitano F, Pasqualetti M, Usiello A, Santini E, Pacini G, Sciamanna G, et al. Dopamine D2 receptor dysfunction is rescued by adenosine A2A receptor antagonism in a model of DYT1 dystonia. Neurobiol Dis. 2010;38:434–445. doi: 10.1016/j.nbd.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi S, Mataluni G, De Bartolo P, Prosperetti C, Foti F, De Chiara V, et al. Cerebellar control of cortico-striatal LTD. Restor Neurol Neurosci. 2008;26:475–480. [PubMed] [Google Scholar]
- 61.Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez-Alegre P, Paulson HL. Aberrant cellular behavior of mutant torsinA implicates nuclear envelope dysfunction in DYT1 dystonia. J Neurosci. 2004;24:2593–2601. doi: 10.1523/JNEUROSCI.4461-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci U S A. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim CE, Perez A, Perkins G, Ellisman MH, Dauer WT. A molecular mechanism underlying the neural-specific defect in torsinA mutant mice. Proc Natl Acad Sci U S A. 2010;107:9861–9866. doi: 10.1073/pnas.0912877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grundmann K, Reischmann B, Vanhoutte G, Hubener J, Teismann P, Hauser TK, et al. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Giles LM, Chen J, Li L, Chin LS. Dystonia-associated mutations cause premature degradation of torsinA protein and cell-type-specific mislocalization to the nuclear envelope. Hum Mol Genet. 2008;17:2712–2722. doi: 10.1093/hmg/ddn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon KL, Gonzalez-Alegre P. Consequences of the DYT1 mutation on torsinA oligomerization and degradation. Neuroscience. 2008;157:588–595. doi: 10.1016/j.neuroscience.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller U. The monogenic primary dystonias. Brain. 2009;132:2005–2025. doi: 10.1093/brain/awp172. [DOI] [PubMed] [Google Scholar]
- 69.Gavarini S, Cayrol C, Fuchs T, Lyons N, Ehrlich ME, Girard JP, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–553. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaiser FJ, Osmanoric A, Rakovic A, Erogullari A, Uflacker N, Braunholz D, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Ann Neurol. 2010;68:554–559. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- 71.Lohmann K, Uflacker N, Erogullari A, Lohnau T, Winkler S, Dendorfer A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20:171–175. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RA, Sillitoe RV, Beesley PW, Blake DJ. SGCE missense mutations that cause myoclonus-dystonia syndrome impair epsilon-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–342. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- 73.Yokoi F, Dang MT, Zhou T, Li Y. Abnormal nuclear envelopes in the striatum and motor deficits in DYT11 myoclonus-dystonia mouse models. Hum Mol Genet. 2012;21:916–925. doi: 10.1093/hmg/ddr528. [DOI] [PMC free article] [PubMed] [Google Scholar]