Abstract
Thromboxane A2 (TxA2) is an arachidonic acid metabolite that stimulates platelet aggregation and vasoconstriction when released from platelets and other cell types during tissue trauma. More recent research has demonstrated that TxA2 can also stimulate vagal and spinal sensory nerves. The purpose of this study was twofold. One, we compared the expression of the TxA2 receptor (TxA2R) in neurons from two sensory ganglia: the nodose ganglion (NG) containing cell bodies of vagal afferent nerves and the thoracic dorsal root ganglion (DRG) containing cell bodies of spinal afferent nerves. Two, we determined if TxA2R co-localizes with mRNA for the nociceptive marker, TRPV1, which is the receptor for the noxious substance capsaicin. We found a greater percentage of neurons in the NG that are positive for TxA2R expression than in the DRG. We also found that there was no correlation of expression of TxA2R with TRPV1. These data suggest that while TxA2R is expressed in both vagal and spinal neurons, TxA2 may elicit stronger vagal or parasympathetic reflexes in the rabbit when released during tissue trauma depending on the location of release. Our data also indicate that TxA2 is likely to stimulate both nociceptive and non-nociceptive neurons thereby broadening the types of neurons and reflexes that it may excite.
Keywords: TxA2, capsaicin, dorsal root ganglion, nodose ganglion, vagal sensory nerves, thoracic sensory nerves, sympathetic, parasympathetic
Introduction
Thromboxane A2 (TxA2) is a highly labile metabolite of arachidonic acid that stimulates contraction of smooth muscle (e.g., vasoconstriction, bronchoconstriction) and aggregation of blood platelets. As research into TxA2 progresses, the physiological actions of TxA2 have been found to be broader than previously thought including stimulation of excitable cells such as neurons and cardiac myocytes. In studies with the anesthetized rabbit, our laboratory reported that TxA2 could induce arrhythmias likely via a direct action on the heart and could excite isolated adult cardiac myocytes [21]. There is also considerable evidence that TxA2 can stimulate both vagal and spinal afferent nerve fibers. Our laboratory, as well as others, have reported that TxA2 stimulates vagal sensory nerves from the lung [11, 16, 18] as well as the heart [19, 22]. In addition we have shown that TxA2 stimulates spinal sensory nerves (group III and IV) from the hindlimb [13] while Fu et al. has shown that TxA2 stimulates spinal afferent nerves innervating the heart [5, 7]. Since TxA2 is released from platelets and other cell types during tissue trauma or events that activate platelets, the stimulation of sensory nerves by TxA2 could play an important role in activation of reflexes during hemorrhage, inflammation, ischemia, tissue injury, or trauma.
The mechanism by which TxA2 stimulates sensory nerves is not completely understood; however, TxA2 has been shown to directly stimulate neurons via its membrane bound receptor, TxA2R. Inhibition of TxA2R with various pharmacological antagonists have inhibited TxA2-induced neural stimulation using in vivo experiments of peripheral nerve recordings as well as using in vitro experiments with cultured neurons [1, 5, 21]. While the exact intracellular signaling mechanism for TxA2 excitation is not yet known, the mechanism likely involves calcium. Andoh et al. [1] demonstrated that the TxA2R agonist, U46619, induces calcium increases in cultured DRG neurons from the mouse. This is not surprising as we have also observed increases in intracellular calcium in isolated cardiac myocytes following application of U46619 [21]. TxA2R is well known to be linked to the Gq pathway in which there is activation of protein kinase C (PKC) as well as formation of inositol trisphosphate (IP3) and subsequent release of intracellular calcium from IP3 receptors located on the sarcoplasmic reticulum. Interestingly, in our study of cardiac myocytes, we demonstrated an inhibition of the U46619-induced calcium increase by inhibitors of the IP3 pathway [21] while Fu et al. [7] have shown a decrease in TxA2 activation of cardiac nerves after inhibition of PKC.
While the intracellular mechanisms are being investigated, there are still questions about the presence and distribution of TxA2R in neuronal populations. Using single cell RT-PCR techniques, we previously reported that a subpopulation of the sensory cells that were sampled from overnight cultures of NG of the rabbit contained measureable levels of TxA2R mRNA [23] and two other studies have presented immunohistochemistry data for TxA2R in dorsal root ganglion (DRG) neurons [1, 7]. Nevertheless, important questions remain concerning this subpopulation of cells that expressed TxA2R. For example, the relative abundance of TxA2R expression in the NG versus DRG may provide insights into the relative importance of TxA2 in stimulating somatic versus visceral afferents and activation of parasympathetic vs. sympathetic reflexes. It is also unknown whether the expression of TxA2R is restricted to a particular type of afferent neuron (e.g., nociceptive) or is more broadly expressed in a wide variety of sensory neurons. In this study, by measuring TxA2R expression in NG, DRG, and co-expression with the nociceptive marker, TRPV1, we can begin to narrow down the types of neurons stimulated and subsequent potential reflexes activated by TxA2 when it is released during tissue trauma.
Methods
Culturing of cells from sensory ganglia
Adult male rabbits (n = 56) were anesthetized and subsequently euthanized for harvesting of NG and DRG. In order to avoid possible variability between gender only male rabbits were used in these experiments. All procedures involving the use of animals in this investigation were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC # 42-02). Standard dissecting procedures were used to harvest DRG (from the thoracic region of the spinal cord) and NG. The ganglia were placed in Dulbecco’s Modified Eagle’s Medium (DMEM) with L-glutamine, penicillin streptomycin, and serum as previously described [23]. The DRG and NG were then treated for 2 hr with collagenase I (Worthington) and then 20 min with trypsin (Invitrogen). The cells were resuspended in 2 ml DMEM, placed on plates precoated with poly-L-lysine and laminin (Sigma), and cultured overnight (approximately 15–20 hours) at 36.5° C in a humidified atmosphere of 95% air and 5% CO2.
Single cell RT-PCR
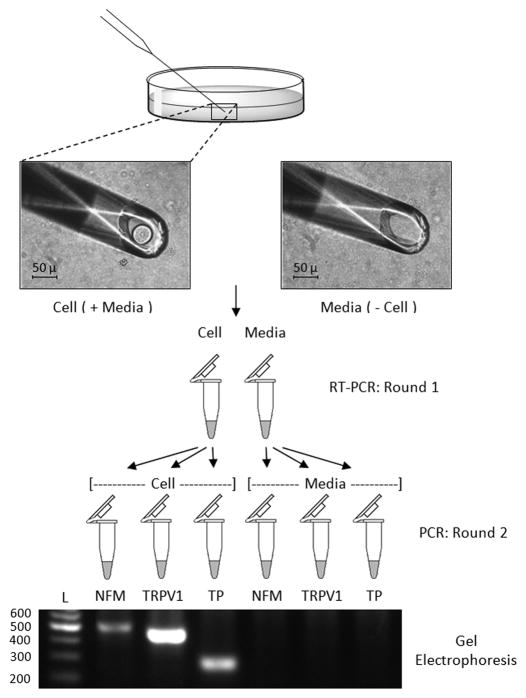
Glass micropipettes (interior tip diameter approximately 50 – 70 μm) were mounted into a micromanipulator (model MM-3 3D-course, 1D-fine, Narishige International) and attached to rubber tubing that was in turn attached to a 1 ml syringe with a 3-way stopcock. Cultures were washed and covered with fresh DMEM. Using an inverted microscope (model CK, Olympus), individual cells were withdrawn into the micropipette with a minimal amount of surrounding media using gentle suction. Cells that were withdrawn into the pipette from the culture dish were selected based on a morphology that we have found to be characteristic of neurons (20–60 μm, round shape and smooth exterior surface). The cells were immediately expelled from the pipette into a PCR tube containing RT-PCR reaction buffer (One-Step SuperScript III RT-PCR kit, Invitrogen), RNaseOUT ribonuclease inhibitor (Invitrogen), and primer sets for neurofilament medium (NFM) (0.15 μM), TRPV1 (0.2 μM) and TxA2R (0.3 μM) in a total volume of 25 μl. Sequences of the primers for NFM and TxA2R have been previously published [23]. The TRPV1 primer sequences for the first round were: Forward: CTCCTGCTCAACATGCTCATCG Reverse: GCGGCCACAGAGTCCTTGAA. New micropipettes were used for each cell. As a control procedure, media from the region where the extracted cell was located in the culture dish was withdrawn into a fresh micropipette and added to a reaction tube. Additionally, controls in which no media or cells were placed in the buffer (no-template controls) and controls in which the RT enzyme was replaced with Taq DNA polymerase (Taq-only controls) were also prepared. The mixture containing either cells or medium were placed into the 96 well thermocycler (PTC – 200, MJ Research) and run at the following conditions: 60º C for 20 min (RT step), 95º C for 2 min, and then 40 amplification cycles of 95º C for 40 s, 56º C for 30 s, and 72º C for 40 s. After the reaction was complete, 1 μl from the RT-PCR reaction was placed in three separate PCR tubes and a second round of PCR carried out separately for each of the three genes using Platinum Taq PCR Supermix (Invitrogen). A nested approach was employed for amplification of TxA2R, NFM, and TRPV1 gene transcripts that were expected to yield 229 bp, 496 bp and 411 bp products respectively. Primer sequences for the second round have been published for NFM and TxA2R [23] with the exception that a new nested reverse primer for NFM was used with the sequence: CCTCTGCAATGGCTGTCAGT. Primer sequences for TRPV1 in the second round were: Forward: CATGGGCGAGACGGTCAACA; Reverse: ACAAAGGGCCTCAGGTGGAC. The second round of PCR had the following conditions: 95º C for 2 min followed by 25 amplification cycles, each of 95º C for 40 s, 56º C for 30 s, and 72º C for 40 s. PCR products (6 μl) from the second round of amplification were separated on an agarose gel to determine the presence or absence of a band of appropriate size. A flow chart of these procedures is summarized in Figure 1.
Fig. 1. Isolation of cultured neurons for detection of TxA2R and TRPV1 gene expression.
Single cells or media-only controls from NG and DRG cultures were isolated and placed directly in RT-PCR buffer which contained primer sets for NFM, TxA2R (TP), and TRPV1. After RT-PCR, 1 μl of sample was taken for a second round of PCR and placed in tubes which separately contained nested primers for each specific gene of interest. The PCR products were visualized by gel electrophoresis.
Verification of the PCR products from the second round was accomplished by digesting the products (6 μl) with restriction enzymes for 2 hours at 37º C followed by separation of PCR products with gel electrophoresis. Verification of TxA2R and NFM products have been previously reported [23] while the TRPV1 product from the second round of PCR was verified by digestion with NlaIII with visualization of PCR products carried out by gel electrophoresis on 3–4% agarose gels.
Chi-square analysis was used to determine if the number of TxA2R+ and TRPV1+ cells were different between NG and DRG and if there was an association of TxA2R expression with TRPV1 expression. Chi-Square analysis was conducted in a classical manner in which observed frequencies were compared against a calculated expected frequency. Here the expected frequency is what is obtained if the percentage of TXA2R+ cells does not differ between two populations of cells. In other words, the null hypothesis is that TxA2R expression is not different between NG and DRG or that TxA2R expression is not different between TRPV1+ and TRPV1- cells. P values < 0.05 were considered significant.
Results
Single cell RT-PCR products
An agarose gel image displaying PCR products from the second round of RT-PCR for an individual cell from the DRG that contained transcripts for all three genes is presented in Fig 1. Note that no PCR products were detected in samples of only media indicating that the detection of gene transcripts originated from the cell and not the accompanying media.
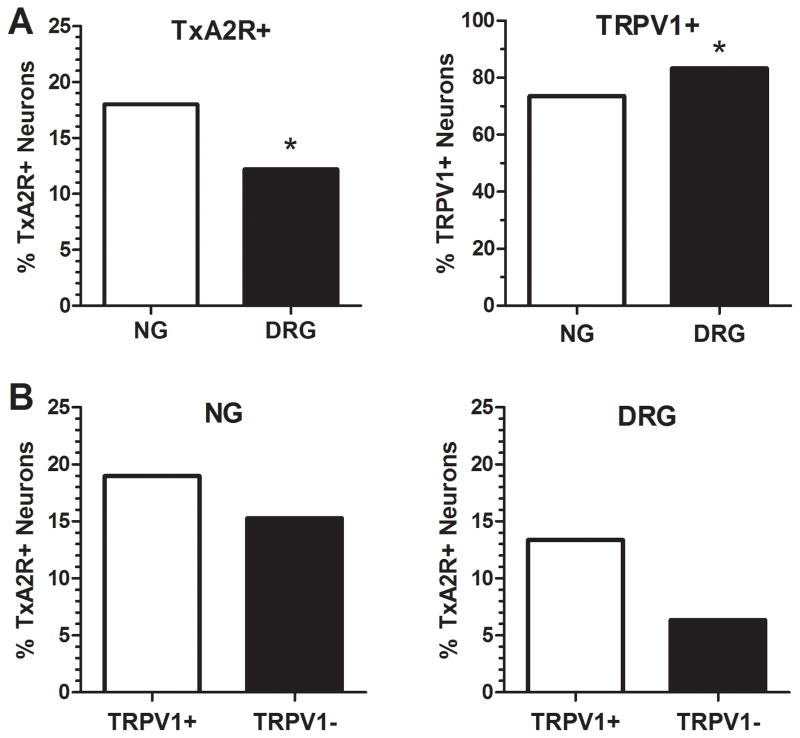
A total of 322 cells were extracted from 24 cultures of NG ganglia while 377 cells were sampled from 32 cultures of DRG. The frequency of cells that contained TxA2R transcripts was greater for neurons cultured from the NG (18.0% of the total number of neurons) compared to neurons from the DRG (12.2% of the total number of cells) (P=0.03). Conversely, the frequency of cells containing the TRPV1 gene transcript was detected with greater frequency in neurons from the DRG (83%) compared to the NG (74%) (P=0.002, Fig. 2A).
Fig. 2. Difference in expression of TxA2R+ and TRPV1+ neurons between NG and DRG.
Panel A: Chi-square analysis revealed that there was a significantly higher number of neurons containing mRNA for TxA2R in NG (n=322) than DRG (n=377) (Chi-square=4.63, DF=1; P= 0.03) and a significantly higher number of TRPV1+ neurons in DRG than NG (Chi-square= 9.76, DF=1; P=0.002). Panel B: Expression of TxA2R mRNA in TRPV1 mRNA containing neurons (TRPV1+, n=237 for NG and n=314 for DRG) vs. TRPV1- neurons (n=85 for NG and n= 63 for DRG). Chi-square analysis revealed no statistical difference between TxA2R mRNA expression in TRPV1+ or – neurons from the NG (Chi Square= 0.58, DF=1; P=0.44) or DRG (Chi-square= 2.42, DF=1; P=0.12).
The presence of TxA2R mRNA in neurons from the NG or DRG that also contained TRPV1 transcripts is presented in Fig. 2B. In the NG, there were 45 TxA2R+ neurons in a total of 237 TRPV1+ neurons (19.0%) and there were 13 TxA2R+ neurons in a total of 85 TRPV1- neurons (15.3%). In the DRG, there were 42 TxA2R+ neurons in a total of 314 TRPV1+ neurons (13.4%) and 4 TxA2R+ in a total of 63 TRPV1- neurons (6.4%). As noted in Fig. 2B, a Chi-square analysis revealed no association of mRNA for TxA2R and mRNA for TRPV1 within cells from either the NG (P=0.45) or DRG (P=0.12).
Discussion
Differences in the expression of mRNA for TxA2R within NG and DRG
To our knowledge, the present study is the first to measure and compare the expression of TxA2R mRNA in individual neurons cultured from both the DRG and NG. First, the identification and presence of TxA2R transcripts in both the NG and DRG is not surprising. We have previously reported the presence of TxA2R mRNA in cells cultured from the NG [23], and two other groups have detected TxA2R in DRG using immunohistochemistry [1, 7]. There is also functional data from a number of studies from our lab and others which show that TxA2 stimulates vagal sensory nerves from the heart and lung [3, 11, 16, 19, 22] and spinal afferents from the heart and hindlimb [6–8, 13]. Since the cell bodies for vagal afferents are located in the NG and the cell bodies for spinal afferents are located in the DRG, the presence of TxA2R gene transcripts in DRG and NG provide further evidence for the sensitivity of cardiac vagal and spinal afferents to TxA2.
Given all of these findings, the question becomes is TxA2 expressed in one sensory pathway over another, potentially signaling a dominance of excitation of parasympathetic (via the vagus) or sympathetic reflexes (via spinal nerves). In this study, we have found a greater expression of TxA2R in NG than thoracic DRG, which suggests that TxA2 may stimulate parasympathetic reflexes over sympathetic reflexes depending on innervation of the organ or region. This idea is supported by several studies. In previous work in the cat, injections of U46619 into the inferior vena cava induced pulmonary vagal (parasympathetic) reflexes that included rapid shallow breathing [18]. Similarly, Pickar noted that intravenous injections of U46619 inhibited a somatic motor reflex via stimulation of vagal afferent nerves [16]. As far as cardiovascular reflexes, our previous experiments with rabbits demonstrated that cardiac injections of the TxA2 mimetic, U46619, elicited a predominately vagal (parasympathetic) response (transient bradycardia and arterial hypotension) as opposed to a sympathetic response (increased blood pressure and heart rate) [22]. There are also reports that a reflex hypotension and bradycardia are commonly observed during coronary ischemia especially when the ischemia occurs on the inferior posterior part of the heart [10, 17, 20, 24, 25]. It is possible that TxA2 is one substance that may mediate these vagal/parasympathetic reflexes during myocardial ischemia.
In contrast, Longhurst’s laboratory has found that TxA2 stimulates sympathetic afferents during cardiac ischemia [5, 7] and have observed sympathoexcitatory reflexes mediated by TxA2 during myocardial ischemia [7]. It should be noted, however, that in this study the animals underwent a bilateral cervical vagotomy prior to experimentation so that no vagal reflexes were present. Nevertheless, this study still highlights the possibility that TxA2 may be able to stimulate different autonomic reflexes depending on the type of trauma, location of trauma, species differences, type of anesthesia, or other factors. While the exact efferent responses elicited by TxA2 in various types of trauma is yet to be fully elucidated, the finding that a higher number of cells within the NG contain mRNA for TxA2R when compared to thoracic DRG cells is a novel finding that may suggest a higher sensitivity of vagal afferents to TxA2 in comparison to thoracic spinal afferents.
Lack of co-localization of TxA2R and TRPV1 in sensory neurons
A second objective of these studies was to determine if gene transcripts for these two receptors were co-localized, i.e., was mRNA for TxA2R more likely to be present in nociceptive neurons. A rationale for the prevalence of TxA2R expression in nociceptive fibers arises from the fact that TxA2 is released during tissue trauma, cyclooxygenase inhibitors are analgesic, and U46619 can stimulate nociceptive neurons [6, 11–13, 22].
We used TRPV1 (capsaicin receptor) as a marker of nociceptive neurons since it is well accepted that capsaicin stimulates nociceptive neurons. We found that TRPV1 transcripts were more frequently contained in DRG cells (83% of the DRG neurons contained mRNA for TRPV1) compared to sensory neurons cultured from the NG (74% of the NG neurons contained the TRPV1 transcript). Although we are not aware of direct comparisons between NG and DRG with regards to either capsaicin sensitivity or the presence of TRPV1 mRNA, previous reports do support the high frequency of cells expressing TRPV1 receptors in these two sensory ganglia. Bielefeldt et al. reported that 80% of NG neurons contain mRNA for the capsaicin receptor [2]. Chung et al. reported that 60% of the cells cultured from the NG of the rat responded to capsaicin [4]. Lastly, Helliwell et al. reported that 80% of the DRG cells responded to capsaicin [9]. If one assumes that unmyelinated afferent fibers are nociceptive in function, these cellular data also agree with histological examinations of the vagus, where it has been reported that greater than 80% of the vagal afferent fibers are unmyelinated [15]. The higher prevalence of capsaicin sensitive afferents in spinal (somatic or sympathetic) afferent fibers (DRG) versus visceral afferents that travel in the vagus (NG) may suggest greater sensitivity of these spinal afferent nerves to nociceptive agents.
Data from this study provide evidence that TxA2R is as likely to occur in neurons that are TRPV1- (TRPV1 mRNA was below detectable limits) as neurons that were TRPV1+ (detectable levels of TRPV1 mRNA were present). The presence of TxA2R in TRPV1+ cells is supported by past studies in which vagal or spinal afferent nerves responded positively to capsaicin or phenylbiguanide also responded positively to TxA2 mimetics [6, 11, 22]. The presence of TxA2R in TRPV1- cells is also not unexpected given a previous report from our laboratory demonstrating that U46619 can stimulate myelinated nerves from the lung that likely innervate rapidly adapting stretch receptors in the airways [3]. The presence of TxA2R within non-nociceptive broadens the potential stimulation of sensory nerves by TxA2. For example, inflammation of joints, tendons, and other somatic tissues that is accompanied by the release of TxA2 could lead to stimulation of proprioceptor sensory nerves from these organs.
NFM transcripts as a marker for neurons and TRPV1 as a nociceptive marker
We only included data from cells that contained transcripts for NFM thereby ensuring measurement of TxA2R and TRPV1 gene transcripts within sensory neurons as opposed to other cell types such as glial cells. It is well known that neurofilaments are comprised of three neurofilament proteins: light (NFL), medium (NFM) and heavy (NFH). The density of neurofilament proteins does vary with the type of neuron [14], however, all subunits are expressed to some degree in all neurons and therefore the presence of NFM provides strong evidence that the cell is a neuron. As stated above, we have used TRPV1 as a marker for nociceptive neurons. We do recognize that it is possible that there may be nociceptive neurons that do not express TRPV1. Nevertheless, we feel that the number of nociceptive neurons that we are not accounting for is small considering the large percentage of TRPV1+ neurons within both ganglia in our study.
mRNA versus protein
Our conclusions are also based on the presence or absence of gene transcripts within these individual neurons. Although we recognize that mRNA does not always translate into protein, we feel confident that TxA2 transcripts are transcribed into functional protein in these neurons. There are many findings from the nerve stimulation studies [7, 8, 19, 22] as well as in vitro DRG cells [1] that TxA2 mimetics directly stimulate neurons via a TxA2R receptor dependent manner. For our particular studies we were interested in characterizing neurons as being TxA2R positive or negative as accurately as possible. We feel that the single cell RT-PCR method we have chosen was the best approach for this study. In our experience commercially available antibodies for TxA2R have resulted in non-specific binding based on Western blotting which may have made the use of protein analysis such as flow cytometry or immunolocalization of neurons inaccurate for this type of single cell analysis. Any potential non-specific binding could have altered our correlations or interpretation of data.
Conclusion and Significance
In conclusion, these experiments provide novel data on the presence of TxA2R and TRPV1 mRNA in sensory neurons cultured from the NG and thoracic DRG of the rabbit. Our data provide additional support for the stimulation of sensory nerves by TxA2 including stimulation of both vagal and spinal afferent nerves. The magnitude of this stimulation may differ, however, between vagal and spinal afferents since we have found TxA2R expression differs between NG and DRG. Lastly, since we have found that the expression of TxA2R is not restricted to TRPV1 containing nociceptive cells, TxA2 stimulation may extend to other afferent nerves innervating a variety of sensory organs such as mechanoreceptors thereby allowing activation of a broad spectrum of reflex responses.
Our experiments provide additional evidence that the understanding of protective reflexes such as vagal reflexes that slow heart rate and airway reflexes that limit distribution of noxious agents to gas exchange regions of the lung through rapid, shallow breathing must include TxA2 within the spectrum of agents that activate these reflexes. These findings may also hold significance for the use of cyclooxygenase inhibitors and TxA2R antagonists. General use of these inhibitors may alter reflexes that may be protective to the heart, lung, and other organs and needs to be considered in dosing aspirin and the more recent COX-2 inhibitors.
Highlights.
TXA2 is an inflammatory mediator that can stimulate neuronal reflexes
TXA2 receptors are expressed more in nodose than dorsal root ganglion neurons
TXA2 does not always localize with the nociceptive marker TRPV1
Acknowledgments
MJW was supported by an American Heart Association Scientist Development Grant (11SDG5330016). JH was supported by MBRS IMSD program (NIH R25 GM062232). WG was supported by Post Baccalaureate Research Education Program Grant (NIH R25 GM078441).
Abbreviations
- TxA2
Thromboxane A2
- TxA2R
Thromboxane Receptor
- DRG
Dorsal Root Ganglion
- NG
Nodose Ganglion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, Nojima H, Narumiya S, Kuraishi Y. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J Invest Dermatol. 2007;127:2042–2047. doi: 10.1038/sj.jid.5700810. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K. Differential effects of capsaicin on rat visceral sensory neurons. Neuroscience. 2000;101:727–736. doi: 10.1016/s0306-4522(00)00375-4. [DOI] [PubMed] [Google Scholar]
- 3.Carrithers JA, Liu F, Shirer HW, Orr JA. Mechanisms for the tachypneic response to the thromboxane A2 mimetic U-46,619 in rabbits. Am J Physiol. 1994;266:R321–327. doi: 10.1152/ajpregu.1994.266.2.R321. [DOI] [PubMed] [Google Scholar]
- 4.Chung E, Gu Q, Kwong K, Arden WA, Lee LY. Comparison of capsaicin-evoked calcium transients between rat nodose and jugular ganglion neurons. Auton Neurosci. 2002;97:83–88. doi: 10.1016/s1566-0702(02)00045-0. [DOI] [PubMed] [Google Scholar]
- 5.Fu LW, Longhurst JC. Bradykinin and thromboxane A2 reciprocally interact to synergistically stimulate cardiac spinal afferents during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H235–244. doi: 10.1152/ajpheart.00782.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu L-W, Longhurst JC, editors. Regulation of cardiac afferent excitability in ischemia. Vol. 194. Springer-Verlag; Berlin Heidelberg: 2009. pp. 185–225. [DOI] [PubMed] [Google Scholar]
- 7.Fu LW, Guo ZL, Longhurst JC. Undiscovered role of endogenous thromboxane A2 in activation of cardiac sympathetic afferents during ischaemia. J Physiol. 2008;586:3287–3300. doi: 10.1113/jphysiol.2007.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu LW, Longhurst JC. Bradykinin and thromboxane A2 reciprocally interact to synergistically stimulate cardiac spinal afferents during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H235–244. doi: 10.1152/ajpheart.00782.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helliwell RJA, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neuroscience Letters. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 10.Iwamura N, Bishop VS. Afferent pathways of reflex hypotension and bradycardia during coronary occlusion. Am J Physiol. 1980;239:H172–180. doi: 10.1152/ajpheart.1980.239.2.H172. [DOI] [PubMed] [Google Scholar]
- 11.Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol. 1992;87:383–396. doi: 10.1016/0034-5687(92)90019-s. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 13.Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res. 1997;744:175–178. doi: 10.1016/s0006-8993(96)01211-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 15.Mei N, Condamin M, Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209:423–431. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- 16.Pickar JG. The thromboxane A2 mimetic U-46619 inhibits somatomotor activity via a vagal reflex from the lung. Am J Physiol. 1998;275:R706–712. doi: 10.1152/ajpregu.1998.275.3.R706. [DOI] [PubMed] [Google Scholar]
- 17.Quigg M. Distribution of vagal afferent fibers of the guinea pig heart labeled by anterograde transport of conjugated horseradish peroxidase. J Auton Nerv Syst. 1991;36:13–24. doi: 10.1016/0165-1838(91)90125-m. [DOI] [PubMed] [Google Scholar]
- 18.Shams H, Scheid P. Effects of thromboxane on respiration and pulmonary circulation in the cat: role of vagus nerve. J Appl Physiol. 1990;68:2042–2046. doi: 10.1152/jappl.1990.68.5.2042. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Wang W, Schultz HD. Activation of cardiac afferents by arachidonic acid: relative contributions of metabolic pathways. Am J Physiol Heart Circ Physiol. 2001;281:H93–H104. doi: 10.1152/ajpheart.2001.281.1.H93. [DOI] [PubMed] [Google Scholar]
- 20.Thames MD, Klopfenstein HS, Abboud FM, Mark AL, Walker JL. Preferential distribution of inhibitory cardiac receptors with vagal afferents to the inferoposterior wall of the left ventricle activated during coronary occlusion in the dog. Circ Res. 1978;43:512–519. doi: 10.1161/01.res.43.4.512. [DOI] [PubMed] [Google Scholar]
- 21.Wacker MJ, Kosloski LM, Gilbert WJ, Touchberry CD, Moore DS, Kelly JK, Brotto M, Orr JA. Inhibition of thromboxane A2-induced arrhythmias and intracellular calcium changes in cardiac myocytes by blockade of the inositol trisphosphate pathway. J Pharmacol Exp Ther. 2009;331:917–924. doi: 10.1124/jpet.109.157677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wacker MJ, Tehrani RN, Smoot RL, Orr JA. Thromboxane A(2) mimetic evokes a bradycardia mediated by stimulation of cardiac vagal afferent nerves. Am J Physiol Heart Circ Physiol. 2002;282:H482–490. doi: 10.1152/ajpheart.00624.2001. [DOI] [PubMed] [Google Scholar]
- 23.Wacker MJ, Tyburski JB, Ammar CP, Adams MC, Orr JA. Detection of thromboxane A(2) receptor mRNA in rabbit nodose ganglion neurons. Neurosci Lett. 2005;386:121–126. doi: 10.1016/j.neulet.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 24.Walker JL, Thames MD, Abboud FM, Mark AL, Klopfenstein HS. Preferential distribution of inhbititory cardiac receptors in left ventricle of the dog. Am J Physiol. 1978;235:H188–192. doi: 10.1152/ajpheart.1978.235.2.H188. [DOI] [PubMed] [Google Scholar]
- 25.Webb SW, Adgey AA, Pantridge JF. Autonomic disturbance at onset of acute myocardial infarction. Br Med J. 1972;3:89–92. doi: 10.1136/bmj.3.5818.89. [DOI] [PMC free article] [PubMed] [Google Scholar]