Abstract
Objective
HIV-risk behaviors were examined at 4- and 12-month follow-up for 230 newly-admitted methadone patients randomly assigned to receive either methadone only (n=99) or methadone with drug abuse counseling (n=131) in the first four months.
Methods
The AIDS Risk Assessment was administered at baseline (treatment entry) and at 4- and 12-month follow-up. Linear mixed model analysis examined changes in HIV drug- and sex-risk behaviors over the 12 months in the total sample, drug-risk behaviors in the subsample that reported injecting drugs at baseline (n=110), and sex-risk behaviors in the subsample that reported engaging in unprotected sex at baseline (n=130).
Results
Significant decreases over time were found in the frequencies of injecting, injecting with other injectors, and sharing cooker, cotton, or rinse water in the total sample and the injector subsample (Ps<0.05). Decreases were also found in the frequencies of having sex without a condom either with someone who was not a spouse or primary partner or while high (Ps<0.05) in the total sample and the frequencies of having sex without a condom and having sex without a condom while high in the unprotected-sex subsample (Ps<0.05). No significant treatment group main effects or treatment group X time interaction effects were found in any of the HIV-risk behaviors in the total sample or either subsample (Ps>0.05).
Conclusions
During the first 12 months of treatment, providing drug abuse counseling with methadone compared to providing methadone alone was not associated with significant changes in HIV-risk behaviors for methadone maintenance patients.
Keywords: Opioid addiction, Methadone maintenance, HIV-risk behaviors
Heroin dependence is associated with HIV-risk behavior through sharing of injection equipment and unprotected sexual encounters and is a leading cause of HIV infection throughout the world (Sullivan et al., 2005). In some areas, such as Eastern Europe and Central Asia, the number of individuals living with HIV has nearly tripled since 2000, primarily due to a rapid increase in the number of new HIV infections among drug injectors (United Nations Program on HIV AIDS, 2010). Methadone treatment has been shown in numerous clinical trials conducted over several decades to be a highly effective treatment that significantly reduces opioid use (Mattick et al., 2009). Research following the availability of the HIV antibody test found that long tenure on methadone maintenance in New York City was a protective factor against HIV infection (Novick et al., 1990). Subsequent longitudinal cohort studies have examined HIV-risk behavior among individuals receiving methadone coupled with standard levels of counseling commonly provided in such treatment programs in pre-post designs (Maddux and Desmond, 1997; Lott et al., 2006) and in cohort designs comparing methadone with standard counseling and out-of-treatment samples (Metzger et al., 1993; Kwiatkowski and Booth, 2001). A Cochrane Review of 33 studies involving more than 10,400 participants found that methadone treatment was associated with significant reductions in certain drug- and sex-related HIV-risk behaviors, including injection and sharing of injection equipment, having multiple sex partners, and trading sex for drugs or money (Gowing et al., 2008). However, there have been very few randomized clinical trials of methadone treatment that have explored the impact of drug abuse counseling on HIV-risk behaviors.
Several studies have found no significant differences in illicit opioid use when comparing methadone treatment provided under direct administration without drug counseling to such treatment with counseling (Senay et al., 1973; Calsyn et al., 1994; Gruber et al., 2008; Schwartz et al., 2011). In a previous trial, participants randomly assigned to receive methadone without counseling for up to 4 months had significantly greater average reduction in frequency of injection and unprotected sex at 4-month follow-up than participants on a waiting list (Wilson et al., 2010). The question remains whether methadone patients who do not receive counseling during the first four months are less likely to reduce their HIV-risk behaviors than methadone patients who receive counseling. This study compared HIV drug- and sex-risk behaviors over 12 months for individuals randomly assigned to receive either methadone with emergency counseling only (termed interim methadone [IM]) for the first four months or methadone with standard drug counseling. Because counseling in standard methadone treatment may contain HIV-risk reduction messages and skills training and previous research has shown that methadone coupled with standard counseling has been associated with significant reductions in some HIV-risk behaviors, we hypothesized that participants who received methadone with counseling would generally show significantly greater reductions in HIV-risk behaviors compared to participants who received interim methadone.
METHODS
The study was conducted in two community-based methadone treatment programs in Baltimore, Maryland and described elsewhere in detail (Schwartz et al., 2011). Participants were enrolled in the study between May 2008 and January 2010. Individuals who otherwise would have been placed on a waiting list were randomly assigned on a 1:1 basis to receive Interim Methadone (IM) for up to 120 days or Standard Methadone (SM) with counseling at one of the sites. The other site had a third Condition, termed Restored Methadone (RM), in which counseling was provided by a counselor with half the typical caseload in order to “restore” the smaller caseloads that were available in the early days of methadone treatment in the US. Individuals at this site were randomly assigned on a 1:1:1 basis to receive IM, SM, or RM.
Participants
The study sample consisted of 230 heroin-dependent adults who were seeking enrollment in one of two methadone treatment programs (MTPs) but for whom a treatment slot was not available for at least two weeks outside of study participation. Inclusion criteria were: being 18 years of age or older; meeting federal criteria for methadone maintenance (i.e., being opioid dependent for a minimum of 12 months); and willingness to provide written informed consent. Exclusion criteria were pregnancy or evidence of an unstable medical or psychiatric condition requiring acute care.
As shown in the CONSORT diagram in Figure 1, 244 individuals were randomly assigned to a treatment group (108, 107, and 29 in the IM, SM, and RM groups, respectively). However, 9 were excluded from IM (4 required acute psychiatric treatment, 4 were not admitted to MTP, and 1 was not heroin-dependent), 3 were excluded from SM (not admitted to MTP), and 2 were excluded from RM (1 had previously enrolled in the study at the other site and 1 had a relative assigned to the same group). Thus, a total of 99 randomly-assigned participants received IM, 104 received SM, and 27 received RM and were interviewed at baseline. At the 4-month follow-up, 97 IM participants (98%), 93 SM participants (89%), and 26 RM participants (96%) were interviewed. At the 12-month follow-up, 93 IM participants (93.9%), 92 SM participants (88.5%), and 25 RM participants (92.5%) were interviewed.
Figure 1.
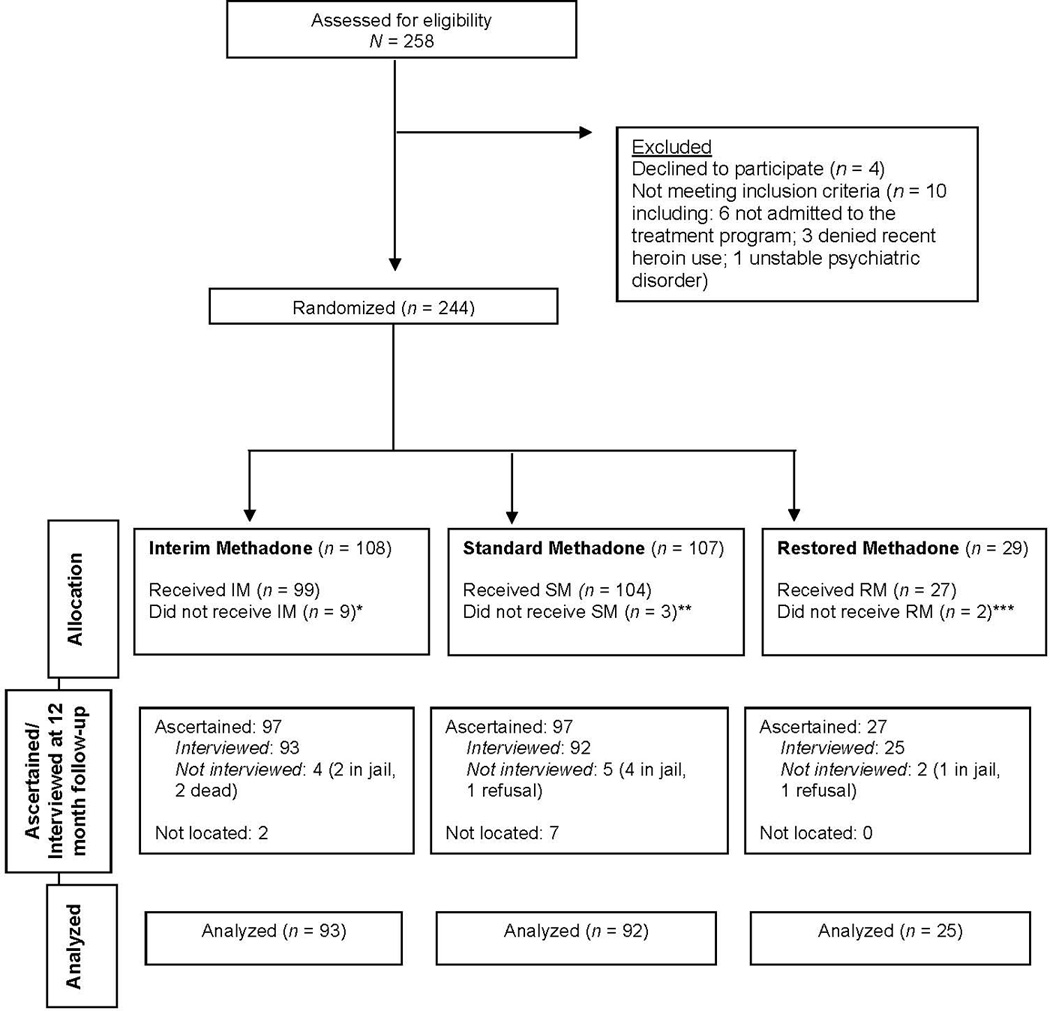
Consort Diagram
* Excluded: unstable psychiatric illness (4), not being admitted by MTP (4), and not being heroin-addicted (1)
** Excluded: not admitted by MTP (3)
*** Excluded: sister enrolled in same condition so excluded by treatment program from condition, (1), previously enrolled in study at other site (1)
The Friends Research Institute’s IRB as well as the IRBs of the organizations of the participating MTPs approved the protocol.
Treatment Groups
There were three treatment groups as follows:
Interim Methadone (IM)
Consisted of up to 120 days of administered methadone with only emergency counseling available (e.g., for suicidal ideation). During IM, participants could request changes in their methadone dose, but were ineligible to earn take-home doses. The mean dose for IM participants was 79.4 mg (SD=18.5) at 4 months and 91.8 mg (SD=21.3) at 12 months. IM participants were required to provide at least three urine samples for drug testing.
IM participants were eligible to be transferred to standard methadone treatment as slots in the treatment program became available, although the majority of participants who were transferred did so towards the very end of their 120 day transfer deadline. Of the 99 IM participants, 88 (88.7%) were on IM for at least 110 days. Of the 91 (91.8%) participants who completed IM, 94 (94.5%) were on IM for at least 110 days.
No emergency counseling was reported among IM participants. Though some IM participants had their first counseling session prior to 120 days, counseling levels were still extremely low among IM participants in the first 4 months (mean [SD] total number of counseling sessions was 0.69 [1.7]). From 4 to 12 months, IM participants had a mean of 11.7 (9.7) counseling sessions.
Standard Methadone (SM)
Consisted of administered methadone and the standard level of counseling. Patients at one site were expected to attend weekly individual counseling for the first 30–60 days, then bi-weekly or monthly as needed. Groups were voluntary, though mandatory for patients still using drugs after six months of treatment. At the other site, patients were expected to attend weekly individual or group counseling for the first 90 days, then at least monthly, as well as 5 group orientation sessions. Patient-to-counselor ratio was generally between 40:1 and 50:1 during SM.
Participants were eligible to earn take-home doses if they were adherent to treatment recommendations and had negative urine drug tests, which were collected more frequently than for the IM group. The mean (SD) methadone dose for SM participants was 76.3 (15.4) at 4 months and 79.4 (22.3) at 12 months. Mean total number of counseling sessions was 8.3 (4.8) in the first 4 months and 10.4 (15.4) between 4 and 12 months.
Restored Methadone (RM)
Consisted of administered methadone and drug abuse counseling provided by a counselor (chosen by the Clinic Director at that site) whose caseload for the purposes of the study did not exceed 25 patients. The RM counselor was instructed to meet with participants as frequently as they requested and/or as deemed necessary by the counselor. RM was available at only one of the sites because of constraints in funding. As with SM, RM participants were eligible to earn take-home doses. The mean methadone dose for RM participants was 68.1 (SD=12.6) at 4 months and 64.7 (SD=15.6) at 12 months. Mean total number of counseling sessions was 17.7 (5.5) in the first 4 months and 17.5 (14.5) during the period from 4 to 12 months.
Routine HIV-Risk Reduction Information at MTPs
At intake (prior to randomization), participants in all three groups at both sites were given a 12-item quiz by the intake counselor that assessed the participant’s knowledge regarding exposure to HIV through risky drug use and sexual behaviors. The counselor then reviewed the responses with the participant and discussed any high-risk behaviors. In addition, during the first four months of treatment, participants in SM and RM (but not IM) received non-standardized HIV-risk reduction information as part of their routine drug abuse group counseling. Counselors generally discussed HIV-risk reduction with participants in routine individual counseling sessions, and one of the sites offered a voluntary HIV educational group session. Details of the content of the counselor-patient discussions of HIV-risk reduction were not systematically documented at either study site, and therefore were not available to the researchers. Free condoms were available in counselors’ offices and waiting areas near medication windows in both clinics, along with pamphlets providing HIV educational material.
Measures
All measures were administered by research assistants at baseline (treatment entry) and 4- and 12-month follow-up. Baseline assessments were conducted prior to random assignment, and follow-up assessments were unblinded. Measures included:
Addiction Severity Index (ASI)
This is a semi-structured interview that has been widely used in addiction research studies (McLellan et al., 1992). Participant demographic and background information were obtained from the ASI.
AIDS Risk Assessment (ARA)
This instrument inquires about HIV sex- and injection-risk behaviors during the past 30 days. The ARA has been used among methadone patients to assess HIV-risk behaviors in relation to gender and cocaine use (Joe and Simpson, 1995), as well as to assess risk levels over time during (Camacho et al., 1996) and after (Camacho et al., 1997) methadone treatment.
Five ARA items were used to represent injection risk, including the number of times participants did the following behaviors in the past 30 days: injected drugs, used dirty needles, injected with other injectors, and shared same cooker, cotton, or rinse water. The number of people with whom participants shared works was the final HIV injection-risk item. Responses were coded as continuous variables.
Six items were used to represent sex risk, including the frequency of sex without a condom, and frequency of sex without a condom: with someone who is not the participant’s usual partner, with another injector, with someone who smokes crack, while the participant or partner was high, or while trading sex for drugs or money. All responses were coded as continuous variables.
Statistical Analysis
For the purposes of this study, the SM and RM groups were combined due to the small number of participants (n=27) in the RM group.
Initial analysis involved simple comparisons of the IM group and the combined SM/RM group on demographics using one-way analysis of variance for continuous variables and χ2 tests of independence for categorical variables. Changes in HIV-risk behaviors over time were determined using a Generalized Linear Mixed Model approach, with Treatment Group, Time, and their interaction (Treatment Group X Time) included as effects in the model. The Treatment Group main effect tests whether the groups differ in their HIV-risk behaviors collapsed over Time. The Time main effect tests whether these behaviors change over time collapsed across Treatment Groups. Finally, the Treatment Group X Time interaction tests whether there are differential rates of change in these behaviors between the Treatment Groups over time.
Analysis was conducted for the total sample (N=230) and for two defined subsamples. The “injector” subsample consisted of the 110 participants (53 IM participants and 57 SM/RM participants) who reported at baseline that they had injected drugs in the past 30 days (regardless of their sex-risk behaviors). Analyses for this subsample were limited to the five risky injection items. The “unprotected-sex” subsample consisted of the 130 participants (58 IM participants and 72 SM/RM participants) who had reported having sex without a condom in the past 30 days at baseline (regardless of their drug-risk behaviors). Analyses for this subsample involved only the six risky sex items.
RESULTS
Participant Characteristics
Table 1 shows participant characteristics for the total sample and the IM group v. the combined SM/RM group. Participants were 70% male, 77.4% African American, had a mean age of 43.2 years, and 13.5% were married. They had 11.3 years of education and worked 4.9 days in the 30 days before baseline. Nearly half the sample (47.8%) had reported injecting heroin and/or cocaine, and 55.7% reported cocaine use at baseline. There were no differences between treatment groups at baseline in the variables shown in Table 1.
Table 1.
Participant Sociodemographic Characteristics and Drug Use at Baseline (N = 230)
| Variable | Total Sample (N = 230) |
Interim Methadone (n = 99) |
Standard/Restored Methadone (n = 131) |
Test Statistic |
P |
|---|---|---|---|---|---|
| Male, n (%) | 161 (70.0%) | 69 (69.7%) | 92 (70.2%) | χ2(1) = 0.01 | 0.93 |
| Race, n (%) | χ2(1) = 0.46 | 0.50 | |||
| African American | 178 (77.4%) | 78 (78.8%) | 100 (76.3%) | ||
| White | 49 (21.3%) | 19 (19.2%) | 30 (22.9%) | ||
| Native American | 2 (0.9%) | 1 (1.0%) | 1 (1.0%) | ||
| Asian/Pacific Islander | 1 (0.4%) | 1 (1.0%) | 0 (0.0%) | ||
| Mean age (SD) | 43.2 (8.0) | 43.6 (8.2) | 43.0 (7.8) | F(1, 228) = 0.39 | 0.53 |
| Married, n (%) | 31 (13.5%) | 12 (12.1%) | 19 (14.5%) | χ2(1) = 2.0 | 0.16 |
| Mean no. of years of education (SD) |
11.3 (1.9) | 11.3 (1.6) | 11.3 (2.1) | F(1, 228) = 0.02 | 0.90 |
| Mean no. of days worked in last 30 days (SD) |
4.9 (8.8) | 4.4 (8.4) | 5.3 (9.0) | F(1, 228) = 0.63 | 0.43 |
| Injected at baseline, n (%) | 110 (47.8%) | 53 (53.5%) | 57 (43.5%) | χ2(1) = 2.3 | 0.13 |
| Used cocaine at baseline, n (%) |
128 (55.7%) | 54 (54.5%) | 74 (56.6%) | χ2(1) = 0.09 | 0.77 |
Note: Test statistic for Race was obtained by collapsing data into two categories: White (n = 49) v. African American/other (n = 181).
Total Sample Drug and Sex Risk
As shown in Table 2, there was a Time main effect for 3 of the 5 drug-risk items, such that the total sample reported significantly reducing its frequency of: injecting (P<0.001), sharing cooker, cotton, or rinse water (P=0.020), and injecting with other injectors (P<0.001) over the 12 months.
Table 2.
Total Sample Means (Standard Errors) for Treatment Group X Time Interaction Effects and Time Main Effects for Past 30 Day Risky Drug-Use and Sex Items (N = 230)
| Variable | Interim Group |
Standard/Restored Group |
Time | Time Main Effect Test Statistic |
P |
|---|---|---|---|---|---|
| Drug-use items, Mean (SE) | |||||
| Number of times injected | F(2, 212) = 76.9 | <.001 | |||
| Baseline | 47.3 (5.3) | 41.7 (4.6) | 44.5 (3.5) | ||
| 4-month | 1.0 (1.1) | 3.0 (1.0) | 2.0 (.76) | ||
| 12-month | 3.3 (1.5) | 4.5 (1.4) | 3.9 (1.0) | ||
| Number of times injected with dirty needles | F(2, 221) = 2.8 | .062 | |||
| Baseline | .51 (.35) | .57 (.30) | .54 (.23) | ||
| 4-month | .04 (.03) | .00 (.03) | .02 (.02) | ||
| 12-month | .00 (.00) | .00 (.00) | .00 (.00) | ||
| Number of times shared cookers, cotton, or rinse water |
F(2, 225) = 4.0 | .020 | |||
| Baseline | 1.9 (.71) | .60 (.62) | 1.2 (.47) | ||
| 4-month | .04 (.03) | .00 (.03) | .02 (.02) | ||
| 12-month | .05 (.04) | .02 (.03) | .04 (.02) | ||
| Number of times injected with other injectors | F(2, 154) = 19.6 | <.001 | |||
| Baseline | 10.8 (2.7) | 11.6 (2.4) | 11.2 (1.8) | ||
| 4-month | .23 (.24) | .36 (.21) | .29 (.16) | ||
| 12-month | .73 (.50) | .95 (.44) | .84 (.33) | ||
| Number of people with whom shared works | F(2, 224) = 1.3 | .272 | |||
| Baseline | .13 (.20) | .29 (.18) | .21 (.13) | ||
| 4-month | .01 (.01) | .00 (.01) | .01 (.01) | ||
| 12-month | .01 (.01) | .01 (.01) | .01 (.01) | ||
| Sex items, Mean (SE) | |||||
| Number of times had sex without condom | F(2, 216) = 1.1 | .335 | |||
| Baseline | 5.2 (.77) | 5.0 (.67) | 5.1 (.51) | ||
| 4-month | 5.1 (.74) | 5.1 (.67) | 5.1 (.50) | ||
| 12-month | 6.6 (1.0) | 5.4 (.90) | 6.0 (.68) | ||
| Number of times had sex without condom with non-primary sex partner |
F(2, 228) = 4.2 | .016 | |||
| Baseline | .37 (.25) | .37 (.22) | .37 (.16) | ||
| 4-month | .05 (.03) | .03 (.03) | .04 (.02) | ||
| 12-month | .42 (.20) | .18 (.17) | .30 (.13) | ||
| Number of times had sex without condom with another injector |
F(2, 133) = 1.2 | .298 | |||
| Baseline | .33 (.24) | .62 (.21) | .48 (.16) | ||
| 4-month | .11 (.23) | .39 (.20) | .25 (.15) | ||
| 12-month | .46 (.26) | .23 (.23) | .35 (.17) | ||
| Number of times had sex without condom with crack user |
F(2, 219) = 1.7 | .177 | |||
| Baseline | .69 (.32) | .66 (.28) | .68 (.21) | ||
| 4-month | .05 (.22) | .38 (.20) | .22 (.15) | ||
| 12-month | .50 (.24) | .16 (.22) | .33 (.16) | ||
| Number of times had sex without condom while participant or partner was high |
F(2, 203) = 32.5 | <.001 | |||
| Baseline | 4.6 (.75) | 4.6 (.65) | 4.6 (.50) | ||
| 4-month | .57 (.31) | .53 (.28) | .55 (.21) | ||
| 12-month | .80 (.35) | .96 (.31) | .88 (.23) | ||
| Number of times had sex without condom while trading sex for drugs/money |
F(2, 220) = 2.0 | .135 | |||
| Baseline | .08 (.09) | .11 (.09) | .08 (.06) | ||
| 4-month | .02 (.01) | .00 (.01) | .01 (.01) | ||
| 12-month | .45 (.23) | .03 (.21) | .24 (.16) | ||
Note: N = 230 at baseline, 216 at 4-month follow-up, and 210 at 12-month follow-up.
Regarding sex risk (Table 2), there was a Time main effect for 2 of the 6 items. The total sample reported significant decreases from baseline to 12 months in frequencies of having sex without a condom either with someone who was not a spouse or primary partner (P=0.016) or while the participant or sexual partner was high (P<0.001).
There were no significant Treatment Group main effects or Treatment Group X Time interaction effects for any HIV drug- or sex-risk items in the total sample (all Ps>0.05).
Injector Subsample
Table 3 shows Treatment Group X Time and Time means for the subset of participants who reported injecting drugs in the 30 days prior to baseline (n=110). Similar to findings for the total sample, this subsample reported significant decreases over 12 months in their frequency of injecting (P<0.001), sharing cooker, cotton, or rinse water (P=0.029), and injecting with other injectors (P<0.001).
Table 3.
Subsample Means (Standard Errors) for Treatment Group X Time Interaction Effects and Time Main Effects for Past 30 Day Risky Drug-Use and Sex Items
| Variable | Interim Group |
Standard/Restored Group |
Time | Time Main Effect Test Statistic |
P |
|---|---|---|---|---|---|
| Injector subsample, Mean (SE)* | |||||
| Number of times injected | F(2, 100) = 280.3 | <.001 | |||
| Baseline | 88.4 (5.2) | 95.8 (5.0) | 92.1 (3.6) | ||
| 4-month | 1.9 (2.2) | 6.8 (2.2) | 4.3 (1.6) | ||
| 12-month | 6.1 (3.0) | 9.7 (3.0) | 7.9 (2.1) | ||
| Number of times injected with dirty needles | F(2, 104) = 2.9 | .060 | |||
| Baseline | .94 (.68) | 1.3 (.66) | 1.1 (.47) | ||
| 4-month | .08 (.06) | .00 (.05) | .04 (.04) | ||
| 12-month | .00 (.00) | .00 (.00) | .00 (.00) | ||
| Number of times shared cookers, cotton, or rinse water |
F(2, 107) = 3.7 | .029 | |||
| Baseline | 3.5 (1.4) | 1.4 (1.3) | 2.4 (.97) | ||
| 4-month | .08 (.06) | .00 (.06) | .04 (.04) | ||
| 12-month | .10 (.07) | .06 (.07) | .08 (.05) | ||
| Number of times injected with other injectors | F(2, 72) = 24.1 | <.001 | |||
| Baseline | 20.3 (4.9) | 26.6 (4.7) | 23.4 (3.4) | ||
| 4-month | .42 (.47) | .81 (.47) | .62 (.33) | ||
| 12-month | 1.4 (.96) | 1.5 (.95) | 1.5 (.68) | ||
| Number of people with whom shared works | F(2, 105) = 1.5 | .232 | |||
| Baseline | .25 (.40) | .67 (.38) | .46 (.28) | ||
| 4-month | .02 (.01) | .00 (.01) | .01 (.01) | ||
| 12-month | .02 (.02) | .02 (.02) | .02 (.02) | ||
|
Unprotected-sex subsample, Mean (SE)** |
|||||
| Number of times had sex without condom | F(1, 123) = 3.9 | .022 | |||
| Baseline | 8.8 (1.1) | 9.1 (.97) | 9.0 (.73) | ||
| 4-month | 6.8 (.99) | 7.1 (.92) | 6.9 (.68) | ||
| 12-month | 7.7 (1.6) | 7.9 (1.4) | 7.8 (1.1) | ||
| Number of times had sex without condom with non-primary sex partner |
F(2, 128) = 2.9 | .058 | |||
| Baseline | .64 (.43) | .67 (.38) | .65 (.29) | ||
| 4-month | .05 (.04) | .05 (.04) | .05 (.03) | ||
| 12-month | .22 (.24) | .28 (.22) | .25 (.16) | ||
| Number of times had sex without condom with another injector |
F(2, 82) = 3.1 | .051 | |||
| Baseline | .57 (.41) | 1.1 (.36) | .85 (.27) | ||
| 4-month | .14 (.39) | .72 (.36) | .43 (.27) | ||
| 12-month | .02 (.19) | .30 (.17) | .16 (.13) | ||
| Number of times had sex without condom with crack user |
F(2, 123) = 2.2 | .110 | |||
| Baseline | 1.2 (.54) | 1.2 (.49) | 1.2 (.37) | ||
| 4-month | .09 (.37) | .69 (.35) | .39 (.26) | ||
| 12-month | .54 (.39) | .19 (.36) | .37 (.26) | ||
| Number of times had sex without condom while participant or partner was high |
F(2, 88) = 52.1 | <.001 | |||
| Baseline | 7.8 (1.1) | 8.4 (.99) | 8.1 (.74) | ||
| 4-month | .33 (.42) | 1.0 (.40) | .67 (.29) | ||
| 12-month | .50 (.44) | 1.6 (.41) | 1.1 (.30) | ||
| Number of times had sex without condom while trading sex for drugs/money |
F(1, 127) = 1.0 | .371 | |||
| Baseline | .14 (.16) | .19 (.14) | .17 (.10) | ||
| 4-month | .03 (.02) | .00 (.02) | .02 (.02) | ||
| 12-month | .00 (.04) | .05 (.03) | .02 (.03) | ||
n = 110 at baseline, 103 at 4-month follow-up, 96 at 12-month follow-up.
n = 130 at baseline, 123 at 4-month follow-up, 119 at 12-month follow-up.
There were no significant Treatment Group main effects or Treatment Group X Time interaction effects for any of the five HIV drug-risk items in this subsample (all Ps>0.05).
Unprotected-Sex Subsample
Treatment Group X Time and Time means for the unprotected-sex subsample (n=130) are shown in Table 3. This subsample reported significant decreases over 12 months in frequencies of having sex without a condom (P=0.022) and having sex without a condom while high (P<0.001) in the last 30 days.
As with the total sample and the injector subsample, there were no significant Treatment Group main effects or Treatment Group X Time interaction effects for any HIV sex-risk items in this subsample (all Ps>0.05).
Supplemental Analyses
To explore the effects of dose on HIV-risk behaviors, a set of correlations were conducted for the total sample examining the relationship between 4- and 12-month mean dose and 4- and 12-month HIV-risk behaviors, respectively. We chose to examine the relationship in this way since including dose as a covariate in the main analyses would limit the sample to only those participants still in treatment.
Results showed no significant correlations among the 4-month variables, with the exception of a small but significant inverse relationship between dose and frequency of using dirty needles (P=0.034). No significant correlations were found between 12-month dose and 12-month variables.
Because some of the IM patients transferred early and a few SM/RM participants did not have counseling in the first 4 months, an additional set of supplemental analyses was conducted examining correlations between whether participants had any counseling at the 4- and 12-month follow-up and their 4- and 12-month HIV-risk behaviors. Findings showed no significant correlations at either time point.
DISCUSSION
This study compared HIV drug- and sex-risk behaviors among newly-admitted methadone maintenance patients randomly assigned to receive either four months of methadone with emergency counseling only or methadone with standard counseling. Both the total sample and the injector subsample significantly reduced their frequencies of injecting, use of same cooker, cotton, or rinse water, and injecting with other injectors over the 12 months. This result would suggest that methadone treatment—with or without counseling—coupled with minimal HIV-risk information provided at the start of treatment can be effective in reducing drug-related HIV-risk behaviors.
Both the total sample and the unprotected-sex subsample reported statistically significant reductions in having sex without a condom while high over the 12 months. Additionally, the total sample reported reductions in sex without a condom with someone who was not a spouse or primary partner, and the unprotected-sex subsample reported significant reductions in the frequency of sex without a condom. The reported change in the frequency of having unprotected sex with someone who is not a primary partner may represent an important reduction in HIV risk, although it is not a reason for complacency, since unprotected sex with a primary sexual partner is no guarantee of safety. Frequencies of other sexual-risk behaviors also decreased over this time but were so low at baseline that statistical significance could not be shown. The findings of little change in condom use per se yet reduced frequency of some sexual high-risk behaviors are consistent with the Cochrane review of 33 studies evaluating opioid agonist treatment and HIV risk (Gowing et al., 2008).
In this study, there were neither significant Treatment Group main effects nor significant Treatment Group X Time interaction effects for self-reported drug- or sex-related HIV-risk behaviors for the total sample or for the two subsamples. Nearly half (46.5%) of the participants in the present study had prior methadone treatment experience and fully 86.5% had been in some form of substance-abuse treatment previously. It is possible that these individuals had already gained the HIV-prevention knowledge they needed to successfully reduce their HIV risk once this knowledge was coupled with sufficient doses of methadone to reduce their illicit drug use. In a previous study that found significant advantages for regular counseling over methadone plus minimal counseling with respect to drug use (McLellan et al., 1993), methadone dosage for the three comparison treatment groups averaged 60 mg. In the present study, the average dose for the total sample by 4 months was 76.6 mg and 84.2 mg by 12 months. It is possible that as the methadone dose approaches more optimal levels it becomes harder to demonstrate the impact of counseling.
As reported elsewhere (Schwartz et al., 2011), this trial also found that all three treatment groups reduced self-reported heroin and cocaine use and criminal activity, and opiate and cocaine positive urine tests over time, but there were no significant treatment group differences in the degree of improvement. The present study and our previously-reported work (Schwartz et al., 2006; Wilson et al., 2010) indicate that, in terms of drug use, self-reported criminal behavior, and self-reported HIV-risk behavior, there may not be measurable disadvantages to IM patients. Also, methadone is provided without counseling in many parts of the world, and, in those contexts, has been found to reduce both drug use and HIV-related behaviors (Gossop et al., 1999).
There are some limitations to this study. First, the HIV-risk behaviors reported were obtained during face-to-face interviews rather than audio computer-assisted interviews, which have been shown to increase reporting of risky behaviors (Metzger et al., 2000; Rogers et al., 2005). Nevertheless, if there was underreporting, it is likely that it would have occurred equally in both treatment groups or that the group that received counseling may have underreported more than the methadone-only group since they were likely to have received more HIV-risk prevention messages in counseling. Second, research assistants were not blinded to study group at the follow-up interviews. Finally, this study was conducted in one US city and it is not clear to what degree the quality and quantity of counseling studied here is representative of that provided in other localities. Therefore, it is not known to what extent these findings can be generalized.
CONCLUSIONS
The findings here indicate that methadone treatment even without regular counseling confers substantial benefits to patients who otherwise would most likely continue to use illicit opioids and engage in HIV-risk behaviors (Schwartz et al., 2006). However, most of those individuals who can benefit from opioid agonist treatment cannot afford to pay for it without some kind of public subsidy or insurance coverage. When provided by an established methadone treatment program, offering some patients interim methadone is, at the margin, substantially less costly. Currently, Federal regulations in the US prevent existing programs from providing this less costly form of agonist treatment even to individuals who cannot afford both the opioids and the counseling. These regulations also prohibit IM patients who stop using illicit drugs from receiving take home medications, thus preventing the use of an established incentive to decrease illicit drug use. If there are reductions in available funding for drug treatment because of the worldwide economic downturn, it would appear reasonable to reconsider the use of methadone without required counseling.
Our findings also emphasize the need for more effective HIV-risk interventions in MTPs, especially those aimed at reducing sexual risk. MTPs should routinely implement new evidence-based sexual-risk interventions as they are developed for drug abuse treatment settings (Calsyn et al., 2010; Tross et al., 2008). They also might consider referring patients who continue to inject drugs to syringe exchange programs. Finally, making anti-retroviral medications available to HIV infected MTP patients or their partners in HIV-discordant couples may be an effective way to prevent HIV transmission (Donnell et al., 2010).
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Drug Abuse (NIDA) grant 2R01 DA 13636 “Entry into Comprehensive Methadone Treatment via Interim Maintenance” (PI Schwartz) and The Abell Foundation, which provided support for the counselor in the Restored Methadone condition. We thank Dr. John Urbaitis, Suzanne Harrison, and Janet Caputo at Sinai Hospital Addiction Recovery Program, and Dr. Eric Weintraub, Jewell Benford, and Wayne Clemons at the University of Maryland Drug Treatment Center. We also thank our NIDA Project Officer, Dr. Thomas Hilton, and, finally, Melissa Irwin for assistance with manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA or The Abell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Calsyn DA, Crits-Christoph P, Hatch-Maillette MA, et al. Reducing sex under the influence of drugs or alcohol for patients in substance abuse treatment. Addiction. 2010;105(1):100–108. doi: 10.1111/j.1360-0443.2009.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsyn DA, Wells EA, Saxon AJ, et al. Contingency management of urinalysis results and intensity of counseling services have an interactive impact on methadone maintenance treatment outcome. J Addict Dis. 1994;13:47–63. doi: 10.1300/j069v13n03_05. [DOI] [PubMed] [Google Scholar]
- Camacho LM, Bartholomew NG, Joe GW, Cloud MA, Simpson DD. Gender, cocaine, and during-treatment HIV risk reduction among injection opioid users in methadone maintenance. Drug Alcohol Depend. 1996;41:1–7. doi: 10.1016/0376-8716(96)01235-5. [DOI] [PubMed] [Google Scholar]
- Camacho LM, Bartholomew NG, Joe GW, Simpson DD. Maintenance of HIV risk reduction among injection opioid users: A 12 month posttreatment follow-up. Drug Alcohol Depend. 1997;47:11–18. doi: 10.1016/s0376-8716(97)00056-2. [DOI] [PubMed] [Google Scholar]
- Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Lehmann P, Strang J. Methadone treatment practices and outcome for opiate addicts treated in drug clinics and in general practice: Results from the National Treatment Outcome Research Study. Br J Gen Pract. 1999;49:31–34. [PMC free article] [PubMed] [Google Scholar]
- Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database System Rev. 2008;(2) doi: 10.1002/14651858.CD004145.pub3. CD004145. [DOI] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe GW, Simpson DD. HIV risks, gender, and cocaine use among opiate users. Drug Alcohol Depend. 1995;37:23–28. doi: 10.1016/0376-8716(94)01030-o. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Booth RE. Methadone maintenance as HIV risk reduction with street-recruited injecting drug users. J Acquir Immune Defic Syndr. 2001;26:483–489. doi: 10.1097/00126334-200104150-00014. [DOI] [PubMed] [Google Scholar]
- Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: A comparison of levomethadyl acetate [corrected] buprenorphine, and methadone. J Subst Abuse Treat. 2006;31:187–194. doi: 10.1016/j.jsat.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Maddux JF, Desmond DP. Outcomes of methadone maintenance 1 year after admission. J Drug Issues. 1997;27:225–238. [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD002209.pub2. CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIV/NET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: An 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Novick DM, Joseph H, Croxon TS, et al. Absence of antibody to human immunodeficiency virus in long term, socially rehabilitated methadone maintenance patients. Arch Intern Med. 1990;150:97–99. [PubMed] [Google Scholar]
- Rogers SM, Willis G, Al-Tayyib A, et al. Audio computer assisted interviewing to measure HIV risk behaviours in a clinic population. Sex Transm Infect. 2005;81(6):501–507. doi: 10.1136/sti.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Highfield DA, Jaffe JH, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry. 2006;63:102–109. doi: 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O'Grady KE, Gandhi D. Interim methadone treatment compared to standard methadone treatment: 4-month findings. J Subst Abuse Treat. 2011;41(2):21–29. doi: 10.1016/j.jsat.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senay EC, Jaffe JH, diMenza S, Renault PF. A 48-week study of methadone, methadyl acetate and minimal services. In: Fisher S, Freeman A, editors. Opiate Dependence: Origins and Treatment. New York, NY: Halstead Press; 1973. pp. 185–201. [Google Scholar]
- Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: The role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100:150–158. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- Tross S, Campbell AN, Cohen LR, et al. Effectiveness of HIV/STD sexual risk reduction groups for women in substance abuse treatment programs: results of NIDA Clinical Trials Network Trial. J Acquir Immune Defic Syndr. 2008;48(5):581–589. doi: 10.1097/QAI.0b013e31817efb6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Program on HIV AIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. Geneva, Switzerland: Joint United Nations Program on HIV AIDS; 2010. [Accessed March 7, 2011]. Available from: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. [Google Scholar]
- Wilson ME, Schwartz RP, O'Grady KE, Jaffe H. Impact of interim methadone maintenance on HIV risk behaviors. J Urban Health. 2010;87:586–591. doi: 10.1007/s11524-010-9451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


