Abstract
The core symptoms of autism are deficits in social interaction and language, and the presence of repetitive/stereotyped behaviors. We demonstrate that behaviors related to these symptoms are present in a mouse model of an environmental risk factor for autism, maternal infection. We stimulate the maternal immune system by injecting the viral mimic poly(I:C) during pregnancy, and analyze the social and communicative behaviors of the offspring. In one test, young pups respond to a brief separation from the mother with ultrasonic vocalizations (USVs). We find that, compared to pups born to saline-injected mothers, pups born to maternal immune activation (MIA) mothers produce a lower rate of USVs in the isolation test starting at day 8. The quality of the vocalizations is also different; analysis of sound spectrograms of ten day-old pups shows that male pups from MIA mothers emit significantly fewer harmonic and more complex and short syllables. These communication differences are also apparent in adult offspring. Compared to controls, adult MIA males emit significantly fewer USVs in response to social encounters with females or males, and display reduced scent marking in response to female urine. Regarding a second autism symptom, MIA males display decreased sociability. In a third test of characteristic autism behaviors, MIA offspring exhibit increased repetitive/stereotyped behavior in both marble burying and self-grooming tests. In sum, these results indicate that MIA yields male offspring with deficient social and communicative behavior, as well as high levels of repetitive behaviors, all of which are hallmarks of autism.
Keywords: acoustic communication, speech disorder, olfactory communication, neurodevelopmental disorder, social preference
1. Introduction
Autism is a neurodevelopmental disorder characterized by social impairments, communication difficulties and repetitive/stereotyped behaviors. While there is a substantial genetic component in autism, there is also evidence that environmental factors can contribute to the complex pathogenesis of autism spectrum disorders (ASD). Recent, large twin studies show a substantial concordance for autism between dizygotic as well as monozygotic twins (Rosenberg, et al., 2009; Hallmayer et al., 2011). Moreover, the significantly higher risk for dizygotic twins compared to non-twin siblings suggests a role for the maternal environment. In fact, epidemiologic studies provide evidence that maternal exposure to stress, infection, anti-depressant medications, thalidomide or valproic acid (VPA) increases the risk for ASD in offspring (reviewed by Hyman et al., 2005; Kinney et al., 2008; Patterson, 2009, 2011a). In particular, a recent study of over 10,000 records in the Danish Medical Register found a strong association between autism and maternal viral infection in the first trimester and a less robust but significant association between autism and maternal bacterial infection in the second trimester (Altadottir et al., 2010). There is also evidence of elevated levels of the chemokine MCP-1 in the amniotic fluid in ASD cases (Abdallah et al., 2011), and elevated levels of IFNγ, IL-4 and IL-5 in maternal serum are associated with increased risk for ASD in the offspring (Goines et al., 2011). These new results greatly extend prior work on the connection between maternal infection and autism.
Animal models displaying certain autistic features can be generated by maternal environmental challenges such as infection and exposure to VPA. Such models can be useful tools for testing epidemiologic findings and investigating the molecular mechanisms underlying the neuropathology (reviewed by Iwata et al., 2010; Hsiao et al., 2011; Patterson, 2011b). Deficits in communication are among the most prominent and functionally important symptoms in ASD, and acoustic and chemical signals are two primary modes of communication in rodents. The number and quality of USVs and the chemical composition of urine can convey information regarding health and fitness. Male responses to female urine can involve USVs and marking the surrounding territory (scent marking). A deficit in social interaction is also a critical symptom of ASD, and there are a number of paradigms used to assess this feature in rodents. One test that was originally introduced to assay social affiliation and pair bonding in voles utilizes a three chamber apparatus. In mice, this can include a test for preference for an unfamiliar mouse over an unfamiliar object (Silverman et al., 2010). The third cardinal symptom of autism involves repetitive/stereotyped behavior, which can be assayed in mice by quantifying self-grooming and compulsive burying of marbles placed in the nest (Thomas et al., 2009).
In the present study, we used these various assays to determine how closely the core symptomatology of autism can be reproduced in a model of an environmental risk factor for ASD. This model involves activating the immune system of pregnant mice in order to mimic the effects of maternal infection. Young and adult offspring of these mice were assessed for relevant behaviors in several paradigms.
2. Methods
2.1. Generation of animals
Female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were obtained from the California Institute of Technology breeding facility and were housed in ventilated cages under standard laboratory conditions. Mice were mated overnight, and the presence of a vaginal plug marked that day as embryonic day 0.5 (E0.5). Pregnant females were not disturbed, except for weekly cage cleaning, until E10.5 when they were weighed and pseudo-randomly assigned to one of two groups, poly(I:C) or saline. Each group initially contained five or six pregnant females. The maternal poly(I:C) treatment did not alter litter size (8.67±0.56 and 7.83±0.54 for control and experimental groups, respectively; t(10) = 1.07, p = 0.309). All pups from a single litter remained with the mother until weaning at P21, at which time male mice were housed with same-sex littermates in groups of two to four. All experimental mice underwent the same sequence of behavioral tests at the same times during development (n = 21 (5 litters) and 22 (5 litters) for control and experimental groups, respectively). All behavioral tests were conducted in behavioral testing rooms between 09.00 and 17.00 h during the light phase of the circadian cycle. Mice were habituated to a testing room at least one hour prior to the start of the behavioral test. Order of testing was: (1) Pup USVs during the isolation test at age 10 days; (2) PPI at age 6 weeks (Supplemental materials); (3) open field at 7 weeks (Supplemental materials); (4) adult male USV responses to female and male stimuli at age 8–9 weeks; (5) three chamber social test at 10 weeks; (6) scent marking at 11 weeks; (7) marble burying at age 12 weeks; (7) self-grooming at age 13 weeks; and (8) olfactory sensitivity at age 13–14 weeks. A separate experiment was carried out with another cohort of mice (n = 29 (6 litters) and 29 (6 litters) to test the USV profile during the second postnatal week. This was done for two reasons: first, to minimize effect of handling and stress on the USV structure; second, to lessen the effect of repeated stress during isolation in early postnatal life on adult behavior.
2.2. Maternal administration of poly(I:C)
One group of mice was given intraperitoneal injections of 5 mg/kg poly(I:C) (potassium salt; P9582; Sigma, St. Louis, MO) or saline on E10.5, 12.5 and 14.5. The manufacturer supplies poly(I:C) at 10% of the total weight of the salt, and the dosage was based on the weight of poly(I:C) itself. The inflammatory cytokine response in pregnant mice induced by an acute systemic challenge of poly(I:C) is time-limited, and the time of maternal immune challenge influences the pattern of behavioral abnormalities in the offspring (Meyer et al., 2006). Therefore, we challenged the maternal immune system three times during pregnancy to determine if the offspring develop a broader range of behavioral deviances. However, where comparisons are possible, the behavioral results from the current offspring do not appear to be significantly different from our prior results using a single poly(I:C) injection on E12.5. Mice born to triple-injected mothers have the deficit in prepulse inhibition (PPI) and reduced locomotion in open field (Fig. S1) that we have seen in single-injected mothers and influenza-infected mothers (Shi et al., 2003; Smith et al., 2007; Hsiao and Patterson, 2011).
2.3. Pup USVs during the isolation test
Pups from mothers injected with saline or poly(I:C) were tested for USVs every other day from day 6 to day 14 in the isolation test as described (Hofer et al., 2002). During the test, the dam was removed from the home cage and placed in a separate cage away from the litter. 15 min after removing the dam, male pups were individually removed from the nest in random order and gently placed into an empty 15 × 15 cm white Plexiglas box. Ambient temperature in the room was 23.5–23.6°C. In the pilot experiment the pups' axillary temperature was measured prior and after testing and found to be decreased after isolation from 34.43±0.19°C to 31.97±0.29°C in 6 day-old pups. In a pilot experiment we tested P6 pups from MIA and control mothers in the isolation test and found no difference in the temperature decrease between the groups (data not shown). As it is known that small decreases in pup core temperature in the home cage before testing have no significant effect on their USV isolation performance (Hofer et al., 2002), we did not measure the body temperature in order to minimize the effect of handling on pup behavior. USVs were recorded for 3 min using an UltraSoundGate Condenser Microphone (CM 16; Avisoft Bioacoustics, Germany) suspended 7 cm from the testing surface. The microphone was sensitive to frequencies of 0–250 kHz with a flat frequency response (±6 dB) between 25 and 120 kHz. It was connected via an AvisoftUltraSoundGate 116 USB Audio device (Avisoft Bioacoustics) to a personal computer, and the recordings were carried out with a sampling rate of 250 kHz. The vast majority of USVs were detected within the 0–150 kHz range. The recordings were analyzed using AvisoftSASLab Pro software (see below). After recording, the pups were weighed and placed back into the nest. Body weight was included as an indicator of pups' nutritional status and maturational age. The mother was returned to the pups after they all had been tested. The testing box was cleaned with water after each pup recording.
2.4. Adult male USV responses to female and male stimuli
Eight-to-ten week-old males were separated and housed singly for a week before testing. To provide a standardized history of sexual experience that is known to facilitate USVs (McGinnis and Vakulenko, 2003), adult male subjects were exposed to unfamiliar adult females for 15–20 min every day for five days. On the day of testing, the male was placed in a clean cage (30 × 16 × 14 cm) for 10 min habituation. The male was then tested for USVs in the presence of an unfamiliar female in the estrous phase for 3 min. The female was scored as in estrous when the vaginal area appeared wide, open and red. USVs were recorded as described above. After 5 min rest following removal of the female, the male subject was tested for USVs in the presence of an unfamiliar male intruder for 3 min. During male-male encounters, host males show characteristic behaviors such as active approaching, sniffing and following the intruder. USVs were analyzed using AvisoftSASLab Pro software as described below.
2.5. Analysis of USVs
For acoustical analysis, recordings were transferred to Avisoft-SASLab Pro (version 4.38; Avisoft Bioacoustics) and a fast Fourier transformation was conducted (512 FFT-length, 100% frame, Hamming window and 75% time window overlap). Detection of USVs was provided by manually setting a threshold-based algorithm and a hold-time mechanism (hold time: 5 ms). Since no USVs were detected below 30 kHz, a lower cut-off frequency of 30 kHz was used to reduce background noise outside the relevant frequencies. The accuracy of detection was verified by an experienced investigator. The total number of vocalizations emitted, total vocalization time as well as duration and dominant frequency for each syllable type were measured automatically. Spectrographic analysis was conducted as originally described by Scattoni et al. (2008) with some modifications that were also made by Grimsley et al. (2011). All USVs were assigned to one of 11 structural groups, primarily according to their pitch contour shape, which is determined by the frequency modulation and duration of the acoustic signal (Fig. S2). Syllables that were described as composite by Scattoni et al. (2008) were classified as harmonics because these syllables often had a third harmonic component at 150 kHz. If the syllable was compound and contained multiple components such as harmonic and frequency steps, it was classified as a frequency step syllable with a harmonic component. In a pilot experiment, we compared the distribution of USV syllables collected during the entire 3 min testing period and from every other 30 sec period during the same 3 min testing and found that both methods of analysis yield similar data (Fig. S3). Thus, for the further analysis, USVs were collected from every other 30 sec period during the 3 min testing period (average number of vocalizations per mouse was 250–300) unless an animal produced fewer than 100 vocalizations, in which case USVs were collected from the total 3 min of testing. The total number of USV syllables was normalized to 100%.
2.6. Three chamber social test
Ten week-old mice were tested in a 60 × 40 cm Plexiglass box divided into three chambers as described previously (Smith et al., 2007). Mice could freely move between chambers through a small opening (6 × 6 cm) in each divider. Mice to be tested were placed in the center chamber, and an overhead camera recorded their movements. Mice were allowed 10 min to explore the empty box in order to evaluate bias for either of the side chambers. For each animal, we confirmed that there was no side bias. Then an unfamiliar, same-sex mouse was placed in a wire cage (11 cm height, 10.5 bottom diameter, bars spaced 1 cm apart; Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH) in one side chamber. Another empty wire cage was placed in the opposite side chamber as a non- social object. Mouse behavior was recorded for 10 min. The time spent in each of the three chambers was measured, and the preference index for social object was calculated as [timesocial/(timesocial + timenon-social)]×100−50 (Jamain et al., 2008). All testing chambers were cleaned vigorously with 70% ethanol followed by Process NPD before and after testing each mouse.
2.7. Scent marking test
This test was done according to the protocol described by Wohr et al. (2011a). Subject mice were placed in a clean cage (30 × 16× 14 cm) containing a sheet of absorbent paper that covered the entire base (Strathmore Drawing Paper Premium, 400 series; Strathmore Artist Papers, Neenah, WI, USA). Female urine was collected from adult C57/B6 females in estrous. Male urine was collected from sexually experienced adult C57/B6 males. The adult male subject was habituated for 60 min to the clean cage lined with fresh paper. After the habituation period, the mouse was placed back in the home cage. A new sheet of paper was then placed in the cage and 15 uL of female urine was aliquotted onto the center. The mouse was placed back in the testing cage for 3 min. Scent marks deposited on the paper during habituation or exposure to female/male urine were visualized using 0.5% ninhydrin in 95% ethanol. Developed papers were scanned and analyzed for total density using ImageJ (NIH, USA). The data are shown as the ratio of density of the experimental group to the control group.
2.8. Marble burying test
This test was done as described by Thomas et al. (2009) with small modifications. In a pilot experiment under these conditions, we found that naive C57 males bury 50% of the marbles in a 10 min period so this testing time was used in further experiments so as to be able to detect either increases or decreases in the number of buried marbles. Clean cages (27 × 16.5 × 12.5 cm) were filled with a 4-cm layer of chipped cedar wood bedding. Males were habituated to this cage for 10 min and then returned to the home cage. Twenty navy blue glass marbles (15 mm diameter) were gently laid on top of the bedding, equidistant from each other in a 4 × 5 arrangement. Animals were placed back into the testing cage and the number of marbles buried (> 50% marble covered by bedding material) in 10 min was recorded.
2.9. Self-grooming test
Self-grooming was evaluated in a 6.5 cm diameter × 10 cm tall, clear glass beaker covered with a filter top. After 10 min habituation in the beaker, self-grooming was measured for a further 10 min. The investigator sat 1 m away from the apparatus and recorded cumulative time spent in grooming (scratching fur with any foot). All testing beakers were cleaned vigorously with water followed by Process NPD before and after testing.
2.10. Olfactory sensitivity test
The method for measuring olfactory sensitivity was adapted from Witt et al. (2009). On day 1, the subject was habituated to a clean empty cage for 10 min, and then a 2 cm × 2cm damp Stathmore paper was placed in the cage and the time spent exploring the paper during 3 min period was recorded. On day 2, the paper was dampened with 10% vanilla scent in water instead of plain water. The data are presented as the time spent exploring the scented paper minus the time spent exploring the plain water paper.
2.11. Statistical analysis
Statistical analyses were carried out with Prism 4.0b software. Normally distributed data were plotted in the figures as means ± SEM. The means of two groups were compared by Student's t test. Where data were not normally distributed (assessed by the Kolmogorov–Smirnov test), they were plotted as medians plus 25% and 75% quartiles, and range. Two groups of such data were compared with the Mann-Whitney test. All statistical tests were two-tailed, except the number of vocalizations in the male-male encounter, with α ≤ 0.05. The differences among groups with two variables were assessed using two-way ANOVAs followed by Bonferroni posthoc tests. Significant differences emerging from the above tests are indicated in the figures by *p < 0.05, **p < 0.01 and ***p < 0.005.
3. Results
3.1. Pups born to MIA mothers display a deficit in the number of USVs and an altered syllable repertoire
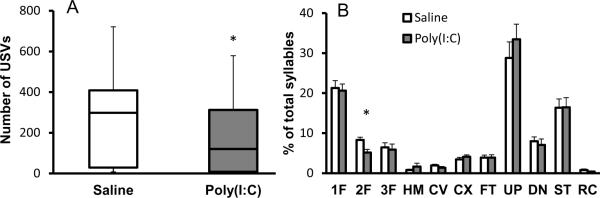
Neonatal rodents emit USVs when separated from their mothers or littermates in order to attract the mother for retrieval to the nest. In most mouse strains, the rate of pup USVs increases during the first 5–6 postnatal days, reaching a peak on days 8–10, and decreasing to zero by the end of the second postnatal week (Motomura et al., 2002). We evaluated male MIA and control offspring for USV production in the isolation test during second postnatal week. Compared to controls, male pups from MIA mothers emit fewer vocalizations (F(1, 56) = 17.74, p < 0.0001; Bonferroni posthoc test p < 0.05 for P8, P10 and P12 pups) and the total vocalization duration is less starting from day 8 (F(1, 56) = 15.74, p < 0.0005; Bonferroni posthoc test p < 0.05 for P8, P10 and P12 pups) (Fig. 1A, B).
Figure 1.
Male pups from MIA mothers emit altered number and quality of USVs in the isolation test. Pups were isolated from the nest and tested individually for USVs every other day from day 6 to day 14. Compared to control pups, the MIA pups emit fewer vocalizations (A), call for a shorter duration of time (B). 10 day-old pups from MIA mothers also produce significantly more short and complex syllables and fewer harmonic syllables than controls (C) Significant differences are indicated by *p < 0.05, **p < 0.01 and ***p < 0.005. USV classification: FS, frequency step; HM, harmonic; CV, chevron; CX, complex; FT, flat; UP, upward; DN, downward; ST, short; RC, reversed chevron.
In order to minimize the effect of repetitive handling and isolation on pup USVs, data for structural analysis of the USVs were collected from pups tested only on P10. The proportion of each syllable type is shown in Fig. 1C. Since there is no difference in the proportion of one, two and multiple frequency step syllables, we present them as one group termed frequency step syllables. Although a two-way ANOVA test does not indicate a difference in the distribution of USV syllables between treatment groups (treatment, F(1, 41) = 0.292, p = 0.58), significant effects of syllable type (F(8, 180) = 79.97, p < 0.0001) and treatment × syllable type interaction (F(8, 180) = 3.67, p < 0.0006) are evident. However, the Bonferonni posthoc test shows a treatment–dependent effect on the proportion of syllables in three of the nine syllable types. Compared to controls, MIA offspring produce 60% fewer harmonic syllables (p < 0.05) and significantly more short (MIA 13% versus control 7%, p < 0.05) and complex syllables (MIA 13% versus control 9%, p < 0.05) (Fig. 1C). ANOVA analysis of the duration of each syllable type also shows a difference between MIA and control offspring (treatment, F (1, 41) = 4.32, p < 0.05). The Bonferonni posthoc test reveals a difference between the treatment groups in complex, downward and upward syllables (p < 0.05) (Table 1). However, no difference is found in the dominant frequency of any of the USV syllables (treatment, F (1, 41) = 0.022, p = 0.88 (Table 2).
Table 1.
Duration of USV syllables of MIA and control offspring in three social encounters.
| 1F | 2F | 3+F | HM | CV | CX | FT | UP | DN | ST | RC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P10 pup | |||||||||||
| Saline | 41.1±3.3 | 49.5±4.5 | 68.3±7.8 | 59.5±4.1 | 22.9±.31 | 49.2±1.9 | 18.5±2.0 | 11.0±0.5 | 26.8±2.5 | 3.8±0.7 | 29.9±2.1 |
| Poly(I:C) | 37.0±2.9 | 48.9±3.5 | 71.5±6.8 | 53.4±4.5 | 20.2±1.5 | 40.6±1.2* | 16.1±1.5 | 13.9±1.3* | 19.7±2.3* | 4.2±0.7 | 23.0±3.0 |
| Male-female | |||||||||||
| Saline | 58.8±3.9 | 74.4±4.0 | 110.8±6.6 | 97.4±5.1 | 43.0±2.1 | 81.5±3.7 | 24.0±2.7 | 20.7±1.0 | 36.8±2.5 | 4.1±0.3 | 33.5±2.1 |
| Poly(I:C) | 52.9±4.1 | 66.3±4.8 | 92.9±6.9* | 97.8±9.2 | 39.0±2.4 | 68.7±4.8 | 25.0±2.4 | 21.9±2.1 | 29.5±3.3 | 5.7±1.7 | 32.7±2.8 |
| Male-male | |||||||||||
| Saline | 37.4±2.5 | 47.0±3.0 | 97.3±11.4 | 73.1±0.7 | 22.3±1.2 | 38.9±3.3 | 10.6±1.1 | 15.2±0.7 | 23.1±2.0 | 5.2±1.2 | 19.4±1.9 |
| Poly(I:C) | 33.9±4.4 | 39.0±0.4 | 82.3±8.7 | 76.3±8.3 | 21.8±0.2 | 34.3±3.3 | 12.1±0.9 | 13.5±1.0 | 17.3±1.3 | 4.2±0.8 | 19.6±1.8 |
Duration for each syllable type is measured in milliseconds. Data are mean values ± SEM. USV classification: 1F, one frequency step; 2F, two frequency step; 3F, three and more frequency step; HM, harmonic; CV, chevron; CX, complex; FT, flat; UP, upward; DN, downward; ST, short.
p < 0.05.
Table 2.
Dominant frequency of USV syllables of MIA and control offspring in three social encounters.
| 1F | 2F | 3+F | HM | CV | CX | FT | UP | DN | ST | RC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P10 pup | |||||||||||
| Saline | 71.1±1.8 | 70.2±2.2 | 59.8±1.9 | 66.8±1.6 | 84.5±0.8 | 85.5±1.6 | 70.8±2.6 | 86.5±2.1 | 80.5±1.4 | 86.0±1.4 | 78.2±3.0 |
| Poly(I:C) | 72.3±2.6 | 68.6±1.5 | 59.3±1.9 | 66.8±1.7 | 85.8±0.8 | 86.4±0.7 | 76.3±2.9 | 90.4±1.1 | 82.1±2.0 | 87.5±1.3 | 81.9±2.8 |
| Male-female | |||||||||||
| Saline | 72.0±0.7 | 71.7±0.6 | 65.4±3.2 | 63.6±3.2 | 77.4±1.2 | 72.4±1.1 | 70.5±4.4 | 7.8±1.4 | 70.0±3.7 | 78.6±1.8 | 73.9±1.1 |
| Poly(I:C) | 72.9±1.2 | 73.8±1.5 | 69.5±1.2 | 62.2±3.5 | 78.5±1.2 | 72.9±1.0 | 68.1±3.7 | 79.0±1.2 | 75.7±1.5 | 78.2±1.8 | 73.1±1.0 |
| Male-male | |||||||||||
| Saline | 79.6±0.8 | 81.7±1.2 | 75.8±2.3 | 77.3±1.3 | 86.1±2.1 | 80.0±1.7 | 84.4±3.6 | 82.4±0.6 | 83.9±1.6 | 87.1±1.5 | 80.3±0.9 |
| Poly(I:C) | 75.6±2.1 | 81.3±2.6 | 69.7±1.1 | 68.6±1.6 | 89.2±3.0 | 80.3±2.3 | 73.4±3.7* | 82.3±1.7 | 78.8±2.3 | 82.2±2.1 | 73.6±2.1 |
Dominant frequency for each syllable type (at peak of frequency) is measured in kHz. Data are mean values ± SEM. USV classification: 1F, one frequency step; 2F, two frequency step; 3F, three and more frequency step; HM, harmonic; CV, chevron; CX, complex; FT, flat; UP, upward; DN, downward; ST, short.
p < 0.05.
Body weight was used as a parameter to evaluate animals' health and it does not differ between these two groups (Fig. S3). Moreover, we find no changes in the development of reflexes (negative geotaxis, grasp reflex and righting reflex) (data not shown). In addition, we analyzed maternal behavior toward pups in response to either nest relocation or to pup retrieval and found no difference between two treatment groups (Fig. S4). Thus, the reduction in the USV production is not due to a delay in gross pup development or evident differences in maternal responsiveness toward the offspring.
3.2. Adult male offspring from MIA mothers display reduced USV responses to various social stimuli
Adult mice produce USVs in several social contexts (Portfors, 2007). Encounters with females induce male USV production, which is most prominent during initial investigatory behavior and correlates well with the level of male sexual arousal (White et al., 1998). This USV production conveys an important communicative function, predominantly to engage the female's attention. Before studying male-female interactions, we used anesthetized animals to conduct a pilot experiment to evaluate the contributions of male and female mice in USV production. We detect USVs in two settings - when both the male and female are awake and when a male is exposed to an anesthetized female. In contrast, few vocalizations (2–3 during 3 min testing period) are detected when the male is anesthetized, although the female mouse interacts physically with the male by sniffing and walking around him (data not shown). Thus, female mice do not contribute significantly in USV production, at least in our test setting. Similar results were reported by several research groups using devocalized or anesthetized males (Maggio and Whitney, 1985; White et al., 1998; Sugimoto et al., 2011). To test vocalizations in adult MIA offspring, we introduced an unfamiliar female to the male and recorded USVs. We find that, compared to control males, MIA offspring produce significantly fewer vocalizations in the presence of a female (t(41) = 2.092, p < 0.05) (Fig. 2A). While analysis of USV syllables using the two-way ANOVA does not show a significant difference in the distribution of syllable types between MIA and control males (treatment, F (1, 41) = 1.68, p = 0.2; syllable type, F (10, 450) = 54.66, p = 0.0001; treatment × syllable type, F (10, 450) = 1.13, p = 0.3), the Bonferonni posthoc test reveals a significant reduction in the MIA offspring in the number of two frequency step (MIA 8% vs control 12%, p < 0.05) and chevron syllables (MIA 10% vs control 13%, p < 0.05) (Fig. 2B). The two-way ANOVA test shows a difference between MIA and control male offspring in duration and dominant frequency of USV syllables during interaction with females (treatment, F (1, 41) = 7.42, p < 0.05 for syllable duration and F (1, 41) = 9.4, p < 0.05 for dominant frequency of syllables). The Bonferonni posthoc test of individual syllables reveals a significant difference in duration of three and more frequency step syllables (p < 0.05) (Table 1, 2). As with the pups, no treatment difference is found in the dominant frequency of any USV syllables (Table 2).
Figure 2.
Adult male offspring from MIA mothers display reduced USV responses in a social encounter with a female. During interactions with a female in estrous phase, MIA males emit fewer vocalizations (A) and also produce significantly fewer two step frequency and chevron syllables (B) than controls. Significant differences are indicated by *p < 0.05, **p < 0.01 and ***p < 0.005. USV classification: 1F, one frequency step; 2F, two frequency step; 3F, three and more frequency step; HM, harmonic; CV, chevron; CX, complex; FT, flat; UP, upward; DN, downward; ST, short.
Before studying male-male interactions, we also conducted a pilot experiment to evaluate the contributions of resident and intruder mice in USV production using anesthetized animals. We detected USVs in only two settings, when both males were awake or when the intruder was anesthetized. In contrast, no vocalizations were recorded when the resident was anesthetized, although the intruder mouse interacted physically with the resident by sniffing and licking him (data not shown). The pilot experiment suggests that resident males respond to an encounter with a male intruder by producing USVs, which likely serve an important defensive function. We next analyzed the responses of adult male offspring of control and MIA mothers to the presence of an unfamiliar male intruder. Compared to controls, MIA offspring produce significantly fewer USVs during this social encounter (Mann–Whitney U = 99, p < 0.05 one-tailed) (Fig. 3A). Since not all tested males produced USVs in the presence of a male intruder, the number of samples used to analyze the sound spectrograms was 16 for control and 19 for experimental groups. While the two-way ANOVA test does not an effect of treatment on the total distribution of all syllables for male-male interaction (treatment, F (1, 33) = 3.31, p = 0.08; syllable type, F (10, 330) = 84.04, p = 0.0001; treatment × syllable type, F (10, 330) = 0.83, p = 0.8), the Bonferonni posthoc test of individual syllable differences reveals a significant reduction in the number of two frequency step syllables emitted by the MIA offspring (MIA 5% vs control 8%, p < 0.05) (Fig. 3B). No difference is seen in the duration of any USV syllables (treatment, F (1, 41) = 2.4, p = 0.12) (Table 1). Two-way ANOVA test shows a difference between MIA and control male offspring in the dominant frequency of all USV syllables during male-male interaction (treatment, F (1, 41) = 16.32, p < 0.001). However, the Bonferonni posthoc test reveals a significant change in the dominant frequency for flat syllables only (p < 0.05) (Table 2).
Figure 3.
Adult male offspring from MIA mothers display reduced USV responses in a social encounter with a male. During interactions with an unfamiliar male intruder, MIA males emit fewer vocalizations (A) and also produce significantly fewer two step frequency syllables (B) than controls. Data for the number of calls are plotted as medians plus 25% and 75% quartiles, and range. Two groups of such data were compared with the Mann-Whitney test. Significant differences are indicated by *p < 0.05 and ***p < 0.005. USV classification as in Fig. 2.
Thus, in all three social paradigms, there is a decrease in the number of USVs emitted by the MIA offspring. In both of the adult social paradigms, MIA offspring produce fewer two frequency step syllables. Our results indicate that MIA not only affects USV production by the offspring, but also changes the quality of their communication in several social settings that have important biological roles in survival and reproduction.
3.3. MIA males display a communication deficit in female urine-induced scent marking
In addition to communication using acoustic signals, mice gain information through olfaction. Scent marks (urinary traces) are used to determine mouse identity, mark territory, and to attract mates (Hurst, 2009). During the 60-min habituation to a new clean cage, the two groups of mice showed no difference in scent marking. The total density of urine deposits for control and experimental males were 3,899±378 and 4,224±525 for control and experimental groups, respectively; (t(41) = 0.51, p = 0.615). Then we used fresh male or female urine to stimulate scent marking from adult male mice in order to assess olfactory communication. Males from both MIA and control groups spend equal time exploring a female urine spot (data not shown), suggesting that they are both processing the olfactory social stimulus. (A further test of olfactory sensitivity is described below). But male offspring from MIA mothers deposit half as many scent marks compared to control animals during the 3 min test period (t(41) = 3.266, p < 0.005) (Figs. 4A, S5). Interestingly, no group difference is found when male urine is used as the stimulus (t(41) = 0.021, p = 0.98) (Fig. 4B). It should be noted, however, that in control males, male urine induces half as many scent marks as does female urine (t(40) = 4.434, p < 0.0001). In sum, MIA males display a communication deficit in female-induced scent marking, which plays a key role in mediating sexual behavior.
Figure 4.
MIA males display a deficit in female urine-induced scent marking. Compared to controls, MIA males deposit fewer scent marks in the presence of female urine (A). However, no group difference is observed in the scent marking induced by male urine (B). A significant difference is indicated by ***p < 0.005.
3.4. MIA offspring display reduced sociability
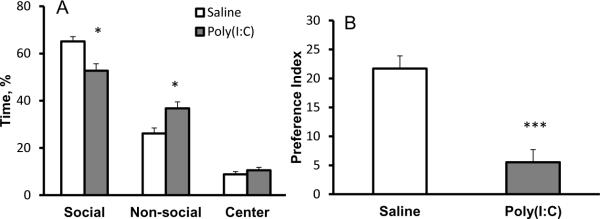
Social behavior is a form of communication between members of the same species, and this type of behavior in rodents is thought to constitute a reasonable model of human interpersonal interaction. The three chamber apparatus can be used to assess autism-related behaviors such as sociability (Silverman et al., 2010). Shortly after a period of habituation, sociability is evaluated as the time the subject mouse spends in the chamber containing a wire cage holding an unfamiliar mouse compared to the time spent in the opposite chamber containing an empty wire cage (novel object). We find that males from saline-injected mothers spend 65% of the time in the social chamber and 26% of the time in the chamber with the novel object. In contrast, offspring of MIA mothers display decreased preference for the social chamber, spending only 50% of the time exploring the social chamber and significantly more time (37%) than conrols exploring a novel object (F(2, 48) =12.73, p < 0.0001; Bonferroni posthoc test p < 0.001 for time in the social chamber and p < 0.01 for time in the chamber with novel object) (Fig. 5A). This results in significantly different ratios of time spent in the two compartments (preference index)compared with the offspring from saline-injected mothers (t(41) =3.283, p < 0.005; Fig. 5B). This result is similar to that obtained with offspring of mothers injected just once with poly(I:C), and where both sexes were tested (Smith et al., 2007).
Figure 5.
Adult male offspring from MIA mothers exhibit decreased sociability in the three chamber social test. Compared to controls, MIA males spend significantly less time in the chamber with an unfamiliar mouse and more time in the chamber with an unfamiliar, non-social object (A). The preference index for social object was significantly lower in the MIA offspring (B). Significant differences are indicated by *p < 0.05 and ***p < 0.005.
3.5. MIA offspring display high levels of repetitive/compulsive behavior in the marble burying test
The marble burying test presents a novel situation that can evoke a highly repetitive and stereotyped response. The marble burying test was previously used to evaluate anxiety but it was recently shown that this behavioral response does not correlate with results from other tests of anxiety. Thus, marble burying is now used to evaluate repetitive and perservative behavior in small rodents (Thomas et al., 2009). We find that the offspring of MIA mothers display extremely high repetitive behavior in this test (t(41) = 8.535, p < 0.0001) (Figs. 6A; S6). Compared to the control group, MIA offspring bury 2.8-fold more marbles during the testing period.
Figure 6.
Offspring of MIA mothers display high levels of repetitive/compulsive behavior. Compared to controls, MIA offspring display extremely high repetitive behavior in the marble burying test (A), and increased self-grooming (B). Significant differences are indicated by *p < 0.05 and ***p < 0.001.
3.6. MIA offspring display elevated levels of self-grooming
Self-grooming can provide another index of repetitive/stereotypic behavior in rodents (Silverman et al., 2010). We tested individual mice in a small glass beaker for 10 min, as it is known that a restricted environment induces repetitive behavior (Lewis et al., 2007). MIA male offspring spend 50% more time on self-grooming than controls (t(41) = 2.335, p < 0.05) (Fig. 6B).
3.7. MIA offspring do not display an obvious olfactory deficit
Since MIA offspring display decreased sociability and communication, we evaluated their olfactory sensitivity. Time spent sniffing a novel, vanilla-scented paper versus a plain water-scented paper was recorded, and no group difference is evident (t(18) = 0.361, p = 0.72) (Fig. 7). Therefore, the social and communication deficits found in the MIA offspring are not likely to be caused by olfactory malfunction.
Figure 7.

MIA offspring do not display an obvious olfactory deficit. The data are presented as the ratio of the time spent exploring a scented paper to the time spent exploring a plain water spotted paper.
4. Discussion
We here show that the offspring of poly(I:C)-treated mothers exhibit the three core features of autism. While prior work from our laboratory had demonstrated deficits in social interaction in MIA offspring, those studies utilized a different poly(I:C) injection schedule, different methods, and did not analyze males specifically (Shi et al., 2003; Smith et al., 2007; Hsiao and Patterson, 2011). The present study is also the first to analyze scent marking, USVs, and repetitive/stereotyped behaviors in the MIA model. Moreover, this is the only model of an environmental risk factor for autism in which all three of the core features have been analyzed thus far.
In the model of a different environmental autism risk factor, maternal VPA, behavioral studies revealed two core autism symptoms, repetitive/stereotypic-like hyperactivity and impairment in social interaction (Schneider and Przewlocki, 2005; Schneider et al., 2006). In the rat model of maternal stress, there are also male-specific behavioral abnormalities: poor sociability (Lee et al., 2007) and sexual behavior (Ward and Reed, 1985), as well as deficits in pup USVs (Morgan et al., 1999; Zimmerberg and Blaskey, 1998). Activation of the maternal immune system with lipopolysaccharide (LPS), which mimics bacterial infection, also yields offspring with decreased social interaction (Hava et al., 2006; Kirsten et al., 2010). In addition, pups from LPS-injected mothers display impaired play behavior and USV communication (Hava et al., 2006; Baharnoori et al., 2010; Kirsten et al., 2010). A neuropathology characteristic of autism, a spatially localized deficit in Purkinje cells in the cerebellum, is also found in the VPA and MIA mouse models (Ingram et al., 2000; Shi et al., 2009). Since the cerebellum is known to contribute to learning, language, sociability and emotionality (Ito, 1998; Thach, 1998), the deficits in communication and social interaction observed in MIA and VPA mice may be associated with abnormalities in the cerebellum as well as other brain regions. Together, these rodent models exhibit a spectrum of autistic-like behaviors.
It should be noted that maternal infection models also display features of schizophrenia. These include enlarged ventricles, enhanced responses to amphetamine and hallucinogens, alterations in dopamine and serotonergic pathways, as well as a number of other behaviors in the offspring that are also found in subsets of autistic subjects such as enhanced anxiety and eye blink conditioning, as well as a PPI deficit (Iwata et al., 2010; Hsiao et al., 2011; Meyer and Feldon, 2010; Patterson, 2011b; Moreno et al., 2011). This is not surprising, as maternal infection is a well-characterized risk factor for schizophrenia as well as autism (Brown and Derkits, 2010). Moreover, these disorders have symptoms in common, such as impaired social interaction, elevated anxiety and abnormal responsivity to stress (Tordjman et al., 2007). On the other hand, schizophrenia and autism obviously have symptoms that differentiate them. Autism occurs in early childhood whereas the psychotic symptoms of schizophrenia appear much later. In addition, delusions and hallucinations are typical of schizophrenic patients while motor stereotypies and lack of language are cardinal symptoms of autism. Thus, the MIA model displays both symptoms in common between these two disorders, as well as symptoms that differentiate them. Presumably the different disease phenotypes associated with maternal infection in human autism and schizophrenia are due to differences in genetic background of the mother and/or fetus, the timing of the infection, the presence of allergies or autoimmune disease in the mother, and possibly the severity of the infection.
The present study shows that MIA offspring display altered emotional behavior early in postnatal life. The male pups display a reduced number of vocalizations when they are isolated from their littermates and mother. Similar findings were reported for pups born to mothers exposed to stress or LPS during pregnancy (Morgan et al., 1999; Baharnoori et al., 2010), and in reelin, Mecp-2 and CNTNAP2 mutant mice (Ognibene et al., 2007; De Filippis et al., 2010; Peñagarikano et al., 2011). The reduction in USV production is not, however, due to a delay in gross pup development or a difference in maternal care. Altered USVs can reflect a reduced response to stress or poor bonding with the mother; the latter was characterized in mice lacking the μ-opioid receptor gene (Moles et al., 2004). The former explanation appears unlikely in the MIA model as we do not see any changes in the pup locomotor activity (circling and pivoting) during the isolation test (data not shown). Interestingly, BTBR pups, a mouse strain with several autism-like features, vocalize more in this test compared to several other strains of mice, which could be due to an elevated emotional response to the novel environment (Scattoni et al., 2008). In addition, the BTBR pups exhibit an altered vocal repertoire, producing a high level of harmonics but minimal syllables with a simple structure (chevron-shaped, upward, downward and short). MIA pups also show some structural changes in their vocalization patterns, but these are opposite what was reported for BTBR pups: lower level of harmonic syllables but increased level of complex and short syllables. Harmonic syllables are known to be prominent in the mouse pup repertoire (Grimsley et al., 2011). They amount to 16% of the pup repertoire, whereas the proportion of this type of syllable is very low in adult male song during male-female and male-male encounters (Fig. 1C, 2B, 3B). It is also interesting that a short reunion with the mother affects pup emotional response to a second isolation as evidenced by increased USV production and augmented proportion of multi-component syllables including harmonics (data not shown; Young et al., 2010). Moreover, harmonic syllables have some similarity to human baby cries (Zeskind et al., 2011), which is known to reflect the emotional state of the baby. Thus, harmonic syllables can be used as an index of pup emotional status, and MIA offspring demonstrate a reduced emotional response to separation from the mother.
Importantly, we find that adult MIA males demonstrate decreased USV responses in social encounters with females or other males. Several mouse strains suggested as being relevant for autism studies (BTBR, NL-3-knockout (KO), NL-4 KO and Shank1 KO) show a similar deficiency (Jamain et al., 2008; Radyushkin et al., 2009; Scattoni et al., 2011; Wohr et al., 2011b). Frequency step syllables are the most frequent in adult male repertoire in both social settings (37–39%), and they likely reflect affiliative/social communication. Sound spectrogram analysis of MIA male vocalizations reveals reduced use of two frequency step syllables in both social paradigms. BTBR males also emit fewer frequency step calls during interaction with females or males, although no changes in USV repertoire were found for NL-3 KO and NL-4 KO mice. Given the differences in syllable preference displayed by MIA versus control males, it will be of interest to examine such preferences more closely over developmental time, and in other social paradigms such as juvenile play. It will also be worthwhile to examine the serial order and groupings of the syllables to determine if these parameters are correlated with social paradigm, age or maternal insult. Such analyses could provide important information as to the relevance of syllable use in mice to the language deficit in autism. The deficit in male USVs in the presence of a female in estrous and the deficit in scent marking in the presence of female urine could suggest a lack of sexual interest. We know of no data on actual mating behavior or successful copulation by adult male MIA offspring. Similarly, the deficit in USVs in the presence of a male intruder suggests a lack of aggressiveness in defense of territory. It will be of interest in the future to test MIA males for dominant/submissive behaviors as well as sexual behavior.
In addition to deficits in auditory communication, MIA males exhibit a reduction in female urine-induced scent marking, which was also found in BTBR and Shank1 KO males (Wohr et al., 2011a; 2011b). The deficits in auditory and olfactory communication tested here would also seem relevant for social interaction. The sociability test we used seems more appropriate for the social deficit in human autism than the social preference or social habituation tests. The latter tests are based on memory of prior social encounters, which has not been shown to be a key feature in autism social pathology. In contrast, the sociability test assesses whether the mouse prefers to be in the presence of a novel mouse versus a novel object. While many factors may be involved in this decision (e.g., fear, curiosity, aggression), it does seem analogous to many human situations that confront autistic individuals. In this light, prior work on interaction with novel objects is of interest. MIA offspring display a deficit in interaction with a novel object (Shi et al., 2003), which is suggestive of anxiety in a novel situation, as is seen in autism. Further work of this type revealed that, compared to controls, MIA offspring display a distinct c-Fos expression pattern in hippoocampal area CA1 following novel object, but not novel location, exposure. Thus, the offspring of MIA mothers may have an abnormality in modality-specific information processing. Indeed, MIA offspring display enhanced discrimination in a novel object recognition, but not in an object location task (Ito et al., 2010). Thus, analysis of object and spatial information processing at both synaptic and behavioral levels reveals a largely selective abnormality in object information processing in this mouse model.
We find that MIA offspring exhibit highly repetitive behavior in marble burying and self-grooming tests. Self-grooming is also increased in several other mouse strains with relevance to autism (BTBR, and Fmr1, MeCP2 and CNTNAP2 KO mice) (McNaughton et al., 2008; McFarlane et al., 2008; Chao et al., 2010; Peñagarikano et al., 2011).
In sum, this study provides new evidence that environmental risk factors such maternal infection during pregnancy yield the offspring with autistic-like behaviors, including communication and social interaction impairments as well as high repetitive behavior. This reinforces prior results indicating the face and construct validity of the model for both autism and schizophrenia.
Supplementary Material
We find that activation of the maternal immune system yields offspring with cardinal symptoms of autism: highly repetitive behavior and deficits in communication and sociability.
Acknowledgments
The authors acknowledge the kind assistance of J. Ko and B. Deverman with reviewing the manuscript, L. Sandoval and R. Sauza for maintaining the animals, and J. Grimsley for providing advice on syllable analysis. This research was supported by the National Institute of Mental Health EUREKA award to PHP and NVM, and an Autism Speaks Dennis Weatherstone Pre-Doctoral Fellowship to EYH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish historic birth cohort. Brain Behav. Immun. 2011 doi: 10.1016/j.bbi.2011.09.003. doi:10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Schendel DE, Ostergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch. Pediatr. Adolesc. Med. 2010;164:470–477. doi: 10.1001/archpediatrics.2010.9. [DOI] [PubMed] [Google Scholar]
- Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq098. doi:10.1093/schbul/sbq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HS, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JLR, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Ricceri L, Laviola G. Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 2010;9:213–223. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFNg, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. doi:10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS ONE. 2011;6:e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotseich L, Crown LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.76. doi:10.1001/archgenpyschiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev. Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Curr. Protoc. Neurosci. 2002:8.14.1–8.14.16. doi: 10.1002/0471142301.ns0814s17. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Bregere C, Malkova N, Patterson PH. Modeling Features of Autism in Rodents. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. Oxford Univ Press; New York: 2011. pp. 935–962. [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL. Female recognition and assessment of males through scent. Behav. Brain Res. 2009;200:295–303. doi: 10.1016/j.bbr.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Arndt TL, Rodier PM. Environmental Agents and Autism: Once and Future Associations. International Review of Research in Mental Retardation. 2005;30:171–194. [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol. Teratol. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cognit. Sci. 1998;2:313–321. doi: 10.1016/s1364-6613(98)01222-4. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SEP, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav. Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Matsuzaki H, Takei N, Manabe T, Mori N. Animal models of autism: an epigenetic and environmental viewpoint. J. Central Nervous System Disease. 2010;2:37–44. doi: 10.4137/JCNSD.S6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Millera AM. Prenatal stress and risk of autism. Neurosci. Biobehav. Rev. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Maiorka PC, Palermo-Neto J, Bernardi MM. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation. 2010;17:240–251. doi: 10.1159/000290040. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav. Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J. Comp. Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav. Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the μ-opioid receptor gene. Science. 2004;340:1983–1985. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, Lopez J, Cadagan R, Martinez-Sobrido L, Garcia-Sastre A, Gonzalez-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan KN, Thayer JE, Frye CA. Prenatal stress suppresses rat pup ultrasonic vocalization and myoclonic twitching in response to separation. Dev. Psychobiol. 1999;34:205–215. doi: 10.1002/(sici)1098-2302(199904)34:3<205::aid-dev5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Motomura N, Shimizu K, Shimizu M, Aoki-Komori S, Taniguchi K, Serizawa I, Saito TR. A comparative study of isolation-induced ultrasonic vocalization in rodent pups. Exp. Anim. 2002;51:187–90. doi: 10.1538/expanim.51.187. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Macrì S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: Interaction of genetic vulnerability and consequences of maternal separation. Behav. Brain Res. 2007;177:142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011a;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Modeling autistic features in animals. Pediatr. Res. 2011b;69:34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab Anim. Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg RE, Law JK, Yenkyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. Pediatr. Adolesc. Med. 2009;16:907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacol. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Schneider T, Turczak J, Przewłocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacol. 2006;31:36–46. doi: 10.1038/sj.npp.1300767. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SEP, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through Interleukin-6. J. Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Okabe S, Kato M, Koshida N, Shiroishi T, Mogi K, Kikusui T, Koide T. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS One. 2011;6:e22093. doi: 10.1371/journal.pone.0022093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. A role for the cerebellum in learning movement coordination. Neurobiol. Learn Mem. 1998;70:177–88. doi: 10.1006/nlme.1998.3846. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty – induced anxiety. Psychopharmacol. 2009;20:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, Laurent C, Roubertoux PL. Animal models relevant to schizophrenia and autism: validity and limitations. Behav. Genet. 2007;37:61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- Ward IL, Reed J. Prenatal stress and prepuberal social rearing conditions interact to determine sexual behavior in male rats. Behav. Neurosci. 1985;99:301–309. doi: 10.1037//0735-7044.99.2.301. [DOI] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol. Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Witt RM, Galligan MM, Despinoy JR, Segal R. Olfactory behavioral testing in the adult mouse. JOVE. 2009 doi: 10.3791/949. doi:10.3791/949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc. Natl. Acad. Sci. USA. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, McMurray MS, Garber KA, Neuspiel JM, Cox ET, Grewen KM, Mayes LC, Johns JM. Development of translational methods in spectral analysis of human infant crying and rat pup ultrasonic vocalizations for early neurobehavioral assessment. Front Psychiatry. 2011;2:56. doi: 10.3389/fpsyt.2011.00056. doi:0.3389/fpsyt.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Blaskey LG. Prenatal stress effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol. Biochem. Behav. 1998;59:819–27. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.