Abstract
Background
Gender differences exist in a variety of cardiovascular and renal diseases, and testosterone may contribute to the discrepancy. Afferent arterioles (Af-Art) are the major resistance vessels in the kidney, and play an important role in the development of renal injury and hypertension.
Objective
The present study aimed to determine the acute effect and underlying mechanism(s) of testosterone on Af-Art.
Methods
The mRNA expression of androgen receptors (AR) in microdissected Af-Art was measured by RT-PCR. An in vitro microperfusion model was used to measure the diameter of Ar-Art in mice. Nitric oxide (NO) was evaluated by an NO-sensitive fluorescent dye, 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate.
Results
Testosterone had no effect on microperfused Af-Art when added into the bath. Therefore we pre-constricted the Af-Art to about 30% with norepinephrine (NE, 10−6mol/L); administration of testosterone (10−9 to 10−7mol/L) subsequently dilated the Af-Art in a dose-dependent manner (p<0.001; n=7). AR mRNA was expressed in microdissected Af-Art measured by RT-PCR. An AR antagonist, flutamide (10−5mol/L), totally blocked testosterone (10−8mol/L)-induced vasodilator effect. NO production of Af-Art wall was increased when testosterone was added into the bath solution after NE treatment, from 278.4 ± 12.1 units/min to 351.2 ± 33.1 units/min (p<0.05; n=3). In the presence of NO inhibition with NG-nitro-L-arginine methyl ester (L-NAME, 3×10−4mol/L), the testosterone-induced dilatation was blunted compared with NE (p<0.05).
Conclusions
We conclude that testosterone dilates pre-constricted mouse Af-Art in a dose-dependent manner by activation of AR and partially mediated by NO.
Keywords: kidney, testosterone, androgen receptors, nitric oxide
Introduction
Gender differences exist in a variety of cardiovascular and renal diseases. Sex steroids are indicated to be involved in these differences. For example, hypertension and chronic coronary artery disease occur more frequently in men than in premenopausal women1, 2. The incidence of end-stage renal disease of all cause is higher in men than in women3. Renal diseases in women progress at a slower rate than they do in blood pressure-and lipid level-matched men 4. Glomerular filtration rate (GFR) in healthy women declines more slowly compared with that in healthy men5. These studies are long-term observations and the underlying mechanisms are suggested to be via a classic pathway which is dependent on androgens binding to the nuclear receptors.
Contrary to the classic genomic effect, androgens also display rapid non-genomic potentially beneficial effects as recently reported in clinical trials and animal studies6, 7. In men with established coronary artery disease, short-term intracoronary administration of testosterone, at physiological concentrations, induces coronary artery dilatation and increases coronary blood flow within minutes8. Also, acute oral administration of testosterone increased cardiac output, and reduced systemic vascular resistance compared with baseline in male patients with stable chronic heart failure9. Both in heart failure patients and in healthy controls testosterone at high concentration (>10µm) dilated subcutaneous resistant arteries ex vivo10. The non-genomic effect usually does not depend on the binding of testosterone to the classic nuclear androgen receptors (AR) with the subsequent process of gene transcription and protein translation11–13. The mechanisms are still unclear. Despite of the contradictions among studies, nitric oxide (NO) seems to be involved in this response because NO synthase (NOS) inhibition has been shown to decrease the dilation effect both in vitro14 and in vivo15. Membrane ion channels including Ca2+16 and K+ channels12 also play a role in the non-genomic effect.
Afferent arterioles (Af-Art) are the major resistance vessels in the kidney17. They are indispensible in regulation of both myogenic response and tubuloglomerular feedback, which are the primary mechanisms for renal autoregulation. These regulatory mechanisms are key factors for control of intraglomerular pressure and salt and water balance, thereby making them important for the development of renal injury and hypertension. Studies showed that testosterone induced relaxation of precontracted rabbit renal arteries in vitro in an endothelium dependent manner,18 however, the acute effect of testosterone on renal microcirculation has not been reported, to our knowledge. In the present study, we aimed to determine the direct and acute effect of testosterone on renal afferent arterioles and the underlying mechanisms. Using an Af-Art microperfusion model in mice, we found that testosterone induced acute vasodilation of the Af-Art via the AR and partially mediated by NO.
Methods
All procedures and experiments were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. All studies are consistent to the Guide for the Care and Use of Laboratory Animals, National Institutes of Health. All chemicals were purchased from Sigma (St. Louis, MO) except as indicated.
Isolation and microperfusion of mouse afferent arterioles
The methods are the same as we previously described to isolate and perfuse the Af-Art with intact glomerulus19, 20. Briefly, male C57BL/6J mice (8 weeks) were anesthetized with ketamine and xylazine, and kidneys were removed and sliced along the corticomedullary axis. Slices were placed in ice-cold minimum essential medium (MEM; Gibco, Grand Island, NY) containing 5% bovine serum albumin and dissected under a stereomicroscope (model SMZ 1500; Nikon). A single superficial Af-Art and its intact glomerulus were microdissected, transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti; Nikon) with Hoffmann modulation using a micropipette and cannulated with an array of glass pipettes. Af-Arts were perfused with MEM from the proximal end in an orthograde direction. Microdissections and perfusions were completed within 60 minutes at 4°C, and the samples were then gradually warmed to 37°C for the rest of the experiment. Once the temperature was stable, a 30-min equilibration period was allowed before taking any measurements. The imaging system consisted of a microscope (Eclipse Ti; Nikon), digital charge-coupled device camera (CoolSnap; Photometrics), xenon light (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10–3; Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
Dose-response curves
Testosterone was purchased from Sigma (testosterone propionate, catalog number T1875), dissolved in ethanol at the concentration of 10mg/ml and stored as stock solution. Working solutions at different concentrations were made from the stock solution in MEM in the following experiments. The final concentration of ethanol was 3.4×10−4 – 3.4×10−8 % (v/v).
After the 30-minute equilibration period, testosterone (10−8mol/L) was added into the bath to test whether it displayed a vasoconstrictor effect. To determine whether testosterone had a vasodilator effect, we pre-constricted the Af-Art with norepinephrine (NE, 10−6mol/L) in the bath for 5 min. Next the bath solution was switched to NE plus testosterone and the diameter of the Af-Art was measured at 5 min thereafter. One concentration was tested firstly, and then 10 min was allowed for elution with MEM before using another concentration in the Af-Art. All the vascular responses were measured at the same time point. Ethanol was used as vehicle in control experiments at the concentration of 3.4×10−4 %, equal to the solution containing testosterone of 10−7 mol/L. To further compare the effect of different concentrations of testosterone to Af-Art, we calculated the percentage of testosterone-induced vasodilation with the following formula: Dilation of the Af−Art %=(DiaNE+TES−DiaNE)/DiaNE×100% (Dia, Diameter of Af-Art). To test if the rapid effect of testosterone was mediated by AR, an AR antagonist, flutamide (10−5mol/L), was added in bath to pretreat Af-Art for 15min, and the above experiment was repeated. To determine the role of NO in testosterone-induced vasodilation, a NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 3×10−4mol/L), was added to the bath for 30 min, and then testosterone was added into the bath. The percentage of Af-Art dilation in the present of flutamide or L-NAME was also calculated in the same order as above.
NO measurement with fluorescence
A NO-sensitive fluorescent dye, 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate was used to measure NO production in the Af-Art. DAF-FM (10−5mol/L) was added into the arteriole perfusate and the perfusion was maintained for 40–60 minutes, and then washed off by MEM for 10 min. Thereafter, NE and NE plus testosterone (10−8mol/L) were added into the bath. DAF-FM was excited at 495 nm and the emitted fluorescence was recorded at 515 nm. Square-shaped regions of interest (ROIs) were set on the wall of Af-Art and mean intensity of ROIs recorded every 5 seconds and corrected for background. The rate of increase in fluorescence intensity was calculated and NO was measured for 5 min.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was used to determine whether ARs were expressed in Af-Art. Af-Arts (n=10 to 20) were microdissected in ice-cold MEM and transferred into RLT buffer (RNeasy Micro Kit, Bio-Rad) for total RNA extraction. Total RNA were amplified by a Message Sensor RT kit. PCR was performed in a BioRad thermal cycler (Bio-Rad, Hercules, CA). Negative controls were performed by omitting cDNA template from the PCR amplification. Sequence of AR primers were 5’-GCCCGGCAAATCTCAACAACTCAA-3’ (forward primer) and 5’-TTAGGGAAAGGAACGTCGTGAGCA-3’ (reverse primer). β-actin served as a "housekeeping" gene. The mixed samples were heated to 95°C for 5 min and then cycled for 40 cycles at 94°C for 30sec, 59°C for 20sec, 72°C for 30sec. Final extension was 8 min at 72°C. Complimentary DNA was electrophoresed on a 1.5% agarose gel, and stained with ethidium bromide. Images were captured using a VersaDoc image analysis system (Bio-Rad, Hercules, CA).
Statistical analysis
Data were collected as repeated measures over time under different conditions. We tested only the effects of interest, using analysis of variance (ANOVA) for repeated measures and a post-hoc Fisher LSD test or a Student’s paired t-test when appropriate. The changes were considered to be significant if p<0.05. Data were presented as mean ± SEM.
Results
Testosterone dilates Af-Art
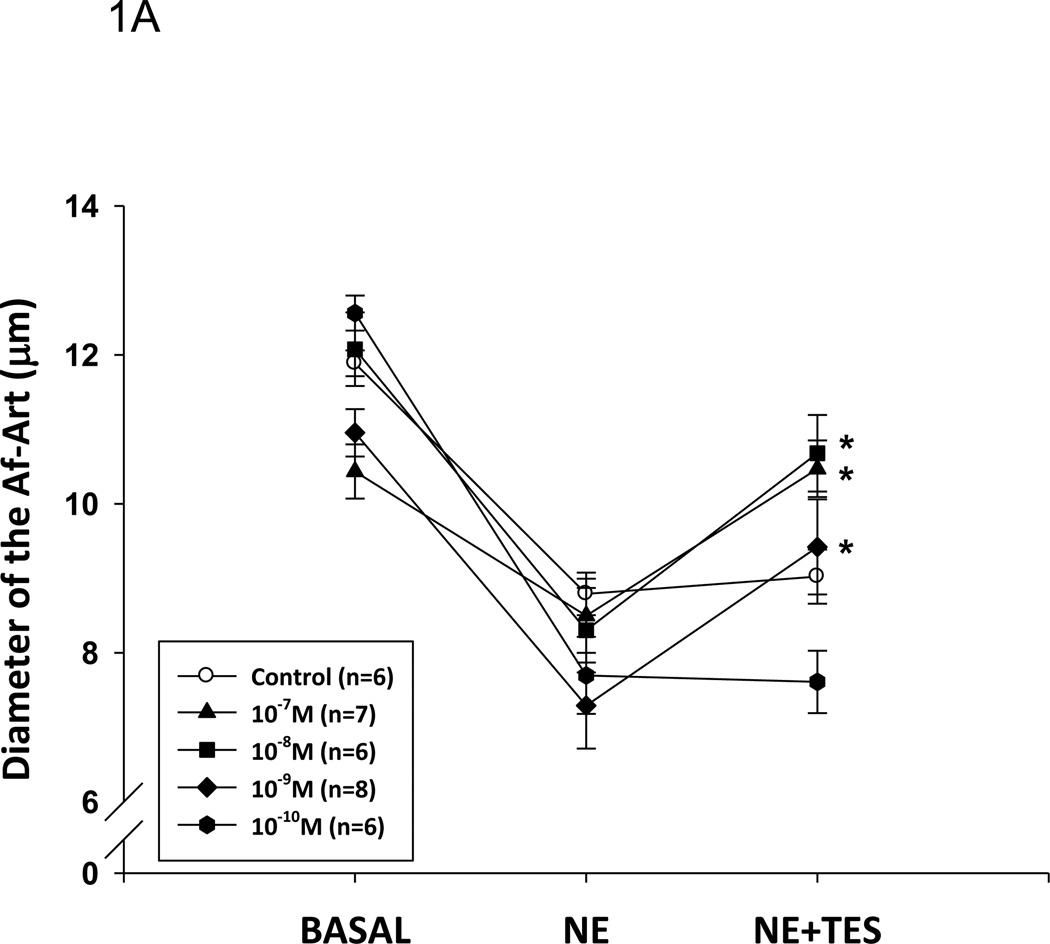
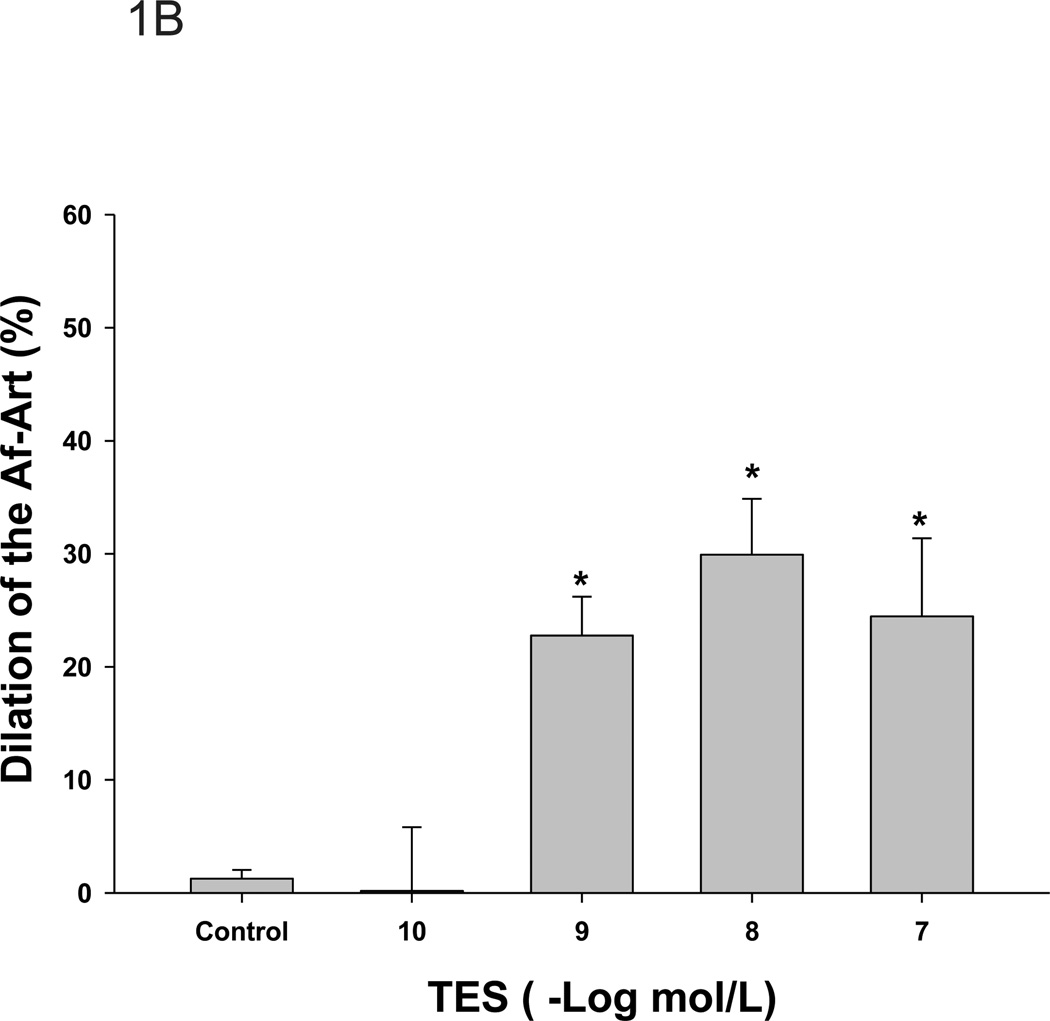
To test the acute effect of testosterone, we added different doses of testosterone to the perfused Af-Art. In non-pre-constricted Af-Art, testosterone had no effect on the Af-Art, indicating that testosterone had no vasoconstrictive effect (from 11.0±0.9um to 12.1±1.2µm, n=4). To test if testosterone had any vasodilatory effect, we pre-constricted the Af-Art with NE and added testosterone, since the microdissected and perfused arterioles lack basal vascular tone. As shown in Figure 1, Af-Art were pre-constricted by about 30%, from 11.6±0.2 to 8.0±0.2 µm, by NE (10−6mol/L) in the bath (aggregate value for all groups; n = 6–8 in each group); testosterone dilated the Af-Art at the concentrations of 10−7 to 10−9 mol/L with a maximum effect at 10−8 mol/L (p<0.001). Testosterone at 10−10mol/L failed to have an effect. All the effects occurred within 5 min after testosterone administration. The vehicle had no constriction or dilatation effect on pre-constricted Af-Art. These data indicate that testosterone causes acute vasodilation of the Af-Art. Testosterone at 10−8mol/L had a significant dilatory effect and is considered to be within the physiological range. Therefore, this concentration was used in the following experiments.
Figure 1. Testosterone dilates the Af-Art.
A. Testosterone (TES) induced a dose-responsive relaxation of preconstricted Af-Art by norepinephrine (NE). Af-Art was dilated 2.1±0.5 µm at 10−7 mol/L; 2.3±0.4 µm at 10−8 mol/L; and 1.9± 0.6 µm at 10−9 mol/L (*p<0.001 v.s. NE). TES at 10−10 mol/L did not induce changes in diameter of the Af-Art. B. The dilatory effect of testosterone expressed in percentage (*p<0.05 v.s. control).
AR are expressed in the Af-Art and involved in the rapid effect of testosterone
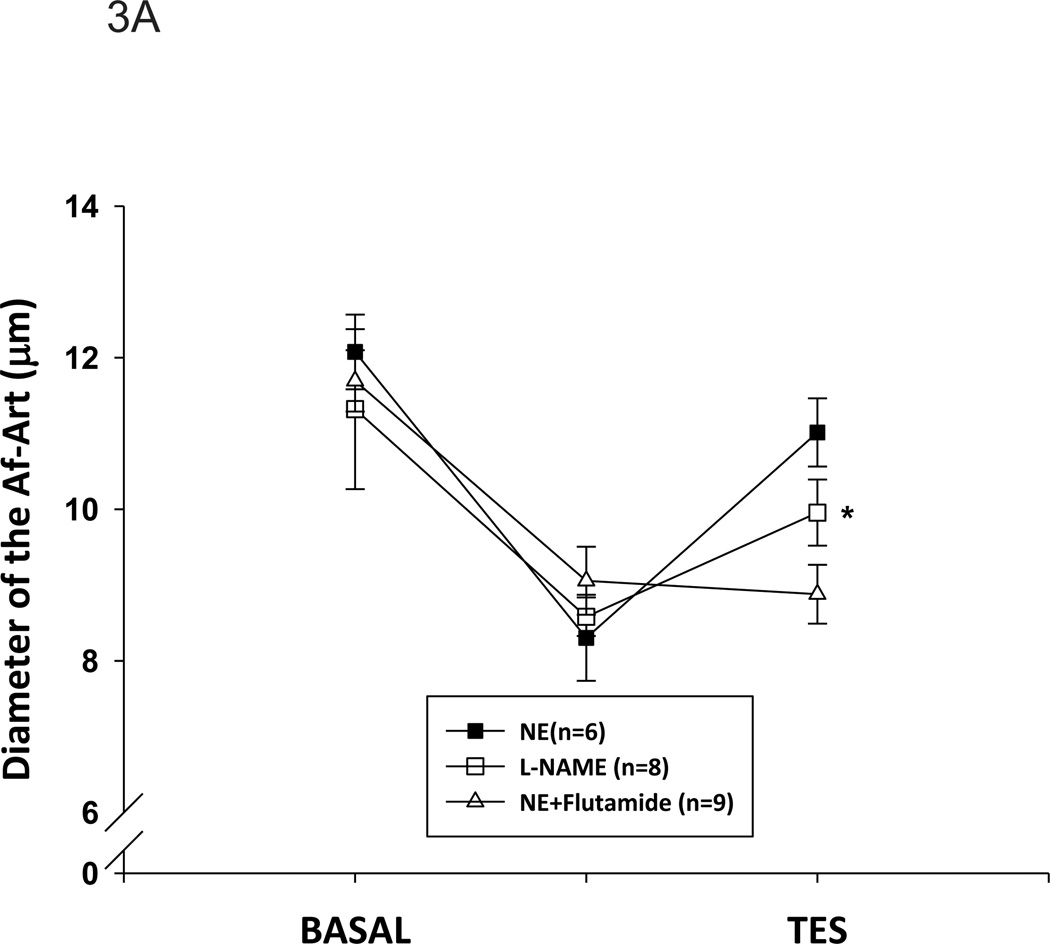
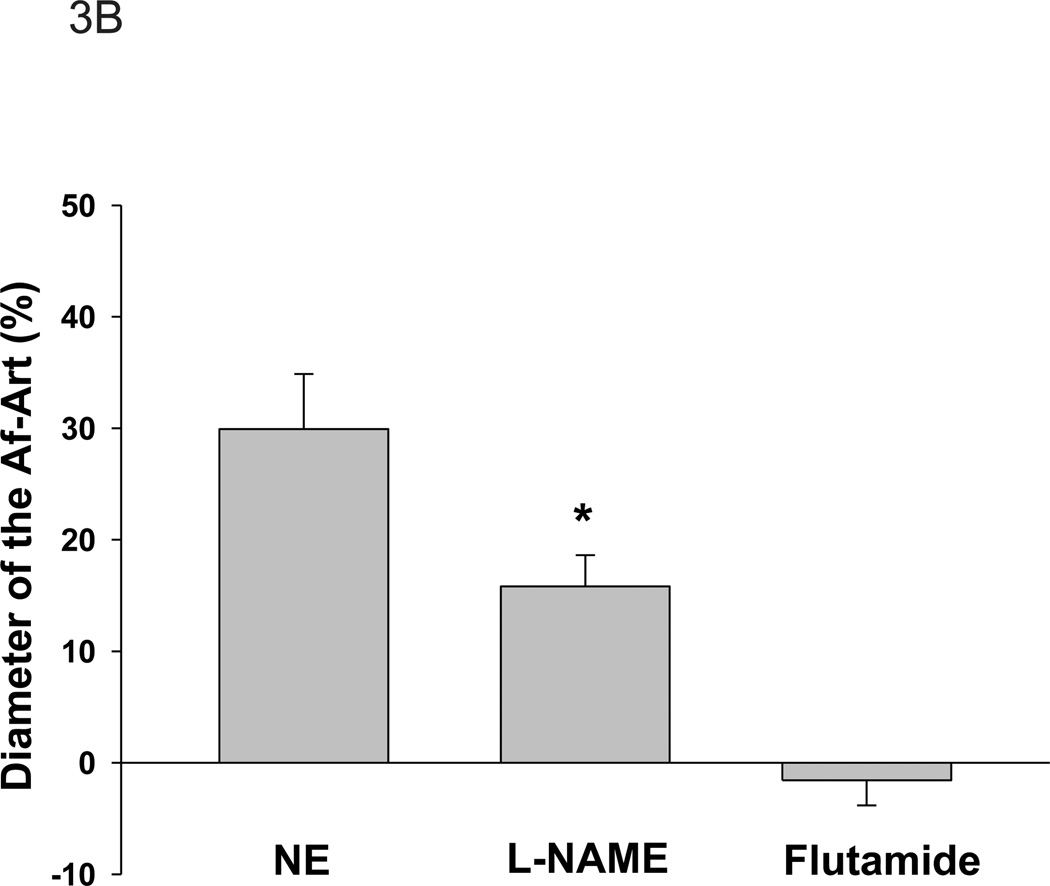
To determine whether AR was involved in the acute effect of testosterone, we first tested whether AR was expressed in mouse Af-Art by RT-PCR. As shown in Figure 2, AR mRNA was found to be present in Af-Art. Next, we tested the effect of testosterone on the Af-Art in the presence of AR antagonist, flutamide, in perfused and pre-constricted Af-Art. Flutamide (10−5mol/L) was added into the bath 15 min before NE administration and was present in the following measurement. As shown in Figure 3A, the diameter of the perfused Af-Art was decreased from 11.7 ± 0.4 to 9.1±0.5 µm by NE. Testosterone (10−8mol/L) caused a vasodilation that was abolished when flutamide was added to the bath (8.9±0.4 µm, n=9). Without flutamide, NE constricted the Af-Art from 12.1 ± 0.5 to 8.3 ± 0.6 µm, and testosterone (10−8 mol/L) dilated it to 10.6 ± 0.5µm (n=6). The dilation percentage was further compared in Figure 3B. These data indicate that the acute dilatory effect of testosterone is mediated by AR that is expressed in the Af-Art.
Figure 2. Androgen receptor mRNA expressed in the Af-Art.
RNA samples from mouse isolated Af-Art were subjected to RT-PCR using the primers to either the AR or β-actin.
Figure 3. Inhibition of androgen receptors or NO reduces testosterone-induced vasorelaxation.
A. After the Af-Art were treated with the androgen receptor antagonist, flutamide (10−5mol/L), testosterone (TES)-induced relaxation of the Af-Art was blocked (n=9). After L-NAME (3×10−4mol/L) was added in the bath, the diameter of Af-Art was decreased from 11.3±1.1 µm to 8.6±0.3 µm. Then testosterone (TES) was added into bath in the presence of L-NAME, the diameter of Af-Art was dilated to 10.0±0.4µm (*p<0.05, v.s. L-NAME; n=8). B. The dilatory effect expressed in percentage. Compared with norepinephrine (NE), in the presence of L-NAME, TES’s dilatory effect was significantly blunted (p<0.05 v.s. TES).
Testosterone enhances NO generation in the Af-Art
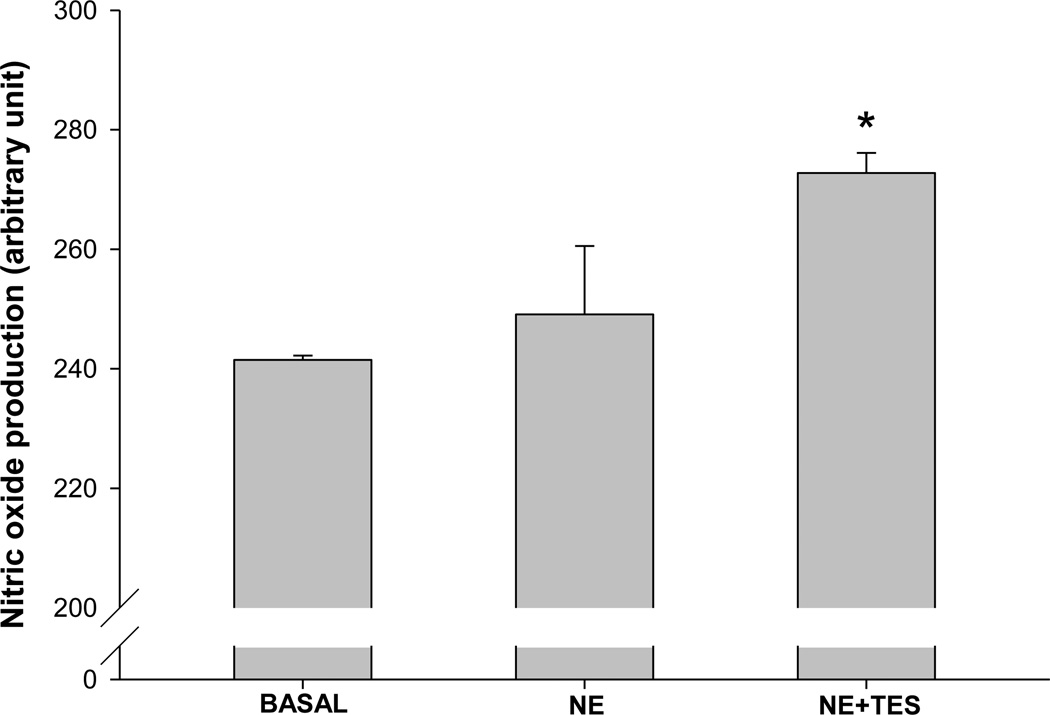
To determine the role of NO in testosterone-induced dilation of the Af-Art, we used fluorescent dye DAF-FM to measure NO production. As shown in Figure 4, after being corrected by background, the basal production of NO was 244.4 ± 10.7 units/min. When NE was added in, NO production was 278.4 ± 12.1 units/min. In the presence of both NE and testosterone, NO production was 351.2 ± 33.1 units/min (p<0.05 vs NE alone; n=3), which increased by 25.7 ± 8.7% compared with NE alone. These data indicated that testosterone rapidly enhanced NO generation in the Af-Art.
Figure 4. Testosterone enhances NO production in the Af-Art measured with DAF-FM.
Comparison of the fluorescent intensity of DAF-FM in isolated perfused Af-Art in basal, norepinephrine (NE) and NE with testosterone (TES). The NE (10−6mol/L) had no significant effect on the NO production. TES (10−8mol/L) increased the NO production significantly (*p<0.05 v.s.NE; n=3).
We then tested whether blockade of NO by inhibition of NOS diminished the effect of testosterone. As shown in Figure 3A, in the presence of L-NAME (3×10−4 mol/L) in the bath, the diameter of Af-Art was decreased from 11.3±1.1 µm to 8.6±0.3 µm. After testosterone was added into bath in the presence of L-NAME, the diameter of Af-Art was 10.0±0.4µm (p<0.05; n=8). The dose of L-NAME has been shown to block the effect of NO in isolated perfused arterioles. 21 Although testosterone still dilated the Af-Art in the presence of L-NAME, the percentage of the dilatation was significantly less compared with NE (p<0.05, Figure 3B), indicating that testosterone induces Af-Art vasodilation, at least, partially via NO.
Discussion
The major finding of the present study is that testosterone acutely dilated the Af-Art. This effect was mediated by the ARs expressed in the Af-Art. Testosterone enhanced NO generation in the Af-Art, while inhibition of NOS attenuated the testosterone-induced vasorelaxation.
The action of testosterone is classically known to be mediated by an intracellular protein, the AR, which belongs to the steroid hormone superfamily. In target tissue testosterone directly binds to the nuclear AR, or is converted to dihydrotestosterone, which also binds the AR. The hormone-receptor complex binds to the androgen response elements, either inducing or suppressing the androgen responsive genes 22, thereby inducing a genomic effect. Testosterone contributed to the exacerbation of hypertension in SHR by reducing pressure-natriuresis,23 and augmented renal vascular responses to angiotensin II in New Zealand genetically hypertensive rats.24 Absence of testosterone prevented endothelial dysfunction and increases in blood pressure secondary to insulin resistance, which involved the Cyp4A/20-HETE system.25 ARs are widely expressed in many tissues besides the reproductive organs. In the kidney, ARs are highly expressed in proximal tubule cells22,cortical collecting ducts26, glomerular endothelial cells and podocytes27. In Af-Art, ARs were found in rats by immunohistochemistry27. In our study, we microdissected the mouse Arf-Art of mice and found the mRNA expression of AR.
In addition to the genomic effects, androgens have rapid, non-genomic effects which occur within minutes in vasculature. Most studies showed a vasodilator effect of testosterone, such as in human umbilical artery28, coronary artery29, rat aorta12, human radial artery30, and internal mammary artery31. Blocking transcription with actinomycin D and translation with cycloheximide does not inhibit testosterone-induced vasodilation13, indicating a non-genomic effect. In contrast, in isolated, perfused rat heart, testosterone (10−10–10−7 mol/L) rapidly blocked the vasodilator effect of adenosine and increased vascular resistance32. The Af-Art is responsible for the predominant pre-glomerular vascular resistance17 which largely determines intraglomerular pressure, glomerular filtration rate, and as such, controls renal hemodynamics and blood pressure. The rapid, direct effect of testosterone on the Af-Art, to our knowledge, has not been investigated. We found testosterone dilated Af-Art within 5 min, which is consistent with other findings on large non-resistant arteries12, 28 and small resistant vessels14, 33. In the present experiments, we performed dose-response studies in the range from 10−10 to 10−7 mol/L, with the lowest effective concentration of 10−9 mol/L, which is considered to be within the physiological range6.
Whether ARs are involved in the non-genomic effect of testosterone is still controversial34. In the present study, AR antagonist flutamide diminished the testosterone induced vasodilation, indicating testosterone’s effect was mediated by AR. In agreement with our findings, Gorczynska and Handelsman found testosterone at higher concentrations (0.3–3×10−3 mol/L) induced a rapid Ca2+ increase, which was blocked by flutaminde in Sertoli cells35; in human prostate cancer cells, dimethylnortestosterone, or 5α-dihydrotestosterone, was shown to increase Ca2+ rapidly, and the effect was blocked by pre-incubation with hydroxyflutamide, suggesting an involvement of the AR36. However, there is some evidence that the testosterone-induced rapid vasodilation was not attenuated by pre-treatment with the AR blocker flutamide11, 12. In addition, in vessels isolated from testicular feminized mice, which lack a functional AR, a testosterone-mediated vasodilation was found 37. These studies do not support the functional role for AR in testosterone’s vasodilating effect. The reasons for above discrepancies are not clear, but may be strain and cell-specific.
Mechanism responsible for the testosterone-induced vasorelaxation have been studied and intensively reviewed6, 7, 34, but the precise mechanism has yet been fully understood. Briefly, the vasodilator effect of Tes is associated with the cell membrane ion channel function in vascular smooth muscle, including the inactivation of L-type voltage-operated Ca2+ channels and the activation of K+ channels. In addition, roles of prostanoids and endothelium derived nitric oxide were also studied. A number of studies showed inhibition of prostanoids by indomethacin did not reduce the vasodilator effect15,38, while Marachelli and colleagues found indomethacin enhanced the relaxant action of testosterone in endothelium-denuded renal arteries18. In endothelium-denuded vessels testosterone (>10 µM) induced vasodilation, but at lower physiological concentrations NO appears to be involved in the vasodilator effect. For example, in human pulmonary arteries from males, only vessels with intact endothelium developed testosterone induced vasodilation within the physiological range (10−9 mol/L), while endothelium-denuded vessels required a concentration as high as 3×10−5 mol/L to show a dilatory response33. Inhibition of NOS with L-NAME significantly inhibited maximal relaxations to testosterone in the rat mesenteric arterial bed14. In our study, we first employed a NO sensitive fluorescent dye to specifically measure the NO production, which showed testosterone enhanced NO generation in the Af-Art. Then we used L-NAME to inhibit NOS activity and found an attenuated effect of testosterone-induced vasorelaxation. However, these data indicate that the acute effect of testosterone is partially NO dependent, since the vasodilatory effect of testosterone was not completely abolished by L-NAME. The other mechanism that mediates testosterone-induced vasodilatation of the Af-Art is to be determined. We feel that metabolites of arachidonic acid are the most possible candidates. Testosterone may either increase dilatory factors like prostaglandins and EETs or decrease constrictive factors like 20-HETE and TXA2.
Conclusions
We found a rapid vasorelaxation effect of testosterone on renal Af-Art, medicated by the AR, and partially involving activation of NOS. The acute effect of testosterone might be beneficial in acute renal injury, when an acute increase in GFR is necessary to abrogate renal ischemia.
Acknowledgements
The authors acknowledge the support of the National Heart, Lung and Blood Institute RO1HL-086767 (to R. Liu) and American Heart Association Postdoctoral Fellowship Award 11POST7750023 (to Y. Fu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 2.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 3.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5 Suppl A:S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000 February;11(2):319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 5.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006 September;21(9):2577–2582. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 6.Perusquia M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1301–H1307. doi: 10.1152/ajpheart.00753.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildiz O, Seyrek M. Vasodilating mechanisms of testosterone. Exp Clin Endocrinol Diabetes. 2007 January;115(1):1–6. doi: 10.1055/s-2007-949657. [DOI] [PubMed] [Google Scholar]
- 8.Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 9.Pugh PJ, Jones TH, Channer KS. Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J. 2003 May;24(10):909–915. doi: 10.1016/s0195-668x(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 10.Malkin CJ, Jones RD, Jones TH, Channer KS. Effect of testosterone on ex vivo vascular reactivity in man. Clin Sci (Lond) 2006 October;111(4):265–274. doi: 10.1042/CS20050354. [DOI] [PubMed] [Google Scholar]
- 11.Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995 February 15;91(4):1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]
- 12.Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol. 2001 December;91(6):2742–2750. doi: 10.1152/jappl.2001.91.6.2742. [DOI] [PubMed] [Google Scholar]
- 13.Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol. 2000 April;129(7):1301–1308. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol. 2002 February;135(3):735–740. doi: 10.1038/sj.bjp.0704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996 November 15;94(10):2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 16.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J Endocrinol Invest. 2002 May;25(5):455–458. doi: 10.1007/BF03344037. [DOI] [PubMed] [Google Scholar]
- 17.Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol. 1990;258:F94–F102. doi: 10.1152/ajprenal.1990.258.1.F94. [DOI] [PubMed] [Google Scholar]
- 18.Marrachelli VG, Miranda FJ, Centeno JM, Burguete MC, Castello-Ruiz M, Jover-Mengual T, Perez AM, Salom JB, Torregrosa G, Alborch E. Mechanisms involved in the relaxant action of testosterone in the renal artery from male normoglycemic and diabetic rabbits. Pharmacol Res. 2010 February;61(2):149–156. doi: 10.1016/j.phrs.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010 June;298(6):H1769–H1775. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int. 2004;66:268–274. doi: 10.1111/j.1523-1755.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Arima S, Juncos LA, Carretero OA. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest. 1993;91:2012–2019. doi: 10.1172/JCI116423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5(2):97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 23.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure- natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Eyster KM, Kost CK, Jr, Kjellsen B, Martin DS. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res. 2010 February 1;85(3):614–621. doi: 10.1093/cvr/cvp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasudevan H, Yuen VG, McNeill JH. Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Mol Cell Biochem. 2012 January;359(1–2):409–418. doi: 10.1007/s11010-011-1035-7. [DOI] [PubMed] [Google Scholar]
- 26.Boulkroun S, Le MC, Blot-Chabaud M, Farman N, Courtois-Coutry N. Expression of androgen receptor and androgen regulation of NDRG2 in the rat renal collecting duct. Pflugers Arch. 2005 November;451(2):388–394. doi: 10.1007/s00424-005-1410-x. [DOI] [PubMed] [Google Scholar]
- 27.Prabhu A, Xu Q, Manigrasso MB, Biswas M, Flynn E, Iliescu R, Lephart ED, Maric C. Expression of aromatase, androgen and estrogen receptors in peripheral target tissues in diabetes. Steroids. 2010 November;75(11):779–787. doi: 10.1016/j.steroids.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perusquia M, Navarrete E, Gonzalez L, Villalon CM. The modulatory role of androgens and progestins in the induction of vasorelaxation in human umbilical artery. Life Sci. 2007 September 1;81(12):993–1002. doi: 10.1016/j.lfs.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol. 2001 October;281(4):H1720–H1727. doi: 10.1152/ajpheart.2001.281.4.H1720. [DOI] [PubMed] [Google Scholar]
- 30.Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci. 2007 March;103(3):309–316. doi: 10.1254/jphs.fp0060883. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz O, Seyrek M, Gul H, Un I, Yildirim V, Ozal E, Uzun M, Bolu E. Testosterone relaxes human internal mammary artery in vitro. J Cardiovasc Pharmacol. 2005 June;45(6):580–585. doi: 10.1097/01.fjc.0000161400.06704.1e. [DOI] [PubMed] [Google Scholar]
- 32.Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yanez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999 May;33(5):691–697. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest. 2009 October;32(9):718–723. doi: 10.1007/BF03346526. [DOI] [PubMed] [Google Scholar]
- 34.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000 December;52(4):513–556. [PubMed] [Google Scholar]
- 35.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995 May;136(5):2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 36.Steinsapir J, Socci R, Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochem Biophys Res Commun. 1991 August 30;179(1):90–96. doi: 10.1016/0006-291x(91)91338-d. [DOI] [PubMed] [Google Scholar]
- 37.Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003 January;148(1):111–120. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- 38.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol. 2006 December;149(8):1083–1091. doi: 10.1038/sj.bjp.0706936. [DOI] [PMC free article] [PubMed] [Google Scholar]