Abstract
The clinical success of non-viral gene delivery reagents is hampered by their inefficient cellular transgene delivery, which is largely influenced by carrier properties that are currently undefined and misunderstood. In an attempt to further define and understand the requirements for a safe and efficient non-viral gene delivery reagent, research labs often engineer and evaluate many putative products with subtle physiochemical differences in order to delineate requirements for improved in vitro and in vivo success. The synthesis of many putative reagents is often time-intensive, laborious and costly. In a previous manuscript published by our lab, different amounts of poly(triethylenetetramine/cystamine bisacrylamide) (p(TETA/CBA) and its pegylated counterpart, poly(triethylenetetramine/cystamine bisacrylamide)- poly(ethylene glycol) (p(TETA/CBA)-g-PEG) were mixed together to easily identify optimal reagent properties and candidates in vitro, while avoiding the synthesis of many putative candidates for study. This report uses the aforementioned facile approach to evaluate reagent properties of products that were obtained via one-pot synthesis, which improved synthetic ease. As such, synthesis time was reduced from 6 days to 3 days and had comparable or improved transfection and viability compared to previous works. Moreover, this synthesis resulted in higher molecular weight products than were used in the previous study and allow for lower polymer doses to be used for complexation, which is useful for systemic delivery that is used herein. The physiochemical properties of the formulations derived using these novel reagents was studied prior to investigating their in vivo biodistribution profiles in a murine colon adenocarcinoma model. Interestingly, negatively charged complexes exhibited greater passive tumor accumulation compared to positively charged complexes following their systemic administration. These studies warrant further investigation for the use of negatively charged drug and gene delivery reagents for passive tumor targeting, and they substantiate the use of polycation/PEG-polycation mixtures for facile product evaluation in order to elucidate design and formulation mandates for the clinical success of non-viral gene delivery formulations.
Keywords: Non-viral gene therapy, polycation mixtures/DNA complexes, poly(ethylene glycol), Dynamic Light Scattering (DLS), Red Blood Cell Lysis/aggregation Assay, Biodistribution
INTRODUCTION
Although polycationic gene carriers have been used in a laboratory setting for over a decade, their clinical application has been stalled because clinical requirements have not been fully met. A better understanding of their synthesis, design and implications of carrier structure and function on biological systems is imperative to their advancement from the lab to the clinic. In addition to ensuring gene carrier safety and efficacy, it is important to design carriers that maintain synthetic ease and avoid laborious production methods that may be inefficient so that scale-up occurs more easily and less expensively [1]. Probably the most important pre-clinical requirement for gene carrier success is their in vivo safety, followed by efficiency. Unlike viral methods of gene delivery, non-viral gene carriers are relatively safe. This is because if designed and/or modified carefully they are relatively non-immunogenic. Other advantages of non-viral gene carriers compared to viral delivery is that they can also be synthesized so that they exhibit multifunctional properties, similar to viral capsids, however, more cost-effectively and with production ease [2–4].
Since the dawn of gene delivery, a large number of non-viral polymers and copolymers have been designed, synthesized and tested for their safety and efficacy in vitro and in vivo. Probably the most rigorously studied polycationic gene carriers is the polyethylenimine class. This is because they were among the first carriers to easily condense nucleic acids into nucleic acid/polycation nanoparticles (polyplexes) that sufficiently protected nucleic acid from serum nuclease degradation and maintained relatively high transfection efficiency in multiple cell types. In recent years, however, the synthesis and design of biodegradable polycations have been focused on because non-degradable polycations, such as PEI systems, often exhibit cellular toxicity. This toxicity results from intracellular accumulation of high-molecular weight species that ultimately interfere with intracellular processes that ultimately lead to cell death [3, 5]. Interestingly, low molecular weight PEIs maintain relatively low cellular toxicity compared to high molecular-weight PEI, however also produce low levels of gene delivery and subsequent transgene expression [6–8]. Moreover, biodegradable polycations have gained attention because they can be synthesized as high molecular weight products for improved nucleic acid condensation, protection and transfection efficiency, all-the-while exhibit low cellular toxicity because they can degrade into small molecular weight species and avoid intracellular accumulation and cell perturbation. This is most noteworthy as low molecular PEI can be modified thusly providing for low cytotoxicity and similar or better transfection efficiency [9–13]. Several other noteworthy classes of biodegradable polycations include: hydrolysable poly(®-amino ester)s, disulfide containing and reducible poly(amidoamine)s (SS-PAAs) and poly(amido ethylenimines) (SS-PAEIs). These classes, in general, have provided similar or improved cellular transgene delivery compared to PEIs, however because of their degradability, they have proven to be significantly less toxic [14–17]. Moreover, degradable carriers including cationic methacrylamide-based polymers have been studied in vitro and in vivo using tumor models, and although their molecular weight exceeds the maximum molecular weight to allow efficient glomerular filtration (45 kDA), they maintain efficient renal clearance due to their extracellular degradation [18–20]. Because, these hydrolysable carriers are prone to extracellular release and are thus difficult to use systemically, carriers designed to degrade via disulfide bond reduction possess unique characteristics that may solve those present in vivo. Serum and extracellular spaces possess low reductive capacity in relation to the intracellular cytosolic space. Cellular enzymes are prepared to deal with these complexes as the enzymes are currently employed to maintain cellular homeostasis in defense against reactive oxygen species. As such, once cellular entry of these complexes has been achieved, they are rapidly degraded and provide for superior transfection and viability compared to comparable non-degradable cationic polymers such as bPEI25k [15]. The polycations used in the studies herein are extremely useful disulfidecontaining candidates because their polymerization is extremely simple, and can be pegylated using a one-pot synthesis, with out the need of purifying the polycation products prior to PEG conjugation.
As mentioned previously, preclinical advancement of polycationic gene carriers is their safety and efficacy in vivo. Many previous reports have shown that cationic polyplexes interact with negatively charged macromolecules found in serum and the extracellular space. These interactions are therapeutically unfavorable because they often lead to particle aggregation and reduced efficacy [20, 21]. Poly(ethylene glycol) (PEG) conjugation to various classes of polycations has been employed to overcome this problem and improve carrier performance in vivo. By avoiding this problem, therapeutically active payload can be delivered and the circulation half-life of polyplex is increased, which can be a major advantage to in vivo gene delivery. This is especially true for cancer therapy where the Enhanced Permeability and Retention (EPR) effect may be exploited by long circulation half-lives of macromolecules [22, 23].
Our lab recently demonstrated a facile method to synthesize potential cationic, polymeric and co-polymeric gene carriers that can be formulated in combination with each other as mixtures at varying mass ratios in order to easily alter carrier physiochemical characteristics and identify optimal candidates for in vitro bioactivity and efficacy [24]. In this present work, a one-pot synthetic approach is executed to improve synthesis ease and further evaluate this approach. This study emphasizes and reiterates the feasibility of using formulated mixtures of cationic polymeric and co-polymer mixtures for in vitro evaluations and, more importantly, how this approach can be applied in vivo to study biodistribution characteristic and putative therapeutic efficacy.
EXPERIMENTAL PROCEDURES
Materials
Hyperbranched polyethylenimine (bPEI, Mw 25 000 (bPEI25k)), triethylenetetramine (TETA) and HPLC grade methanol were purchased from Sigma-Aldrich (St. Louis, MO). N,N’-Cystamine bisacrylamide (CBA) was purchased from Polysciences, Inc. (Warrington, PA). Ultrafiltration devices along with regenerated cellulose membranes (10 kDa) were purchased through Millipore Corporation (Billerico, MA). The reporter gene plasmid, pCMVLuc, was previously designed by inserting luciferase cDNA into a pCI plasmid (Promega, Madison, WI) that is driven by the CMV promoter. The resulting plasmid was purified using Maxiprep (Invitrogen, Carlsbad, CA) protocols. Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin streptomycin, trypsin-like enzyme (TrypLE Express), and Dulbecco’s phosphate buffered saline were purchased from Gibco BRL (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories (Logan, UT).
p(TETA/CBA) synthesis
Synthesis of p(TETA/CBA) was performed with minor adaptation of the previously described method [15]. The polymerization reaction, which began with equimolar triethylenetetramine (TETA) and cystamine bisacrylamide monomers (CBA) in 100% HPLC grade methanol was run at 30°C for 12 hr, at which time excess TETA was added to the reaction mixture and the reaction was allowed to run for an additional 24 hr to ensure that the reaction had been terminated. The pH was subsequently adjusted to 7.0 and the polymer was purified by ultrafiltration using a 10kDa regenerated cellulose membrane. p(TETA/CBA) was obtained by lyophilization using a Labconco FreeZone Plus 2.5L system set at −80C and 0.0016mBar for 36 hr. Composition of the polymer was monitored using 1H NMR (400 MHz, D2O). p(TETA/CBA) ™ 2.61 (COCH2CH2NH, 4H), 2.72 (NHCH2CH2S-S, 4H), 2.90–3.21 (COCH2CH2NHCH2CH2,16H), 3.41 (NHCH2CH2S-S, 4H).
p(TETA/CBA)-g-PEG synthesis
The synthesis of p(TETA/CBA)-g-PEG (TCP) is described as a one-batch synthesis that began with the original polymerization reaction conditions described for p(TETA/CBA). However, following 10 hr reaction time methoxy PEG-NHS was added to the reaction mixture and allowed to react for an additional 2 hr before the addition of excess TETA (100%). As mentioned above, the reaction was allowed to proceed for an additional 24 hr before the pH was lowered to 7.0 and the polymer was purified using ultrafiltration (10 kDa) The composition of TCP was also monitored using 1H NMR (400 MHz, D20). p(TETA/CBA)-g-PEG2k (TCP) ™ 2.61 (COCH2CH2NH, 4H), 2.72 (NHCH2CH2S-S, 4H), 2.90–3.21 (COCH2CH2NHCH2CH2,16H), 3.41 (NHCH2CH2S-S, 4H), 3.45–3.7 (CH2CH20, 4H).
Polymer Characteristics
Absolute molecular weight analysis for p(TETA/CBA) and TCP was performed using AKTA/FPLC (Amersham Pharmacia Biotech Inc.) coupled to a light-scattering detector and using the polymer refractive index increment (dn/dc) for each sample. A Superose 6 110/300 GL column was used for polymer sample separation. Poly[N-(2-hydroxypropyl)methacrylamide] (poly(HPMA)) standards were injected onto the column prior to experimental sample analysis in order to ensure the column was clean and functional, but were not used to derive experimental molecular weight values. Experimental and standard polymer samples were dissolved in degassed and filtered (0.2 υm (Nylon, Alltech)) 0.3 M NaOAc, pH 5.4 with 30% (v/v) acetonitrile eluent buffer. The flow rate was set to 0.4 mL/min.
Polyplex Formation
In all cases polyplex was formed with a known amount of pDNA and a corresponding and desired amount of polymer. The two solutions were combined, lightly vortexed and allowed to equilibrate for 30 min. When mixtures of the two polymers were used to form polyplex, a known amount of pDNA was brought up in solution and a corresponding and desired concentration of p(TETA/CBA) and TCP were prepared. The polymer solutions were mixed prior to polyplex formation to generate a desired, relative amount of each species and the resulting mixtures were then added to the pDNA solution.
Gel Retardation Assay
Polymer/pDNA complexation was investigated by generating polyplex prepared at increasing polymer-to-pDNA ((p(TETA/CBA) or TCP) weight-to-weight ratios (w/w) in HEPES buffered solution (20 mM HEPES, pH 7.4, 5% glucose). Polyplexes were formed using 500ng pDNA and a corresponding amount of polymer. The polymer/pDNA mixtures were then allowed to incubate at room temperature for 30 min prior to being loaded on a 1% agarose gel that was stained with ethidium bromide (EtBr, 5µg/mL) at 104 V for 30 min in TAE (40 mM Trisacetate, 1 mM EDTA) buffer. The pDNA was visualized using GelDoc software.
Light Scattering and ζ-Potential Measurements
Polymer/pDNA (polyplex) particle size (diameter) and surface charge were measured on a Zetasizer 2000 instrument and with a dynamic light scattering (DLS) unit on a Malvern 4700 system, respectively. Polyplexes were prepared by mixing equal volume polymer solution (100uL) at increasing concentrations in HEPES buffer (20 mM, pH 7.4, 5% glucose) with a desired concentration of 40ug pDNA (100uL). The polyplexes equilibrated in solution for 30 min and then subsequently diluted using filtered milliQ water to a 2 mL volume for final analysis.
Polyplex Stability in 90 % Fresh Rabbit Serum
Polyplex stability and resulting pDNA stability against nuclease activity in serum was evaluated using 500ng free pDNA as a control and 500ng pDNA complexed with polymer mixtures pre-formed in HEPES buffer. Polyplex formation was carried out by combining equal volume solutions of pDNA and polymer mixtures using a polymer/pDNA at 24 w/w (N/P 50) and allowed to equilibrate for 30 min. Pre-formed polyplex was then diluted in 90% fresh rabbit serum and incubated at 37°C over time. 25 µl aliquots (125 ng pDNA) were taken at each time point and 10 µl stop buffer (250 mM NaCl, 25 mM EDTA, 2% SDS) was added to each. The samples were frozen at −70°C until further analysis. Once the samples were thawed, they were incubated overnight at 60°C to completely dissociate polycation from the pDNA and 2 µl of 50 mM dithiothreitol (DTT) was added to each sample and incubated at 37°C for an additional 30 min to ensure complete decomplexation. Lastly, the samples were loaded onto a 2% agarose gel stained with ethidium bromide (EtBr) and subjected to electrophoresis at 96 V for 30 min in TAE (40 mM Tris-acetate, 1 mM EDTA) buffer. The gel image was viewed using GelDoc software (n=2).
Cell Culture
Mouse colon adenocarcinoma cells (CT-26) (ATCC) were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin at 37°C in a humidified incubator with an atmosphere containing 5% (v/v) CO2. Sub-culturing of cells was performed when cells reached 70–80% confluency.
In Vitro Transgene Expression
Luciferase reporter gene expression in mouse colon adenocarcinoma cells (CT-26) cell was performed in vitro using respective polymer and pCMVLuc plasmid DNA mixtures. Cells were plated in 24-well plates containing 0.5mL media. Once the cells were approximately 70% confluent, polyplexes were prepared using 0.5 µg pDNA and a respective amount of polymer or polymer mixture in HEPES buffer. Polyplexes were allowed to equilibrate for 30 min and the cells were transfected in the presence of serum (with the exception Figure 4a.) by adding 20 µl polyplex (0.5 µg pDNA) to each well for 4 hr before replacing with fresh culture media. The cells remained in the incubator for a total of 48 hr before they were washed with 1ml PBS and treated with cell culture lysis buffer (Promega). Luciferase quantification was performed using a Luciferase assay system (Promega) on a luminometer from Dynex Technologies, Inc. (Chantilly, VA). The amount of protein in the cell lysate was determined using a standard curve of bovine serum albumin (Sigma) and a BCA protein assay kit (Pierce) (n=6).
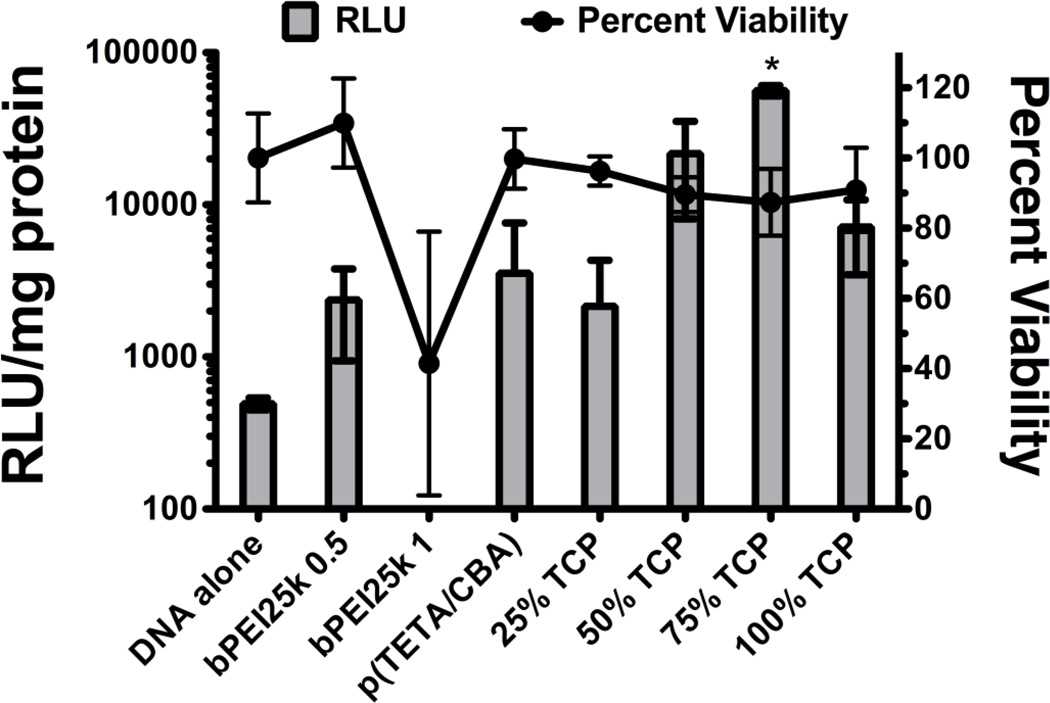
Fig 4.
Transfection efficiency and cell viability for each polymer formulation were assessed in CT-26 cells. bPEI25k was used at 0.5/1 and 1/1 w/w ratio for transfections. The other formulations were used at 12/1 w/w. The RLU/mg protein was not included for bPEI 1/1 due to the significant toxicity observed. Data are represented as mean ± SD, *p < 0.05 for 1-way ANOVA using a Tukey’s post-hoc test (n=6).
Cell Viability Assay
CT-26 cells were plated in 24-well plates and gene transfections were carried out when the cells were approximately 70% confluent. Polyplexes were prepared as they were for the luciferase reporter gene assay. Respective cell cultures were transfected in the presence of serum with the addition of 20 µl equilibrated polyplex in HEPES buffer solution (0.5 µg pDNA) to each well. Cells were left to incubate for a total of 18 hr before analyzing cell viability using an MTT assay (Sigma). Percent cell viability was determined relative to untreated cells (n=6).
Erythrocyte Lysis Assay
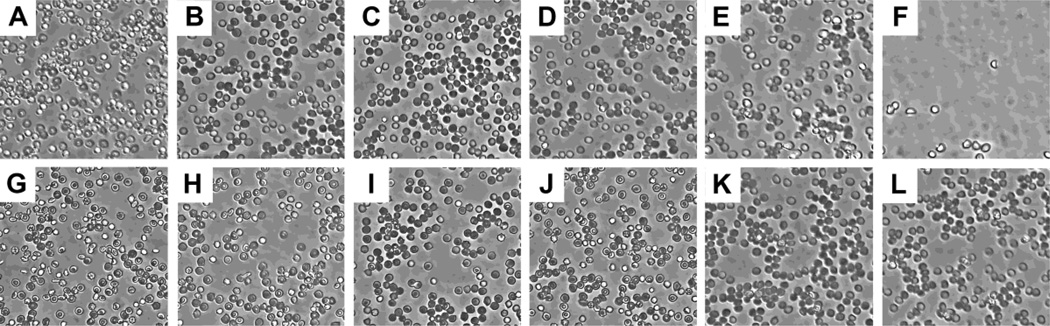
Fresh rabbit erythrocytes were isolated using a ficoll density gradient on a Sorval 6 swing bucket rotor at 900×g for 20 min at room temperature. The supernatant was removed and the Red Blood Cell (RBC) pack was re-suspended in 10ml of 0.5% NaCl and mixed thoroughly by pipetting. The solution was then spun at 600 × g in order to remove any remaining serum proteins. The erythrocytes were then diluted 100x in 20mM HEPES buffer containing 5% glucose. An equal number of cells were plated in each well using a 24-well cell culture dish. Polyplexes were formed as described above at increasing polymer concentrations using 500ng pDNA and added to the culture dish. The study was done in triplicate with representative pictures taken following 4 hr incubation.
Biodistribution study
Mouse colon adenocarcinoma cells (CT-26) were suspended in 100µl sterile PBS and subcutaneously injected (1 × 106 cells) into the right flank of 6-week old, female Balb/C mice. Tumors were allowed to form over 2 weeks or until they reached an average tumor volume around 75–80 mm3. Once sufficient tumors formed, five mice were injected with 40µg of pDNA (7264bp) complexed to 20 or 120µg of polymer at specified weight % formulation(s). The weight-to-weight (w/w) ratios were chosen based on particle size and surface charge. Tissue samples were collected 48 hr following injection of formulation complexes and subsequently homogenized in 1mL protease inhibitor cocktail on a mini bead-beater 96 for 3x at 3 min. A 10µL sample was taken and DNA was isolated using the Wizard SV 96 DNA Purification System. Sample purity was analyzed by UV spectrophotometry using a Nanodrop NM-1000 by 260/280. Taqman primer/probes were designed to amplify a sequence of two adjacent genes within the plasmid to confer specificity. Tissue samples were spiked with serial dilutions of the respective plasmid DNA and amplified using FastStart Universal master mix (w/Rox) and a StepOne Real-Time Thermocycler (Applied Biosystems) to derive a standard curve. The data are represented as percent initial dose (% ID) in each organ at 48 hr.
RESULTS
The objective of the studies herein were to verify the effectiveness of designing a single poly(ethylene glycol) copolymer (TCP) using a simple one-step synthetic approach, which resulted in a polycationic copolymer, TCP, that could be used alone, or in conjunction with the previously synthesized backbone (p(TETA/CBA)10k). If used in combination, gene carrier mixtures can be formulated that maintain different poly(ethylene glycol) (PEG):p(TETA/CBA) amounts. By doing so, efficacy studies are more easily performed to understand the design and formulation requirements that govern ideal non-viral gene carrier properties and their bioactivity in vitro and in vivo. We have previously shown that a graft copolymer of PEG and p(TETA/CBA) (p(TETA/CBA)-g-PEG2k) synthesized by a slightly different method can be used in this manner for similar studies in vitro [24].
Synthesis and Characterization
In order to expand these findings, we synthesized p(TETA/CBA) and TCP separately. 1H NMR confirmed the synthesis of both products and was used to estimate the wt % of PEG in TCP so that accurate wt % formulations and PEG amounts using mixtures of the two potential gene carriers could be evaluated (Data not shown). NMR studies confirmed that a 10 wt % feed ratio of PEG during synthesis resulted in a final conjugation of ~4 wt % PEG following purification. Relative molecular weight analysis using FPLC indicated that similar molecular weight polymers were obtained during synthesis and purification, and that both gene carriers (p(TETA/CBA) and TCP) have narrow polydispersity indexes (PDIs) (Table 1). The degree of branching for p(TETA/CBA) is also in agreement with our previously published result [24].
Table 1.
Characterization of one-pot p(TETA/CBA)-PEG
| Sample | Mn (kDa)a | Mw (kDa)a | PDI (Mw/Mn) | Degreeb Branching |
Relative wt% PEGc |
|---|---|---|---|---|---|
| p(TETA/CBA) | 21.7 | 33.4 | 1.54 | 0.79 | - |
| p(TETA/CBA)-PEG (TCP) | 24.5 | 31 | 1.27 | 0.87 | 4.0 |
Number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity (Mw/Mn) determined using FPLC.
Degree branching was determined by reduction of disulfide bonds with TCEP followed by the protection of free sulfhydryls with NEM and analyzed by MALDI-TOF.
NMR was used to estimate the relative wt% PEG by integrating p(TETA/CBA) and p(TETA/CBA/PEG) peak area under the curve (AUC).
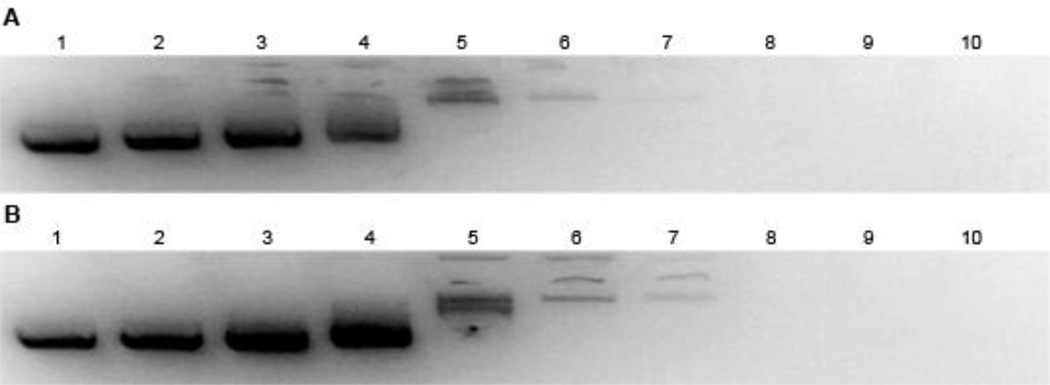
Plasmid DNA (pDNA) complexation using p(TETA/CBA) and TCP were investigated. Results from this study indicate that both gene carriers begin to form interactions with pDNA as polymer concentrations increase. p(TETA/CBA demonstrates partial pDNA complexation at 0.1:1 (p(TETA/CBA)/pDNA) w/w and complete polyplex formation as polymer concentrations are increased to reach 6:1 w/w where no migration or visualization of pDNA is seen (Figure 1a.). Similar complexation is seen using TCP, where complexation of pDNA using TCP begins around 0.2:1 w/w and complete pDNA encapsulation is achieved at 6:1 w/w (Figure 1b).
Fig. 1.
Polymer/pDNA complexation was assessed using a Gel Retardation assay. An equal volume solution of a) p(TETA/CBA) or b) mixture of p(TETA/CBA)/TCP (75% TCP) was added to 500ng pDNA in HEPES buffered saline and allowed to incubate for 30 min before being loaded on a 1% agarose gel, stained with EtBr, and then run for 30 min in TAE buffer. Total polymer amounts in solution used for both experiments (a and b) were 1) 0.0µg 2) 0.025µg 3) 0.05µg 4) 0.1µg 5) 0.5µg 6) 1µg 7) 2µg 8) 3µg 9) 6µg 10) 8µg. Gel images were obtained using GelDoc Software.
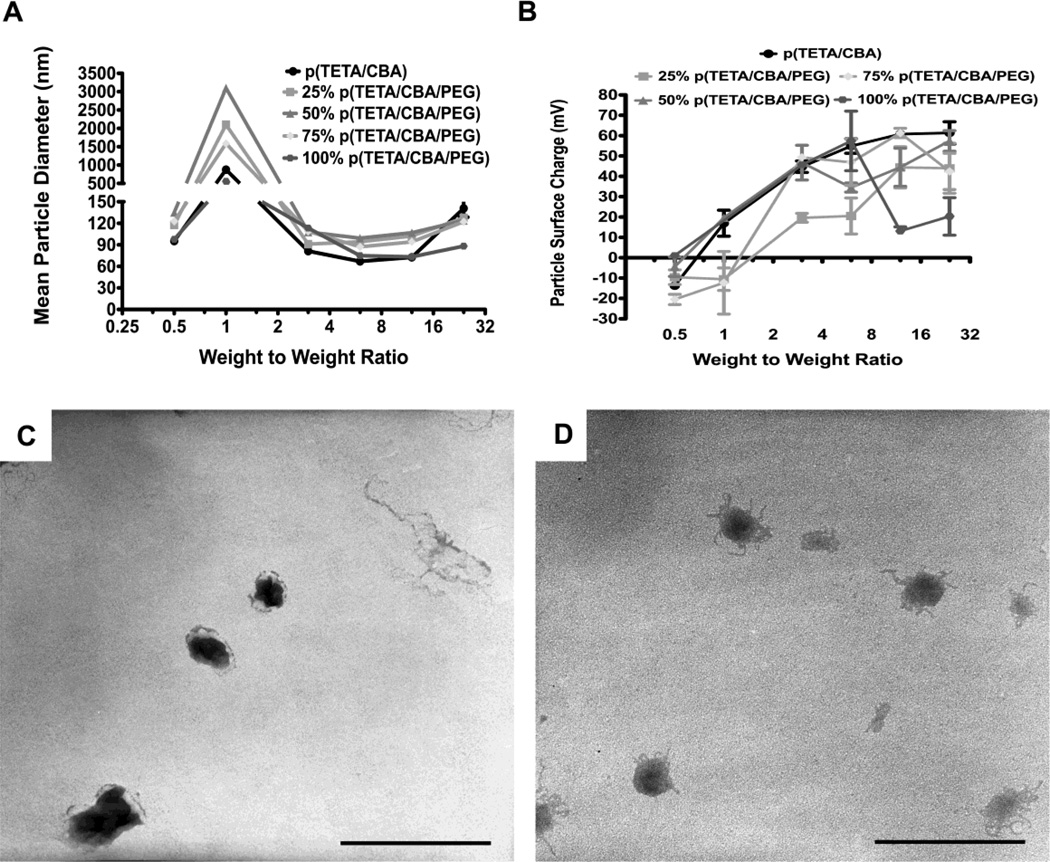
Particle size and surface charge
Given the aforementioned influence of PEG on polyplex formation using TCP alone, mean particle diameter and surface charge were investigated using mixtures of the two gene carriers, in addition to evaluating their individual physiochemical properties using DLS. TEM images were also taken for all formulations and two representative images are shown (Figure 2c,d). Both gene carriers and the mixture formulations (25, 50 and 75% TCP/p(TETA/CBA)) formed polyplex at or below 100nm when a w/w ratio equal to 3:1 and above are used. A dramatic increase in particle size is seen at w/w ratios equal to 1:1 regardless of the formulation mixture, indicating that complete pDNA/polymer complexation does not occur at or below this ratio and inter-complex aggregation may occur at 1:1 w/w due to loosely packed complexes and close to neutral charge (Figure 2a). When particle surface charge was evaluated for each formulation, positive polyplex was formed at 3:1 w/w (Figure 2b.), further indicating that complete complexation occurs at this ratio. Interestingly, at 12:1 w/w ratio for 100% TCP, a dramatic decrease in surface charge was consistently observed. At this polycation concentration complete pDNA encapsulation has occurred and excess polycation exists in solution. These polycations contain PEG chains, and it is possible that there is chain entanglement between excess polymer and polyplex, resulting in a conformational shift in polyplex such that pDNA is exposed and decreases the overall observed surface charge. The same trend was also observed for 75% TCP at 24:1 w/w, which also has a relatively high number of PEG chains.
Fig. 2.
Light Scattering and ζ-Potential Measurements were performed to determine polymer/pDNA (polyplex) particle size (diameter) of putative gene carrier formulations (A) and surface charge (B) at increasing polymer concentrations, indicated by polymer/pDNA weight-to-weight ratios on the graphs. TEM images were taken in order to visualize polyplex and 100% 0.5:1 w/w (C) and 100% 3:1 w/w (D) are shown as representative images. Scale bar equals 200nm.
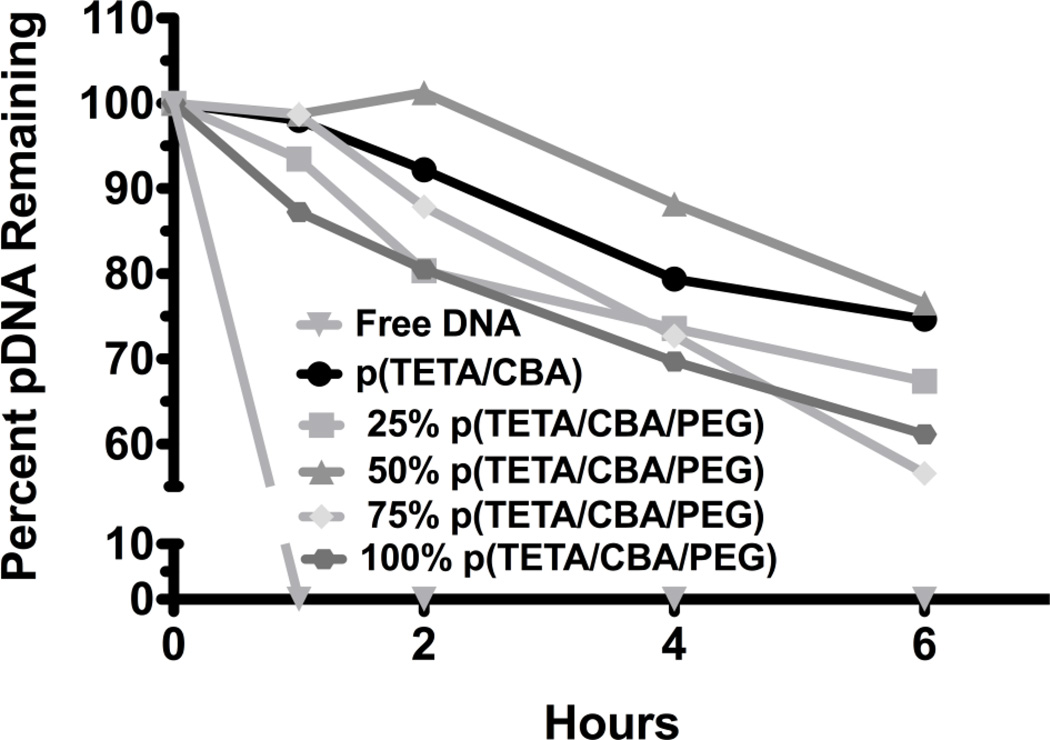
The ability of each polymer formulation to condense and protect pDNA from serum nuclease degradation was evaluated. Following polyplex formation using each polymer formulation at 24:1 w/w, the samples were added to fresh rabbit serum and allowed to incubate at 37°C for 6 hr. All formulations significantly enhance nucleic acid protection from serum nuclease degradation when compared to free pDNA (Figure 3). Increasing amounts of TCP correspond with lower protective effects in serum, although 100% TCP protected 70% of DNA over 6 hr.
Fig 3.
Polymer protection of pDNA from serum nuclease degradation over time. Free pDNA or polymer/pDNA mixtures at increasing polymer concentrations were incubated in 90% fresh rabbit serum. Samples were taken at 0, 1, 2, 4 and 6 hr and evaluated for intact pDNA using gel electrophoresis (1% agarose). Percent intact pDNA was normalized to the amount of intact free (unprotected) pDNA at 0 hrs. using GelDoc software.
Bioactivity and Bioavailability of Formulations
The bioactivity of each formulation was assessed in the mouse colon adenocarcinoma cell line CT-26. To do so, luciferase transgene expression and cell viability were evaluated using each polymer mixture formulation (0, 25, 50 and 75% TCP/p(TETA/CBA)). When p(TETA/CBA) was used alone for transfection, it provided similar transgene delivery to control bPEI25k at 0.5:1 w/w (Figure 4). The transfection efficiency for bPEI25k when performed using 1 w/w was not included because this condition exhibited significant cell toxicity and accurate transgene expression could not be determined for this treatment. The p(TETA/CBA) and TCP formulations exhibited no cellular toxicity even when used at 12:1 w/w, which exemplifies its biocompatibility in this cell line. Moreover, the TCP/TC formulations showed comparable, or greater, transgene delivery and expression compared to the relevant bPEI25k control used at 0.5:1 w/w (Figure 4). More importantly, the 75% TCP reagent provided significantly better transfection than the other formulations when compared to the bPEI 25kDa control (Figure 4).
In order to ensure that the formulations were biocompatible with red blood cells (RBCs) prior to systemic delivery, RBC lysis assay using fresh Erythrocytes was performed. Polyplexes were prepared at various polymer/pDNA w/w ratios and administered to each well containing Erythrocytes. Cells were visualized following 4hr incubation. Increasing concentrations of p(TETA/CBA), but not TCP, correlated with RBC aggregation and lysis (Figure 5). Nearly 85% of the erythrocytes had been lysed by p(TETA/CBA) treatments around 6 w/w over the 4 hr incubation period, whereas, TCP treatments at the same concentration had no noticeable lysis and/or increase in cell aggregation. This indicates the improved biocompatibility with RBC of TCP, which contains PEG chains. Most importantly, however, both p(TETA/CBA) and TCP had no noticeable effect on RBC lysis or aggregation at concentrations above 3:1 w/w, which is the highest w/w ratio used for subsequent in vivo study (Figure 5d,j).
Fig 5.
The erythrocyte lysis assay demonstrates that Red Blood Cell (RBC) lysis and aggregation occur at increasing concentrations of TC (A–F); whereas high concentrations of TCP do not correlate with RBC lysis. Polymer concentrations are as follows: A and G, 0.277µg; B and H, 0.415 µg; C and I, 0.83µg; D and J, 1.66 µg; E and K, 2.5µg; F and L, 3.3µg.
Biodistribution Studies
The biodistribution of 25, 50, 75 and 100% TCP/p(TETA/CBA) formulations following systemic administration via tail vein in a murine adenocarcinoma model was studied,. For this study p(TETA/CBA), alone, was not evaluated because significant RBC lysis and aggregation was observed for this formulation (Figure 5) and previous studies have demonstrated the importance of PEG modification of polycations used for systemic administration [2, 25]. As such, formulation complexes derived using 0.5:1 and 3:1 w/w were systemically injected into tumor bearing mice that possess negative and positive surface charges, respectively, and maintain nanosized complexes (85–120 nm).
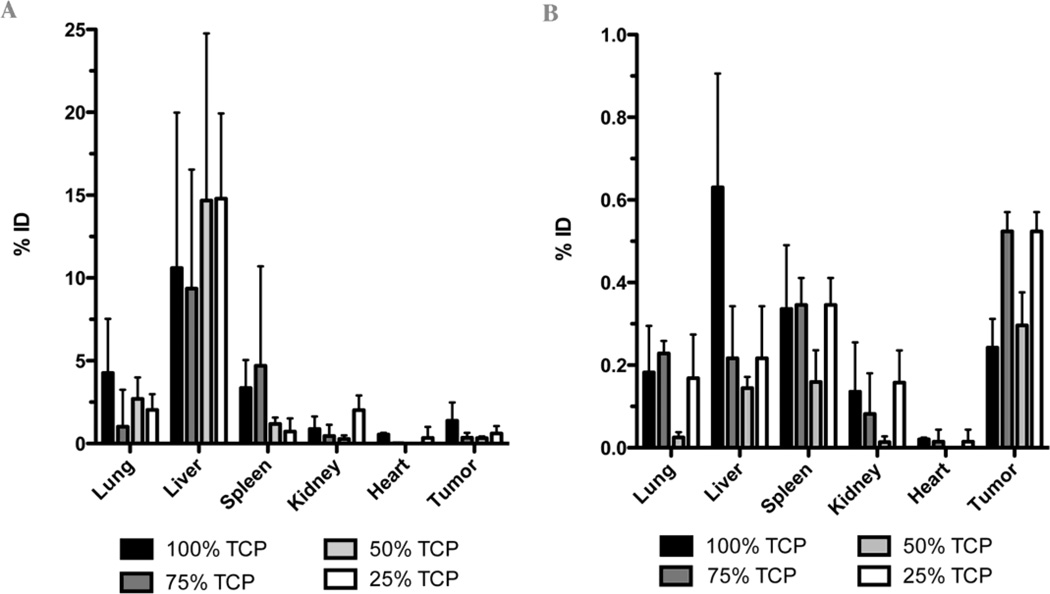
Organ accumulation values determined for lung, liver, spleen kidney, heart and tumor after each respective organ was removed from the mice 48 hr post-injection are shown in Table 2. qPCR evaluation of the injected pDNA at 48 hrs. post-injection indicated that positively charged complexes formed at 3:1 w/w predominantly accumulated in the liver (9.35 ± 7.19 to 14.78 ± 5.14% of injected dose), whereas negatively charged complexes had similar payload deposition between organs with the exception of heart, which possessed nearly no pDNA accumulation. 100 and 75% TCP formulation used at 3:1 w/w exhibited lower liver accumulation but relatively higher spleen accumulation compared to the 50 and 25% TCP, which may be due to their relative size and surface charge. The percent injected dose (% ID) values for the negatively charged complexes were markedly lower than those values obtained for the positively charged complexes, which indicates decreased encapsulation and/or protection of pDNA at 0.5 w/w compared to 3: w/w during systemic circulation. Interestingly, however, negatively charged complexes exhibited greater passive tumor accumulation compared to the positively charged complexes. Moreover, despite the reduced pDNA protection of the negatively charged complexes compared to positively charged complexes in vivo, the % ID values at the tumor site were similar between the two. By achieving similar payload delivery to the tumor site using complexes formed using 6x less polycation would be an advantage in cancer therapy by avoiding putative dose-limiting polymer toxicities.
Table 2.
Biodistribution p(TETA/CBA)-PEG
| Formulation | w/w | Lung | Liver | Spleen | Kidney | Heart | Tumor |
|---|---|---|---|---|---|---|---|
| 25% TCP | 3 | 2.03 ± 0.94 | 14.78 ± 5.14 | 0.72 ± 0.78 | 2.02 ± 0.88 | 0.33 ± 0.67 | 0.61 ± 0.44 |
| 50% TCP | 3 | 2.69 ± 1.30 | 14.67 ± 10.08 | 1.18 ± 0.39 | 0.27 ± 0.21 | 0.01 ± 0.00 | 0.32 ± 0.11 |
| 75% TCP | 3 | 1.01 ± 2.23 | 9.35 ± 7.19 | 4.69 ± 5.99 | 0.46 ± 0.66 | 0.02 ± 0.00 | 0.35 ± 0.28 |
| 100% TCP | 3 | 4.26 ± 3.28 | 10.59 ± 9.38 | 3.35 ± 1.69 | 0.88 ± 0.75 | 0.54 ± 0.99 | 1.37 ± 1.09 |
| 25% TCP | 0.5 | 0.17 ± 0.10 | 0.22 ± 0.12 | 0.35 ± 0.07 | 0.16 ± 0.08 | 0.01 ± 0.02 | 0.52 ± 0.03 |
| 50% TCP | 0.5 | 0.03 ± 0.01 | 0.14 ± 0.03 | 0.16 ± 0.07 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.30 ± 0.08 |
| 75% TCP | 0.5 | 0.23 ± 0.03 | 0.22 ± 0.13 | 0.35 ± 0.06 | 0.08 ± 0.09 | 0.01 ± 0.02 | 0.52 ± 0.05 |
| 100% TCP | 0.5 | 0.18 ± 0.11 | 0.63 ± 0.27 | 0.34 ± 0.15 | 0.14 ± 0.11 | 0.02 ± 0.00 | 0.24 ± 0.07 |
Values are shown as percent injected dose (% ID) 48 hr post-injection and represent the mean ± standard deviation (mean ± SD; n=5).
DISCUSSION
In the present work, a one-pot synthesis is employed in order to generate a pegylated p(TETA/CBA) (TCP) that can be used in conjunction with the polycationic backbone polymer, p(TETA/CBA). By formulating mixtures of the two species, the relative amount of each polymer can be easily altered to influence gene carrier characteristics and evaluate potential effects on carrier properties, bioactivity and biodistribution. Using this facile approach, changes in gene carrier properties are more easily studied and the identification of optimal candidates is expedited.
Pegylation of p(TETA/CBA) mitigated complexation of pDNA with the polycationic gene carrier reagent (Figure 1). This finding is in agreement with previous reports, which showed that complexation capacity is influenced by the amount of PEG that is chemically introduced to the polycationic carrier and/or the length of the PEG chain, as indicated by differences in complex size and stability [24]. DLS studies herein, in conjunction with previous studies published by our lab corroborate these findings, as particle size and complexation correlate with the amount of PEG, in each formulation mixture [26]. This present report indicates that complexes are formed with complex sizes raging between 100–120 nm in diameter for the formulations tested when complexed were generated at 3:1 w/w. Interestingly, similar unimodal particle sizes (90–140 nm) that confer a net-negative surface charge are formed at 0.5:1 w/w. A marked increase in complex size was seen at 1:1 w/w, which indicates that pDNA condensation is not complete below 3:1 w/w and the aforementioned complexes derived at 0.5:1 w/w do not fully encapsulate p(DNA) payload.
In vitro bioactivity was evaluated for p(TETA/CBA) and the respective TCP formulations, which include 25, 50, 75 and 100% by weight of the latter species. Significant cell toxicity is seen using bPEI 25 kDa at 1:1 w/w, while the bioreducible polymer formulations comprised of p(TETA/CBA) and TCP imposed no cellular toxicity at 12:1 w/w. The amount of polycation used in the p(TETA/CBA) and TCP formulations is significantly greater than that used for bPEI controls, thus exemplifying the advantage of using a degradable gene carrier system with respect to biocompatibility and cell toxicity. Mixture formulations of TCP/p(TETA/CBA) are able to deliver viable transgene to CT-26 cells as well or better than the bPEI25k control at 0.5:1 w/w. Cells treated with 75% TCP/pDNA showed a statistically significant increase in transgene expression compared to the bPEI25k control. This formulation exhibited good complexation characteristics with pDNA and due to the amount of PEG incorporated in this formulation likely possesses exceptional steric repulsion of serum proteins in the transfection media to avoid their adverse affect on transfection [27]. All the while, this reagent’s positive surface charge assisted in cell association of complexes and their subsequent endocytosis. The 100% TCP formulation may have provided less transgene expression than the 75% TCP formulation despite sufficient PEG present to sterically reduce serum protein adsorption because of the marked decrease in surface charge seen at this w/w ratio (Figure 2). This formulation also exhibited reduced stability and pDNA protection serum during the initial hours of incubation. Increasing amounts of TCP in polyplex formulations corresponded with lower protective effects in serum over 6 hrs, and strong effects in the first two hours, which may be explained by reduced nucleic acid condensation (Figure 3).
The ability of p(TETA/CBA) and TCP to induce RBC lysis was investigated prior to evaluating the biodistribution profiles of the polymer formulations. Noticeably, increasing concentrations of p(TETA/CBA), and not TCP, induced erythrocyte lysis and aggregation. The difference in the behavior of these two treatment groups may be explained by their respective ability to disrupt RBC membrane stability, where cell membrane disruption via intercalation of polycationic chains is reduced by the presence of PEG chains in the TCP reagent. This reaction is also dependent upon PEG size as well [28]. In addition, oxidant strength has shown to influence RBC viability [29]. As such, another plausible explanation for this observed difference is that TCP exhibits less oxidative stress to the RBC because of the presence of PEG and its steric effect on enzymatic degradation by intracellular reductive enzymes.
A biodistribution study was performed using a murine colon adenocarcinoma model in order to examine the potential differences in organ distribution of pDNA following systemic administration using each gene carrier formulation (25, 50, 75 and 100% TCP) in vivo. Injection of positively and negatively charged complexes was also investigated in order to study potential differences of their biodistribution profiles. Each aforementioned gene carrier formulation was administered using 0.5 or 3:1 w/w, which derives negatively or positively charged complexes, respectively (Figure 2b). Results from this study indicate that all TCP formulations administered at 0.5:1 w/w exhibit significantly lower % ID values in each organ compared to the formulation complexes derived using 3:1 w/w. The reduced % ID values determined in each organ following systemic delivery of the negatively charged complexes are explained by the insufficient protection of pDNA payload from serum nuclease degradation during circulation. Interestingly however, the negatively charged complexes showed greater passive tumor accumulation than positively charged complexes. Moreover, those complexes formed at 0.5:1 w/w that possessed the greatest negative surface charge, 75% TCP (−20 ± 2.5 mV) and 25% TCP (−9.8 ± 3.5 mV), exhibited greater tumor accumulation than 100% TCP (1.1 ± 0.9 mV) and 50% TCP (−4.3 ± 3.8 mV) at 0.5:1 w/w, which is irrespective of PEG content. A similar result was found in a previous study and may be explained by the ability of negatively charged complexes to avoid interaction with glycosaminoglycans (GAGs) present at relatively high levels in the tumor environment. By avoiding interactions with GAGs, these complexes can penetrate tumors more effectively than complexes with a high positive surface charge that often adsorb to GAGs, which leads to their destabilization and tumor delivery [30, 31]. Taken together, these results suggest potential advantages of negatively charged gene and drug delivery reagents for passive tumor targeting and warrant further investigation using polycations or other polymeric delivery reagents. The system recently developed by Park et al. may conform to this and perform admirably, as it releases the amphiphilic structure and still remains complexed [32]. Unfortunately, this was an intratumoral injection, so a direct comparison cannot be made. It was thought that the reducible linkages within the TCP would allow for release of the PEG structures without significantly compromising structural integrity of the complex. The difference between incorporating the PEG with its own reductive bond and the current conjugation scheme was thought to be insignificant, especially in that the complexes were of mixed species. The biodistribution pattern suggests otherwise. Further investigations into reductive release of PEG are warranted.
The biodistribution profile of positively charged complexes demonstrated a high degree of liver accumulation compared to the other major organs that were evaluated. This finding is similar to other previously published results using pegylated polycations as gene delivery reagents [33]. Moreover, positively charged complexes formed using 100 and 75% TCP formulations demonstrated reduced liver deposition compared to 50 and 25% TCP formulations, with an inverse relationship toward their accumulation in the spleen. A correlation between complex physiochemical characteristics and this difference in organ deposition does not exist. However 25% TCP complexes formed at 3:1 w/w did provide the lowest complex size and surface charge compared to the other formulations, which correlated with relatively high liver deposition and significantly low spleen accumulation. This distribution profile suggests potentially low interaction of these complexes with serum proteins and resultant evasion of the reticuloendothelial system (RES), which would mitigate their accumulation in the spleen as seen. Moreover, the relatively small size of these complexes may facilitate their extravasation through liver endothelial fenestrae, as extravasation of nanoparticles is size dependent [34]. While this explanation may explain the observed organ distribution profile, more rigorous studies are required to assert this putative correlation, as the aforementioned differences are subtle and the correlation is not definitive. Nonetheless, these studies demonstrate that mixtures of a synthesized polycation and its pegylated counterpart can be formulated together in order to easily alter and control the relative amount of each species in a gene delivery reagent formulation and delineate changes in gene carrier properties and influence on bioactivity and biodistribution. This facile evaluation method allows researchers utilizing PEG-modified delivery reagents to easily manipulate the amount of PEG in a formulation and avoid the need to synthesize many putative reagents containing various amount of PEG in order to identify the best candidate.
CONCLUSION
The clinical application of polycationic gene carriers is impeded by un-defined design and formulation requirements that optimize safety and efficacy in vitro and in vivo. The work herein demonstrates that a copolymer comprised of polyethylene glycol (PEG) and a branched SSPAEI, p(TETA/CBA) can be easily synthesized and used in combination with the polycationic alone, p(TETA/CBA), in order to easily formulate putative polymeric gene delivery reagents mixtures that possess different physiochemical properties and function in order to identify optimal properties or delivery reagents. By using mixtures of a polycation and modified polycation, the synthesis of many putative gene delivery reagents is avoided, which to date, have been required to study reagent characteristics on biological activity in order to identify products that are functionally viable for in vitro and in vivo use. Most importantly, this approach may be applied to other many polycation-PEG preparations and biodistribution results suggest potential advantages of negatively charged, nano-sized delivery reagents for passive tumor targeting compared to positively charged reagents.
Fig. 6.
Biodistribution of pDNA following systemic injection using 100, 75, 50 and 25% (TCP/TC) formulations as delivery agents in a murine colon adenocarcinoma model. Data are represented as mean ± standard deviation (n = 5) of percent-injected dose (% ID). A) Positively charged complexes formed at 3:1 w/w. B) Negatively charged complexes formed at 0.5:1 w/w.
ACKNOWLEDGMENTS
This work was supported in large part by Samyang Corporation (Korea) as well as NIH grant CA107070. We thank the University of Utah Core Facilities for assistance with microscopy, NMR and Maldi-TOF. We also thank the Jindrich Kopecek lab for the use of their FPLC instrument.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wilkinson M, McConville FX. Is your process scalable? Pharm. Formulation & Quality. 2006;7:24. [Google Scholar]
- 2.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm. Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 3.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc. Natl. Acad. Sci. U S A. 1992;89:7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee P, Reichardt W, Weissleder R, Bogdanov AJ. Novel hyperbranched dendron for gene transfer in vitro and in vivo. Bioconjug. Chem. 2004;15:960–968. doi: 10.1021/bc0342128. [DOI] [PubMed] [Google Scholar]
- 7.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight branched polyethylenimine: effect of molecular weight, on transfection efficiency and cytotoxicity. Pharm. Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 8.Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3:1197–1207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 9.Kang HC, Kang HJ, Bae YH. A reducible polycationic gene vector derived from thiolated low molecular weight branched polyethyleneimine linked by 2-iminothiolane. Biomaterials. 2011;32:1193–1203. doi: 10.1016/j.biomaterials.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Jiang X, Xu L, Wang X, Hennink WE, Zhuo R. Novel reduction-responsive cross-linked polyethylenimine derivatives by click chemistry for nonviral gene delivery. Bioconjug. Chem. 2010;21:1827–1835. doi: 10.1021/bc100191r. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Lee MY, Kim KS, Hahn SK. Target specific tumor treatment by VEGF siRNA complexed with reducible polyethyleneimine-hyaluronic acid conjugate. Biomaterials. 2010;31:5258–5265. doi: 10.1016/j.biomaterials.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Son S, Singha K, Kim WJ. Bioreducible BPEI-SS-PEG-cNGR polymer as a tumor targeted nonviral gene carrier. Biomaterials. 2010;31:6344–6354. doi: 10.1016/j.biomaterials.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Xia W, Wang P, Lin C, Li Z, Gao X, Wang G, Zhao X. Bioreducible polyethylenimine-delivered siRNA targeting human telomerase reverse transcriptase inhibits HepG2 cell growth in vitro and in vivo. J. Control. Release. 2011 doi: 10.1016/j.jconrel.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew. Chem. Int. Ed. Engl. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 15.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjug. Chem. 2006;17:1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 16.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjug. Chem. 2007;18:138–145. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 17.Luten J, Akeroyd N, Funhoff A, Lok MC, Talsma H, Hennink WE. Methacrylamide polymers with hydrolysis-sensitive cationic side groups as degradable gene carriers. Bioconjug. Chem. 2006;17:1077–1084. doi: 10.1021/bc060068p. [DOI] [PubMed] [Google Scholar]
- 18.de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int. J. Pharm. 2007;331:167–175. doi: 10.1016/j.ijpharm.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release. 2008;126:97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Plank C, Mechtler K, Szoka FCJ, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 21.Verbaan FJ, Oussoren C, van Dam IM, Takakura Y, Hashida M, Crommelin DJ, Hennink WE, Storm G. The fate of poly(2-dimethyl amino ethyl)methacrylate-based polyplexes after intravenous administration. Int. J. Pharm. 2001;214:99–101. doi: 10.1016/s0378-5173(00)00642-6. [DOI] [PubMed] [Google Scholar]
- 22.Duncan R. Polymer conjugates for tumour targeting and intracytoplasmic delivery. The EPR effect as a common gateway? Pharm. Sci. Technolo. Today. 1999;2:441–449. doi: 10.1016/s1461-5347(99)00211-4. [DOI] [PubMed] [Google Scholar]
- 23.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, Zhang M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumbach JH, Lin C, Yockman J, Kim WJ, Blevins KS, Engbersen JF, Feijen J, Kim SW. Mixtures of poly(triethylenetetramine/cystamine bisacrylamide) and poly(triethylenetetramine/cystamine bisacrylamide)-g-poly(ethylene glycol) for improved gene delivery. Bioconjug. Chem. 2010;21:1753–1761. doi: 10.1021/bc900522x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer M, Wagner E. pH-responsive shielding of non-viral gene vectors. Expert Opin. Drug. Deliv. 2006;3:563–571. doi: 10.1517/17425247.3.5.563. [DOI] [PubMed] [Google Scholar]
- 26.Mao S, Neu M, Germershaus O, Merkel O, Sitterberg J, Bakowsky U, Kissel T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 27.Lai TC, Kataoka K, Kwon GS. Bioreducible polyether-based pDNA ternary polyplexes: Balancing particle stability and transfection efficiency. Colloids Surf B Biointerfaces. 2011 Sep 22; doi: 10.1016/j.colsurfb.2011.09.026. [Epub ahead of print] PMID: 22000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkel OM, Urbanics R, Bedocs P, Rozsnyay Z, Rosivall L, Toth M, Kissel T, Szebeni J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials. 2011;32:4936–4942. doi: 10.1016/j.biomaterials.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Albright RK, White RP. Red blood cell susceptibility to hydrogen peroxide (H2O2) lysis in chronic hemodialysis patients. Clin. Exp. Dial. Apheresis. 1982;6:223–228. doi: 10.3109/08860228209049855. [DOI] [PubMed] [Google Scholar]
- 30.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 31.Son KK, Tkach D, Hall KJ. Efficient in vivo gene delivery by the negatively charged complexes of cationic liposomes and plasmid DNA. Biochim. Biophys. Acta. 2000;1468:6–10. doi: 10.1016/s0005-2736(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 32.Park K, Hong SW, Hur W, Lee MY, Yang JA, Kim SW, Yoon SK, Hahn SK. Target specific systemic delivery of TGF-beta siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32:4951–4958. doi: 10.1016/j.biomaterials.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Kunath K, von Harpe A, Petersen H, Fischer D, Voigt K, Kissel T, Bickel U. The structure of PEG-modified poly(ethylene imines) influences biodistribution and pharmacokinetics of their complexes with NF-kappaB decoy in mice. Pharm. Res. 2002;19:810–817. doi: 10.1023/a:1016152831963. [DOI] [PubMed] [Google Scholar]
- 34.Snoeys J, Lievens J, Wisse E, Jacobs F, Duimel H, Collen D, Frederik P, De Geest B. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]