Abstract
Exposure to traumatic psychological stress increases risk for disease events and mortality in patients with cardiovascular disease (CVD). While the biological mechanisms of these effects are not known, inflammation may play a key role as it is both elevated by psychological stress and involved in the development and progression of CVD. In a prospective study of patients with stable CVD (n = 979), we examined if higher lifetime trauma exposure was associated with elevated levels of inflammation at baseline and at five-year follow-up, and with greater increases in inflammation over time. Inflammation was indexed by a composite score incorporating the inflammatory markers interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) and resistin. In follow-up analyses, we adjusted for sociodemographic factors, psychiatric disorders and health behaviors that were significantly associated with trauma exposure. Higher trauma exposure was associated with elevated inflammation at baseline (β = .09, p = .01) and at five-year follow-up (β = .09, p = .03). While levels of inflammation increased from baseline to follow-up in the sample, there was no significant association between trauma exposure and rate of change in inflammation. Findings were robust to adjustments for sociodemographic factors and psychiatric disorders, but health behaviors appeared to contribute to the association between trauma and inflammation at follow-up. This is the first large-scale demonstration of an association between lifetime trauma exposure and inflammation. High lifetime exposure to traumatic stress may contribute to an accelerated rate of CVD progression through elevated inflammation.
Keywords: Aging, cardiovascular disease, C-reactive protein, immune system, inflammation, interleukin-6, psychological stress, resistin, traumatic psychological stress, tumor necrosis factor-α
Most people encounter traumatic psychological stressors, involving threat to life or physical integrity, at some point in the lifespan. Such exposures impact both the brain and peripheral bodily systems and increase risk for psychiatric disorders (Brown et al., 2000; Gillespie et al., 2009) as well as for the development and accelerated progression of chronic physical diseases including cardiovascular disease (CVD)(Dong et al., 2004; Glaesmer et al., 2011; Norman et al., 2006; Shedd et al., 2004; Spitzer et al., 2009; Tottenham & Sheridan, 2010). While the pathway from traumatic psychological stress to adverse health outcomes remains poorly understood, there is emerging evidence that inflammation plays a key role.
Both acute and chronic forms of psychological stress have been linked with elevated inflammation (Hansel et al., 2010; Kiecolt-Glaser et al., 2003; Steptoe et al., 2007), and elevations in inflammation in turn have been implicated in the development and progression of many chronic diseases including CVD (Harris et al., 1999; Libby, 2002; Libby et al., 2011; Volpato et al., 2001). To date, however, only a small literature has examined the relationship between exposure to traumatic stress and inflammation, independent of post-traumatic stress disorder (Danese et al., 2007; Dekaris et al., 1993), and no studies have examined this association in patients with CVD, a population for whom inflammation has particular relevance as a promoter of disease progression and a predictor of mortality (Libby, 2006).
A few lines of investigation indicate that traumatic stress exposure is associated with elevated inflammation. First, individuals with post-traumatic stress disorder (PTSD) have been shown to have elevated inflammation in several studies (Spitzer et al., 2010; von Kanel et al., 2010; von Kanel et al., 2007). Second, research has indicated that exposure to specific forms of traumatic stress is associated with elevated inflammation. For example, one large-scale prospective study showed that individuals exposed to two or more forms of severe maltreatment in childhood had significantly higher levels of the systemic inflammatory marker C-reactive protein (CRP) in adulthood (Danese et al., 2007). Other studies have also linked severe adverse early life events with elevated inflammation in adulthood (Carpenter et al., 2010; Kiecolt-Glaser et al., 2011). Moreover, a handful of studies with small samples have examined the impact of specific types of adulthood trauma on inflammation. The results of these studies likewise indicate that exposure to traumatic events such as being a prisoner of war or a victim of intimate partner violence is associated with elevated inflammation (Dekaris et al., 1993; Woods et al., 2005). However, the focus of these studies on the presence or absence of a single type of trauma limits the extent to which conclusions can be drawn about the effects of trauma exposure more generally. Furthermore, to our knowledge, no studies have examined the relationship between cumulative lifetime trauma exposure and inflammation.
There are a number of factors that may contribute to any observed association between traumatic stress and inflammation. First, several sociodemographic factors may predispose individuals to both trauma (e.g., neighborhood violence) and elevated inflammation through exposure to chronic psychological stressors that are known to impact inflammation (e.g., financial strain, discrimination) (Carroll et al., 2011; Morozink et al., 2010; Nazmi & Victora, 2007). Second, in a sizeable minority of cases, exposure to traumatic stress results in post-traumatic stress disorder (PTSD), major depression, or clinically high levels of anxiety (Breslau et al., 1999; Glover et al., 2010; Maes et al., 2000) and all of these disorders have been linked with elevated inflammation in physically healthy individuals (Hoge et al., 2009; Miller et al., 2002; O’Donovan et al., 2010; O’Donovan et al., 2011b), and in some but not all samples of patients with CVD (Ferketich et al., 2005; von Kanel et al., 2010; Whooley et al., 2007). Third, trauma-exposed individuals with and without psychiatric disorders are more likely to engage in potentially harmful behaviors that would increase inflammation (e.g., smoking, substance use) and less likely to engage in healthful behaviors that would reduce inflammation (e.g., physical exercise, medication adherence, sleep) (Bermudez et al., 2002; Cottler et al., 1992; de Assis et al., 2008; Feldner et al., 2007; Llabre & Hadi, 2009; Prather et al., 2009; Shemesh et al., 2004). In the present study of patients with stable CVD, we examined if higher lifetime trauma exposure is associated with elevated levels and a faster rate of increase in inflammation. We also evaluated the role of sociodemographic factors, psychiatric disorders, and health behaviors as contributors to the relationship between lifetime trauma exposure and inflammation.
Methods
Participants
The Heart and Soul Study is a prospective cohort study designed to determine the mechanism of association of psychological factors with risk of CVD events and mortality in patients with stable CVD. Administrative data were used to identify outpatients with documented CVD at two Department of Veterans Affairs Medical Centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System, California), one University medical center (University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had known CVD documented by at least one of the following: a history of myocardial infarction, angiographic evidence of ≥50% stenos is in one of more coronary vessels, prior evidence of inducible ischemia by treadmill or nuclear testing, or a history of coronary revascularization. A total of 1024 participants were enrolled (age range: 45–90 years) and methods have been described previously (Whooley et al., 2008). Five participants were excluded from the present analyses because of missing data on traumatic stress exposure, leaving 1,019 participants of whom an additional 40 were missing data on some inflammatory markers. Thus, our baseline sample for examining the effects of traumatic stress exposure on inflammation was 979. Five-year follow-up data on all inflammatory markers was collected for 626 patients with baseline trauma data and prospective associations are based on this smaller number of participants. This follow-up sample includes 76% of the 829 members of the baseline cohort who were surviving at the time of the follow-up examination.
Procedure
Study participants were instructed not to take aspirin for 1 week, not to eat for 12 hours (except for medications, which they were instructed to take with water), and not to smoke for 5 hours before their study appointments at baseline and at follow-up five years later. If patients had any indicators of recent acute infection, their appointment was rescheduled. At their appointments, participants donated morning blood samples and completed standardized assessments of lifetime trauma exposure, demographic details, psychiatric disorders, and health behaviors and had a thorough clinical review. On the same day, participants underwent resting echocardiogram and exercise treadmill test with a stress echocardiogram.
Methods
Predictor Variable: Traumatic Stress Exposure
Patients reported history of exposure to 18 different types of traumatic events from the Computerized Diagnostic Interview Schedule for the Diagnostic and Statistical Manual-IV (CDIS), a validated computer-based interview, which was administered by trained and calibrated research personnel (Robins et al., 1981). Questions refer to a broad range of traumatic events, which are summarized in Table 1. All responses were coded yes/no, and a cumulative trauma exposure score was calculated (range 0 to 18).
Table 1.
Summary description of items on the lifetime trauma exposure measure
| Item # | Item Description |
|---|---|
| During time in combat: | |
| 1. | Has been held captive/tortured |
| 2. | Has been wounded |
| 3. | Has seen someone seriously injured/killed |
| 4. | Has unexpectedly discovered dead body |
| In non-combat situations: | |
| 5. | Has been shot or stabbed |
| 6. | Has been mugged/threatened with a weapon or robbed |
| 7. | Has been raped/sexually assaulted by a relative |
| 8. | Has been raped/sexually assaulted by a non-relative |
| 9. | Has been in a disaster |
| 10. | Has been exposed to radiation/dioxin/other dangerous materials |
| 11. | Has been experienced an unexpected, sudden death or a close friend/relative |
| 12. | Has been held captive/tortured/kidnapped |
| 13. | Has been diagnosed with a life-threatening illness |
| 14. | Has been in a serious accident |
| 15. | Has seen someone seriously injured/killed |
| 16. | Has unexpectedly discovered a dead body |
| 17. | Learned that any of these terrible things happened to a close friend/relative |
| 18. | Had other terrible experiences |
Outcome Variable: Inflammation
Fasting venous blood samples were collected from the antecubital vein, using a 21-gauge butterfly needle, and placed into serum separator tubes. Serum was then was separated by centrifugation, aliquotedin to individual cryovials and stored at −70° until time of assay. Levels of IL-6, TNF-α, CRP, and resistin were measured in serum derived from these samples at baseline and at five-year follow-up. A Quantikine High Sensitivity Immunoassay kit was used to assess serum concentration of IL-6 (R&D Systems, Minneapolis, MN, USA). The inter-assay CV for the IL-6 assay ranged from 6.5% to 9.6%. The Human Serum Adipokine Panel B LINCO plex Kit (Linco Research, Inc., St. Charles, MO) was used to measure TNF-α. The inter-assay CV for the TNF-α assay ranged from 5.8% to 10.5%. High-sensitivity C-reactive protein levels were measured using the Roche Integra assay (Indianapolis, Indiana) in the first 229 participants and (due to a change in the laboratory) the Beckman Extended Range assay (Galway, Ireland) in the remaining samples. Results from these two assays were highly correlated (r = 0.99 in 185 participants). The Roche Integra hsCRP assay had an inter-assay coefficient of variation (CV) of 3.2%. The Beckman Extended Range hsCRP assay had an inter-assay CV of 6.7%. The Linco Adipokine Panel A multiplex immunoassay was used to measure serum resistin concentrations (Linco Research, Inc., St. Charles, MO). The inter-assay coefficient of variation was 19.7% to 20.4% at a mean resistin level of 8.7 to 8.8 ng/mL. Each sample was assayed in duplicate for all measures, and the average of the 2 measurements was reported. The technicians who performed the assays were blinded to patient characteristics. All of the inflammatory markers IL-6, TNF-α, CRP, and resistin were non-normally distributed and were log transformed to produce a normal distribution.
Demographics
Participants completed standardized questionnaires to determine age, sex, ethnicity, income, and highest education level achieved.
Cardiovascular Disease Severity
Participants underwent resting echocardiography using an Acuson Sequoia Ultrasound System with a 3.5 MHz transducer (Siemens, Mountain View, California). Standard 2-dimensional views were obtained and planimetry with a computerized digitization system was used to determine left-ventricular ejection fraction (LVEF). We defined systolic dysfunction as LVEF ≤ 50%(De Keulenaer & Brutsaert, 2007). Participants also completed a symptom-limited, graded exercise treadmill test according to a Standard Bruce Protocol. Participants were asked to walk on a treadmill beginning at a workload of 20 to 30 watts and increasing by 20 to 30 watts every 3 minutes until reaching dyspnea, symptom-limited fatigue, chest discomfort, or electrocardiographic changes suggestive of ischemia. To achieve maximum heart rate, participants who were unable to continue the Standard Bruce protocol (for orthopedic or other reasons) were switched to slower settings on the treadmill and encouraged to exercise for as long as possible. Total exercise capacity was estimated in metabolic equivalent tasks (METs) achieved. At peak exercise, the development of right or left ventricular dilatation or wall motion abnormalities was detected; inducible ischemia was defined as the presence of new wall motion abnormalities at peak exercise that were not visualized on the baseline rest echocardiogram. Results from all echocardiograms were interpreted by an expert echocardiographer. Methods for these tests have been described in depth elsewhere (Beattie et al., 2003; Gehi et al., 2008).
Comorbidities
Kidney dysfunction was indexed by creatinine clearance and cystatin C levels. Creatinine clearance was assessed from a 24-hour urine sample collected by participants and was calculated using the following formula: urine creatinine (mg/dl) * 24-h urine volume (dl)/serum creatinine (mg/dl) * 1440 (min/d). Serum cystatin C was assessed with a BNII nephelometer (Dade Behring, Inc., Deerfield, IL) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, Inc.). Methods for creatinine clearance and cystatin C assessments have been described elsewhere (Ix et al., 2003). Participants self-reported on their history of diabetes, neurodegenerative disease, cancer and cardiovascular revascularization including coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA) placement.
Medications
Participants were instructed to bring their medication bottles to their appointment, and study personnel recorded all current medications, which were subsequently categorized using Epocrates Rx (San Mateo, California). We examined use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), aspirin, β-blockers, renin-angiotensin inhibitors and corticosteroids.
Psychiatric Diagnoses
Participants were assessed for PTSD, major depression, and anxiety disorders based on criteria outlined in the Diagnostic and Statistical Manual-IV using the CDIS, a validated, standardized psychiatric interview (Robins et al., 1981). For the present study, the CDIS was administered by trained research personnel who attended a four-day CDIS training sessions that included observed interviews to standardize administration. The CIDS has been widely used in epidemiologic studies and has shown good concordance among lay and clinical interviewers (Breslau et al., 1999; Kulka et al., 1988).
Health Behaviors
Standardized questionnaires were used to assess health behaviors, including tobacco use, illicit substance use, alcohol use, physical activity, and medication adherence (Cohen et al., 2009; Whooley et al., 2008). To evaluate illicit substance abuse, participants were asked, “Has a doctor or nurse ever told you that you have drug addiction/abuse?” Alcohol use was measured with the AUDIT-C, a validated screening questionnaire that uses three questions to assess frequency and amount of alcohol use and yields a total score of 0–12. Regular alcohol use was defined as a score of >3 (Bush et al., 1998). Participants were asked to indicate on a 5-point Likert Scale how physically active they had been in the previous month based on their engagement in vigorous physical activity for at least 15 – 20 minutes at a time: not at all; a little (1 – 2 times); fairly (3 – 4 times); quite (1 – 2 times per week); very (3 – 4 times per week); or extremely (more than 5 times per week) and were coded as inactive if they were not at all or a little active. Medication adherence was assessed by asking how often patients took their medications as prescribed in the previous month: all of the time (100%); nearly all of the time (90%); most of the time (75%); about half of the time (50%); or less than half the time (<50%) and were coded as non-adherent if they took their prescribed medications 75% or less of the time (Gehi et al., 2007). Poor sleep quality was defined as a self-rating of very bad or fairly bad sleep quality on a 5-point scale ranging from very bad to very good. BMI was measured by trained technicians and was calculated as weight in kilograms divided by the square of height in meters.
Data Analysis
The sum of log-transformed and z-scored IL-6, TNF-α, CRP and resistin at baseline and follow-up were used as indices of inflammation. Our primary analytic strategy consisted of a series of linear regression models assessing the associations between lifetime trauma exposure and baseline, follow-up, and rate of change over time in, inflammation. We also tested for the presence of non-linear relationships between trauma exposure and inflammatory activity using a linear regression model that includes a quadratic trauma exposure term and by using the “gam” (generalized additive model) module in Stata 12.0, which employs cubic smoothing splines. In secondary analyses, we repeated our linear regression models to examine associations between trauma exposure and the individual inflammatory markers IL-6, TNF-α, CRP and resistin. As appropriate based on associations with lifetime trauma exposure, we adjusted for age, gender, markers of disease severity and comorbidities in the first step of all models and included sociodemographic factors (income, education, ethnicity), psychiatric disorders (PTSD, major depression and generalized anxiety disorder), and health behaviors (BMI, smoking, physical activity, illicit drug use, medication adherence, and sleep quality) sequentially to the models to examine if they accounted for the observed relationship between trauma and inflammation.
T-tests and chi-square tests were used to assess group differences in trauma exposure and Pearson’s correlations were used to examine associations between trauma exposure and potential continuous variables. Paired samples t-tests were used to examine absolute change in the inflammatory index and in the individual inflammatory markers over time. The threshold for statistical significance was set at p< .05 for primary and secondary analyses. However, we set a conservative threshold of p < .10 for the inclusion of covariates associated with lifetime trauma exposure. We included only participants who had complete data for trauma exposure and all inflammatory markers in our analyses. SPSS 18.0 (IBM Inc.) was used for all analyses and Stata 12 was used to create the figures.
Results
Participant Characteristics
Sample characteristics for participants with complete data on trauma and inflammation at baseline (n = 979) and follow-up (n = 626) are displayed in Table 2. At baseline, a large majority of participants in the sample endorsed at least one category of trauma (970/979; 99%). The median number of categories of trauma endorsed was 5 and the range was from 0 to 17 categories. Compared to the group of participants not available at follow-up, those available at follow-up had lower lifetime trauma exposure (t = −2.95, df = 1017, p = .003), younger age (t = −3.17, df= 1017, p = .002), higher exercise capacity (t = 7.74, df = 939, p < .001), less ischemia (t = 3.36, df = 934, p = .001), higher ejection fraction (t = −3.01, df = 1015, p = .003), and lower levels of inflammation (t = 6.34, df = 977, p< .001). However, groups of participants available versus not available at follow-up were not significantly different from one another in gender composition or mean BMI.
Table 2.
Sample characteristics at baseline and follow up
| Baseline | Association with trauma | Follow-up | Association with trauma | |
|---|---|---|---|---|
| N = 979 | p | N = 626 | p | |
| Age M (SD) | 66.72 (10.95) | <.001 | 70.97 (10.21) | .01 |
|
| ||||
| Sex (male) | 797 | <.001 | 514 (82) | .001 |
|
| ||||
| Lifetime Trauma M (SD) | 5.64 (2.78) | -- | 5.48 (2.67) | -- |
|
| ||||
| LVEF ≤50% | 116 (12) | .75 | 85 (14) | .22 |
|
| ||||
| Exercise capacity M (SD) | 7.31 (3.53) | .62 | 6.73(3.02) | .12 |
|
| ||||
| Inducible ischemia M (SD) | 217 (23) | .35 | 150 (24) | .39 |
|
| ||||
| CABG | 349 (36) | .55 | 232 (37) | .99 |
|
| ||||
| PTCA | 381 (40) | .41 | 271 (43) | .18 |
|
| ||||
| Creatinine clearance M (SD) | 81.07 (28.77) | .25 | 70.36 (25.91) | .36 |
|
| ||||
| Cystatin-C M (SD) | 1.20 (.56) | .72 | 1.00 (.46) | .25 |
|
| ||||
| Diabetes | 258 (27) | .41 | 181 (29) | .81 |
|
| ||||
| Arthritis/Gout/Joint Problems | 531 (55) | .004 | 351 (56) | .15 |
|
| ||||
| Cancer | 59 (6) | .34 | 50 (8) | .88 |
|
| ||||
| Neurodegenerative Disease | 7 (1) | <.001 | 9 (1) | .21 |
|
| ||||
| Medications | ||||
| Statins | 630 (66) | .21 | 156 (25) | .03 |
| Aspirin | 757 (79) | .87 | 456 (73) | .79 |
| β-blockers | 563 (59) | .83 | 421 (67) | .96 |
| Renin-angiotensin Inhibitor | 502 (52) | .55 | 409 (65) | .08 |
| Steroids | 22 (2) | .64 | 24 (4) | .97 |
|
| ||||
| Education (< High School) | 300 (31) | .02 | 166 (27) | .08 |
|
| ||||
| Low Income (< $20k/year) | 470 (49) | .20 | 242 (39) | .69 |
|
| ||||
| White | 593 (61) | .004 | 378 (60) | .01 |
|
| ||||
| Tobacco use | 194 (20) | <.001 | 88 (13) | .001 |
|
| ||||
| Poor sleep quality | 272 (28) | .001 | 195 (31) | .003 |
|
| ||||
| Physical inactivity | 358 (37) | .17 | 248 (40) | .10 |
|
| ||||
| Alcohol use | 285 (30) | .86 | 175 (28) | .75 |
|
| ||||
| Illicit drug use | 69 (7) | <.001 | 35 (6) | <.001 |
|
| ||||
| Medication non-adherence | 79 (8) | .33 | 45 (7) | .48 |
|
| ||||
| BMI M (SD) | 28.43 (5.37) | .10 | 28.62 (5.52) | .18 |
|
| ||||
| PTSD | 89 (9) | <.001 | 29 (5) | .007 |
|
| ||||
| Depressive disorder | 216 (23) | <.001 | 66 (11) | .003 |
|
| ||||
| Generalized anxiety disorder | 104 (11) | .02 | 21 (3) | .03 |
|
| ||||
| Interleukin-6 Median (IQR) |
2.62 (1.62–4.20) | .04 | 3.22 (2.17–5.47) | .15 |
|
| ||||
| Tumor necrosis factor-α Median (IQR) |
3.78 (2.56–5.61) | .99 | 5.52 (4.02–7.38) | .42 |
|
| ||||
| C-reactive protein Median (IQR) |
2.27 (.92–4.96) | .08 | 1.40 (.67–3.61) | .74 |
|
| ||||
| Resistin Median (IQR) |
8512.25 (5785.54–12121.52) | .13 | 15,971.34 (11,880.58– 22,174.35) | .26 |
Notes. Numbers refer to n (%) unless otherwise specified. BMI = body mass index; CABG = coronary artery bypass graft surgery; IQR = interquartile range; LVEF = left ventricular ejection fraction; M = mean; SD = standard deviation; MET = metabolic equivalent of task; PTCA = Percutaneous transluminal coronary angioplasty; PTSD = post-traumatic stress disorder. T-tests were used to calculate differences between dichotomous groups in trauma exposure. Pearson’s correlations were used to calculate associations between trauma exposure and continuous variables. P values are all based on unadjusted models. Medians and interquartile ranges for interleukin-6, tumor necrosis factor-α, C-reactive protein and resistin are based on raw data, but the p values for unadjusted associations with trauma exposure are based on log-transformed data.
At baseline, higher lifetime trauma exposure was associated with younger age and male gender as well as with greater prevalence of arthritis, gout or chronic joint problems and neurodegenerative disease at p < .10. These were therefore adjusted in all models of baseline and change over time. There were no associations between trauma exposure and kidney dysfunction, diabetes, history of cancer, or cardiovascular revascularization (CABG or PTCA). At follow-up, higher lifetime trauma exposure was associated with age, sex and use of statins and renin-angiotensin inhibitors at p < .10, and these were therefore adjusted in all models of follow-up. Trauma exposure was not associated with greater prevalence of comorbidities at follow-up or with a history of cardiovascular revascularization.
Traumatic Stress and Inflammation
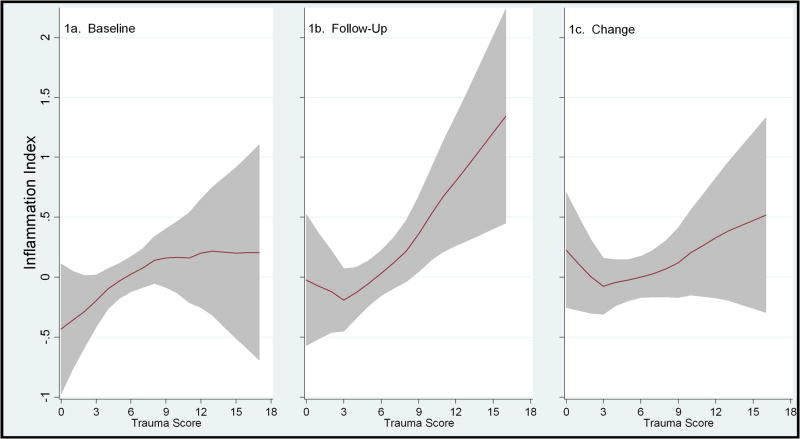
Table 3 displays the results of our primary and secondary analyses of associations between traumatic stress exposure and inflammation. Higher lifetime trauma exposure was associated with elevated inflammation at baseline and at five-year follow-up, adjusting for potential confounds (Figure 1). Over time, the sample as a whole exhibited significant increases in inflammation, t (625)= −3.98, p< .001. However, while there was a tendency for higher trauma exposure to be associated with a greater increase in inflammatory activity from baseline to five-year follow-up, this effect was not statistically significant. Follow-up analyses indicated that nonlinear models did not provide significantly better model fit to the data than the linear models.
Table 3.
Associations between lifetime trauma exposure and inflammatory activity
| FChange | df | B | SE (B) | β | p | |
|---|---|---|---|---|---|---|
| Baseline: | ||||||
| Inflammatory index | 7.73 | 1, 969 | .08 | .03 | .09 | .01 |
| IL-6 | 6.87 | 1, 969 | .02 | .01 | .08 | .01 |
| TNF-α | .04 | 1, 969 | .002 | .01 | .01 | .85 |
| CRP | 5.01 | 1, 969 | .03 | .02 | .07 | .03 |
| Resistin | 4.56 | 1, 969 | .02 | .01 | .07 | .03 |
| Follow-up: | ||||||
| Inflammatory index | 4.52 | 1, 615 | .09 | .04 | .09 | .03 |
| IL-6 | 2.33 | 1, 615 | .02 | .01 | .06 | .13 |
| TNF-α | .05 | 1, 615 | .002 | .01 | .01 | .82 |
| CRP | 4.40 | 1, 615 | .04 | .02 | .09 | .04 |
| Resistin | 3.87 | 1, 615 | .02 | .01 | .08 | .05 |
| Change over time: | ||||||
| Inflammatory index | 2.76 | 1, 618 | .06 | .03 | .05 | .10 |
| IL-6 | 1.30 | 1, 618 | .01 | .01 | .04 | .26 |
| TNF-α | .85 | 1, 618 | .01 | .01 | .03 | .36 |
| CRP | 1.79 | 1, 618 | .01 | .02 | .05 | .18 |
| Resistin | 2.46 | 1, 618 | .01 | .01 | .05 | .12 |
Note. Statistics are based on linear regression models adjusted for potential confounding variables that were significantly associated with trauma exposure, including age, gender, comorbities, and medication use. IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α; CRP = C-reactive protein.
Figure 1.
Figures 1a, 1b and 1c. Figures 1a, 1b and 1c illustrate the relationship between lifetime trauma exposure and inflammation with a line graph created using a running line smoother. 1a shows baseline inflammation, 1b shows follow-up inflammation, and 1c shows change over time. Shaded areas represent 95% point wise confidence intervals, which represent dispersion at individual points in the curve. Note that there are few participants at the extreme ends of low and high lifetime trauma exposure, and that non-linear models did not provide significantly better model fit to the data than non-linear models.
In our secondary analyses, trauma exposure was associated with significantly elevated levels of IL-6, CRP and resistin at baseline and with significantly higher levels of CRP and a trend towards higher levels of resistin at follow-up.
The Contribution of Sociodemographic Factors, Psychiatric Disorders and Health Behaviors
Next, we examined the contribution of sociodemographic factors, psychiatric disorders and health behaviors to the association between trauma exposure and inflammation. Of the potential sociodemographic risk factors and correlates of trauma exposure, higher lifetime trauma exposure was associated with non-White ethnicity and lower than high school educational attainment at both baseline and follow-up, but there were no differences in trauma exposure between participants with and without a household income <$20,000 per year. After additional adjustment for White versus non-White ethnicity and education, the association between lifetime trauma exposure and inflammation remained significant at baseline, FChange (1,965) = 7.95, β = .09, SE = .03, p = .005, and at five-year follow-up, FChange (1,611) = 4.89, β = .09, SE = .03, p = .03, and the association with change over time remained non-significant, FChange (1,613) = 1.92, β = .05, SE = .03, p = .17.
Participants with a current diagnosis of PTSD, major depression, or generalized anxiety disorder reported significantly higher lifetime trauma exposure. However, even after additional adjustment for these diagnoses, the association between lifetime trauma exposure and inflammation remained significant at baseline, FChange (1,961) = 10.49, β = .11, SE = .03, p = .001, and at five-year follow-up, FChange (1,611) = 5.09, β = .10, SE = .04, p = .02, and the association with change over time remained non-significant, FChange (1,610) = 1.76, β = .05, SE = .04, p = .19. The same pattern emerged if we controlled for lifetime history instead of current diagnosis.
Finally, we assessed the contribution of health behaviors to our models. Higher lifetime trauma exposure was associated with more tobacco use, poorer sleep quality, and illicit drug use at baseline and follow-up, but there were no significant associations between trauma exposure and physical inactivity, alcohol use, adherence to medication regimens or BMI. After additional adjustment for tobacco, drug use and sleep quality, the association between lifetime trauma exposure and inflammation remained significant at baseline, FChange (1,956) = 7.76, β = .09, SE = .03, p = .005, but became non-significant at five-year follow-up, FChange (1,576) = 2.37, β = .07, SE = .05, p = .12, and the association with change over time remained non-significant, FChange (1,605) = 1.64, β = .05, SE = .04, p = .20.
Discussion
This study is the first large-scale demonstration of an association between higher lifetime cumulative trauma exposure and elevated inflammation. Importantly, the present study was conducted in a sample of patients with CVD for whom stress-related elevations in inflammation have particular clinical relevance because inflammation is both a mechanism of accelerated CVD progression and a strong predictor of adverse CVD events and mortality (Harris et al., 1999; Libby, 2002; Libby et al., 2011; Volpato et al., 2001). Results specifically indicate that higher lifetime trauma exposure is associated with elevated inflammation as indexed by the combination of IL-6, TNF-α, CRP and resistin, each of which is individually associated with an accelerated rate of CVD progression (Cesari et al., 2003; Dunlay et al., 2008; Jamaluddin et al., 2011; Reilly et al., 2005; Tuomisto et al., 2006; Volpato et al., 2001). The association between lifetime trauma exposure and inflammation was independent of sociodemographic factors and psychiatric disorders. However, we found some limited evidence that poor health behaviors may contribute to the relationship between trauma and inflammation.
The present results expand upon findings indicating that exposure to specific types of traumatic stress is associated with elevated basal and reactivity levels of inflammation in later life (Carpenter et al., 2010; Danese et al., 2007; Dekaris et al., 1993; Kiecolt-Glaser et al., 2011; Woods et al., 2005). Our data extend this literature by suggesting that cumulative exposure to different categories of trauma across the lifespan is also associated with elevated inflammation. In particular, our results indicate that higher cumulative lifetime trauma exposure is associated with elevated inflammation at baseline and at five-year follow-up in a sample of older adults with CVD. Prior evidence indicates that cumulative stress exposure has the most potent and enduring negative effects on psychological and biological systems (Breslau et al., 1999; O’Donovan et al., 2011a; Schnurr et al., 1998; Shrira et al., 2010). In line with these studies, our results indicate that different types of traumatic life experiences may have additive effects on inflammation.
Sociodemographic factors, psychiatric disorders, and health behaviors as measured in the present study accounted for some, but not all variance, in the relationship between traumatic stress and inflammation in our sample. Thus, the complete pathway from traumatic stress to elevated inflammation remains unclear. One alternative pathway is through altered neural and peripheral stress responses. Trauma exposure impacts neural systems, leading to structural and functional abnormalities in the hippocampus, amygdala and prefrontal cortex (Tottenham & Sheridan, 2010). Trauma exposure also influences cognitive-behavioral responses related to these neural systems, and exaggerated threat perception has been observed in individuals exposed to trauma in childhood (Pole et al., 2007) or in adulthood (Lindstrom et al., 2011). Threat, in turn, is a key feature of psychological stressors that elicit biological stress responses, including hypothalamic pituitary adrenal (HPA) axis, sympathetic nervous system (SNS) and inflammatory systems responses, leading to increases in levels of the glucocorticoid hormone cortisol, catecholamines, and inflammatory cytokines (Blascovich & Mendes, 2010; Dickerson et al., 2009; Dickerson & Kemeny, 2004; Steptoe et al., 2007; van Marle et al., 2009). In the setting of repeated and prolonged stress reactivity, basal levels of inflammation may become chronically elevated (Kiecolt-Glaser et al., 2002; Kiecolt-Glaser et al., 2003). Thus, one potential pathway from trauma exposure to elevated inflammation is through exaggerated threat perception and associated repeated and prolonged activation of the inflammatory response.
Limitations
The present findings should be interpreted in the context of a number of limitations. Lifetime traumatic stress exposure is difficult to measure, particularly in a large sample. While the present retrospective assessment method for traumatic stress used a validated measure and permitted the collection of trauma exposure data for a relatively large sample of patients with CVD, it did not yield details on perceived severity of the trauma or on reactions to traumatic events. Lifetime trauma exposure was also considerably higher in our sample than in previous reports (Breslau, 2002; Breslau et al., 1999; Kessler, 2000; Resnick et al., 1993; Stein et al., 2000). However, this high rate of trauma exposure can be explained by several factors. First, the mean age of our cohort is above 65, which is higher than prior studies. As cumulative exposure to traumatic events increases with age, being 60% by age 35 in one study, it can be expected that an older cohort will have a higher prevalence of events (Breslau et al., 1997). Second, our trauma measure was derived from DSM-IV, which is a measure that typically shows a higher rate of trauma exposure due to its expanded list of potential traumatic events, some of which were particularly prevalent in our sample (Breslau, 2002). For example, the unexpected death of a close friend or relative was endorsed by 75% of our sample; life-threatening illness was endorsed by 54% of our sample; and exposure to a natural disaster was endorsed by 79% of our Bay Area sample, many of whom may have been exposed to the 1989 Bay Area earthquake. We also did not collect information on either age at trauma exposure, number of exposures to each specific type of trauma or severity of traumatic events, each of which is likely to play an important role in the relationship between trauma exposure and inflammation (Danese et al., 2007; Kaysen et al., 2010).
Given that the majority of our participants were male, we had limited power to assess gender differences in the relationship between traumatic stress and inflammation. However, the present study is the first to investigate the relationship between cumulative traumatic stress exposure and inflammation and suggests that further research in males and a larger sample of females is warranted. We lost a number of participants from baseline to follow-up, primarily due to a high mortality rate in the cohort. However, a large majority (76%) of the Heart and Soul cohort alive at the follow-up time point were included in our analyses. Finally, as our sample includes older and mostly male patients with CVD, it is unclear to what extent the findings are generalizable to a healthy older sample or to younger subjects.
Conclusion
The present study indicates that higher lifetime exposure to traumatic psychological stress is associated with elevated levels of inflammation in patients with CVD. Inflammation is a potentially modifiable risk factor for CVD and other chronic illnesses, as well as a potential treatment target in patients with CVD. Therefore, a better understanding of how psychological trauma influences inflammation could lead to new methods to improve the long-term health of patients who experience traumatic events. In sum, the psychological, behavioral and biological sequelae of traumatic psychological stress may persist across the lifespan and have an important influence on health in later life.
Research Highlight.
Cumulative lifetime traumatic stress exposure is associated with elevated inflammation in patients with cardiovascular disease.
Acknowledgments
This research was supported in part by NIH/NHLBI grant K23 HL 094765-01, Department of Defense/NCIRE grant DAMD 17-03-1-0532, W81XWH-05-2-0094, and a grant from the Irene Perstein Foundation to Dr. Cohen and by a Society in Science: Branco Weiss Fellowship to Dr. O’Donovan. The Heart and Soul Study was funded by the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), Bethesda, MD, the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, South San Francisco, CA, and the Nancy Kirwan Heart Research Fund, San Francisco, CA. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Medical Center, San Francisco, California. Mr. Metzler is supported in part by grants from the Department of Defense (W81XWH-10-2-0089), the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration. The funding organizations had no role in the design or conduct of the study; data collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript. The authors would like to thank The Heart and Soul Study team for supporting this manuscript, particular Dr. Mary Whooley, Mathilda Regan and Bee Ya Na.
Footnotes
Financial Disclosures
Dr. Neylan has received investigational medication from Actelion and Glaxo Smith Kline. All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, editors. The Handbook of Social Psychology. Wiley; New York: 2010. [Google Scholar]
- Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder, and other psychiatric disorders. Can J Psychiatry. 2002;47:923–929. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Brown ES, Fulton MK, Wilkeson A, Petty F. The psychiatric sequelae of civilian trauma. Compr Psychiatry. 2000;41:19–23. doi: 10.1016/s0010-440x(00)90126-3. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacol. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease: The Health, Aging and Body Composition [Health ABC] Study. Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Marmar CR, Neylan TC, Schiller NB, Ali S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2009;66:1214–1220. doi: 10.1001/archgenpsychiatry.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis MA, de Mello MF, Scorza FA, Cadrobbi MP, Schooedl AF, da Silva SG, de Albuquerque M, da Silva AC, Arida RM. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics. 2008;63:473–478. doi: 10.1590/S1807-59322008000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keulenaer GW, Brutsaert DL. Systolic and diastolic heart failure: different phenotypes of the same disease? Eur J Heart Fail. 2007;9:136–143. doi: 10.1016/j.ejheart.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Dekaris D, Sabioncello A, Mazuran R, Rabatic S, Svoboda-Beusan I, Racunica NL, Tomasic J. Multiple changes of immunologic parameters in prisoners of war. Assessments after release from a camp in Manjaca, Bosnia. JAMA. 1993;270:595–599. [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol Sci. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psych Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625–631. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: a critical review of the empirical literature. Clin Psychol Rev. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Ferguson JP, Binkley PF. Depressive symptoms and inflammation among heart failure patients. Am Heart J. 2005;150:132–136. doi: 10.1016/j.ahj.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Gehi AK, Ali S, Na B, Schiller NB, Whooley MA. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2008;168:1423–1428. doi: 10.1001/archinte.168.13.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaesmer H, Brahler E, Gundel H, Riedel-Heller SG. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosom Med. 2011;73:401–406. doi: 10.1097/PSY.0b013e31821b47e8. [DOI] [PubMed] [Google Scholar]
- Glover K, Olfson M, Gameroff MJ, Neria Y. Assault and mental disorders: a cross-sectional study of urban adult primary care patients. Psychiatr Serv. 2010;61:1018–1023. doi: 10.1176/appi.ps.61.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, Hong S, Camara RJ, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WHJ, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the heart and soul study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen D, Rosen G, Bowman M, Resick PA. Duration of exposure and the dose-response model of PTSD. J Interpers Violence. 2010;25:63–74. doi: 10.1177/0886260508329131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Ann Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka RA, ESW, Fairbank JA, LBR, KJB, RMC, Weiss DS. National Vietnam veterans readjustment study (NVVRSI: readjustment, current status, and initial P7SD prevalence estimates. Veterans Administration; Washington, DC: 1988. [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Lindstrom KM, Mandell DJ, Musa GJ, Britton JC, Sankin LS, Mogg K, Bradley BP, Ernst M, Doan T, Bar-Haim Y, Leibenluft E, Pine DS, Hoven CW. Attention orientation in parents exposed to the 9/11 terrorist attacks and their children. Psychiatry Res. 2011;187:261–266. doi: 10.1016/j.psychres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabre MM, Hadi F. War-related exposure and psychological distress as predictors of health and sleep: a longitudinal study of Kuwaiti children. Psychosom Med. 2009;71:776–783. doi: 10.1097/PSY.0b013e3181ae6aee. [DOI] [PubMed] [Google Scholar]
- Maes M, Mylle J, Delmeire L, Altamura C. Psychiatric morbidity and comorbidity following accidental man-made traumatic events: incidence and risk factors. Eur Arch Psychiatry Clin Neurosci. 2000;250:156–162. doi: 10.1007/s004060070034. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Means-Christensen AJ, Craske MG, Sherbourne CD, Roy-Byrne PP, Stein MB. Associations between psychological trauma and physical illness in primary care. J Trauma Stress. 2006;19:461–470. doi: 10.1002/jts.20129. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011a;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011b;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, Marmar CR. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. J Abnorm Psychol. 2007;116:352–361. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psych. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Spiro A, 3rd, Aldwin CM, Stukel TA. Physical symptom trajectories following trauma exposure: longitudinal findings from the normative aging study. J Nerv Ment Dis. 1998;186:522–528. doi: 10.1097/00005053-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Shedd OL, Sears SF, Jr, Harvill JL, Arshad A, Conti JB, Steinberg JS, Curtis AB. The World Trade Center attack: increased frequency of defibrillator shocks for ventricular arrhythmias in patients living remotely from New York City. J Am Coll Cardiol. 2004;44:1265–1267. doi: 10.1016/j.jacc.2004.04.058. [DOI] [PubMed] [Google Scholar]
- Shemesh E, Yehuda R, Milo O, Dinur I, Rudnick A, Vered Z, Cotter G. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med. 2004;66:521–526. doi: 10.1097/01.psy.0000126199.05189.86. [DOI] [PubMed] [Google Scholar]
- Shrira A, Palgi Y, Ben-Ezra M, Shmotkin D. Do Holocaust survivors show increased vulnerability or resilience to post-Holocaust cumulative adversity? J Trauma Stress. 2010;23:367–375. doi: 10.1002/jts.20524. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Lowe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thrombosis Haemostasis. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular disease, interleukin-6, and risk of mortality in older women: The Women’s Health and Aging Study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AB, Page GG, O’Campo P, Pugh LC, Ford D, Campbell JC. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am J Community Psychol. 2005;36:159–175. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]