Abstract
Objective
Magnetic Resonance Imaging (MRI) has defined neurologic abnormalities in infants with congenital heart disease (CHD) including pre-operative injury and delayed brain maturation. This study utilized qualitative scoring, cerebral biometry, and diffusion imaging to characterize pre-operative brain abnormalities in infants with CHD, including the identification of regions of greater vulnerability.
Methods
Sixty-seven infants with CHD had pre-operative MRI with analysis for brain injury by qualitative scoring and brain development by qualitative scoring, metrics and diffusion imaging.
Results
Qualitative abnormalities were common, with 42% of infants having pre-operative focal white matter lesions. Infants with CHD had smaller brain measures in the frontal lobe, parietal lobe, cerebellum and brainstem (p<.001); with the frontal lobe and brainstem displaying the greatest alterations (p<.001). Smaller brain size in the frontal and parietal lobes correlated with delayed white matter microstructure reflected by diffusion imaging.
Conclusion
Infants with CHD commonly display brain injury and delayed brain development. Regional alterations in brain size are present, with the frontal lobe and brainstem demonstrating the greatest alterations, which may reflect a combination of developmental vulnerability and regional differences in cerebral circulation.
Introduction
Congenital heart disease (CHD) is the commonest congenital lesion in the term infant and has long lasting neurodevelopmental consequences during childhood and adolescence with motor, cognitive, and behavioral impairments.1,2 To provide insight into the nature of cerebral abnormalities in infants with CHD, neuroimaging with Magnetic Resonance Imaging (MRI) has been undertaken in several cohorts. These studies have demonstrated focal white matter abnormalities, periventricular leukomalacia, and infarct in up to 39% of infants prior to surgery.3–6 In addition, evaluations of brain maturation by qualitative scoring7, MR spectroscopy4,6 and diffusion tensor imaging (DTI)6 suggest delays or impairment in brain development. DTI provides information regarding tissue microstructure and development using the measures of Mean Diffusivity (MD) and Fractional Anisotropy (FA).8 With cerebral maturation, MD decreases while FA increases. In contrast, both MD and FA decrease with acute injury.9,10 Infants with CHD have demonstrated higher MD and lower FA compared to healthy controls, consistent with maturational delay.6 In addition, volumetric analysis has been applied to fetal and neonatal MRI of infants with CHD demonstrating reductions in brain volume during the third trimester of pregnancy11 and post-operatively, particularly within the frontal lobe.12
Our study aimed to utilize qualitative scoring, cerebral biometry (a relatively simple measurement technique that bears a strong correlation with volumetry13), and DTI on pre-operative MRI scans to define the nature of pre-operative brain abnormalities in infants with complex CHD. We hypothesized that infants with CHD would display regional alterations in brain growth and abnormalities in white matter microstructure that may differ in relation to their primary cardiac diagnosis. This study is part of a larger prospective longitudinal cohort of CHD infants who underwent MRI pre-operatively, post-operatively, and at 3 months of age, as well as neurodevelopmental follow-up at two years of age.
Methods
Patients
This is a prospective longitudinal study conducted between March 2005 and November 2008 at Starship Children’s Hospital in Auckland, New Zealand involving infants with complex CHD who underwent cardiac surgery at less than 8 weeks of age. Patients were excluded if they were less than 36 weeks gestation or greater than 48 weeks post-menstrual age, had a pre-existing neurologic deficit unrelated to the cardiovascular defect, had a chromosomal abnormality or syndrome, had moderate or severe extracardiac anomalies, had previous cardiac surgery, required ECMO pre-operatively, or were unable to have a pre-operative MRI performed. Infants were separated into one of four cardiac diagnostic groupings based on a classification previously described by Clancy et al: single ventricle circulation (SV), single ventricle circulation with aortic arch anomaly (SVA), two ventricle circulation (2V), and two ventricle circulation with aortic arch anomaly (2VA).14 Informed consent was obtained from all parents, and the study was approved by the ethics committees for Starship Children’s Hospital and Washington University in St. Louis. Thirty-six healthy term infants from Royal Children’s Hospital, Melbourne were recruited for control purposes and underwent brain MRI at that time.
MRI Acquisition
Pre-operative MRI scans were performed when the infants were clinically stable. Those who had balloon atrial septostomy had an MRI after the septostomy was performed. MRIs were performed on a 1.5T Magnetom Avanto (Siemens, Erlangen, Germany) with the following sequences: 1) Coronal T2-weighted turbo-spin echo sequences; 2-mm slice thickness; repetition time (TR) = 4510 ms; echo time (TE) = 79 and 158 ms; flip angle = 150°; field of view (FOV) = 192×192. 2) Transverse T2-weighted sequences; 3-mm slice thickness; TR = 4140 ms; TE = 158 ms; flip angle = 150°; FOV = 160×160. 3) Coronal 3D-FLAIR T1-weighted images with 1-mm slice thickness; TR = 10 ms; TE = 4.8 ms; flip angle = 15°; FOV = 192×192. 4) Diffusion weighted images (DWI); 4-mm slice thickness in 20 directions with b amplitudes of 0 and 1000 mm2/s; TR = 3200–3600 ms; TE = 96 ms; flip angle = 90°; FOV = 128×128. Control data from 36 healthy-term infants were used for comparison in metric analysis (T2-weighted images only). The images were obtained using a 1.5T Sigma System MR imaging system (GE Healthcare, Milwaukee, Wis) with T2-weighted, dual-echo, fast spin-echo sequences; 2-mm slice thickness; TR = 4000 ms; TE = 60/160 ms; FOV = 220×160. No data for the DTI acquisition from term born controls without CHD were acquired or available.
Qualitative Scoring
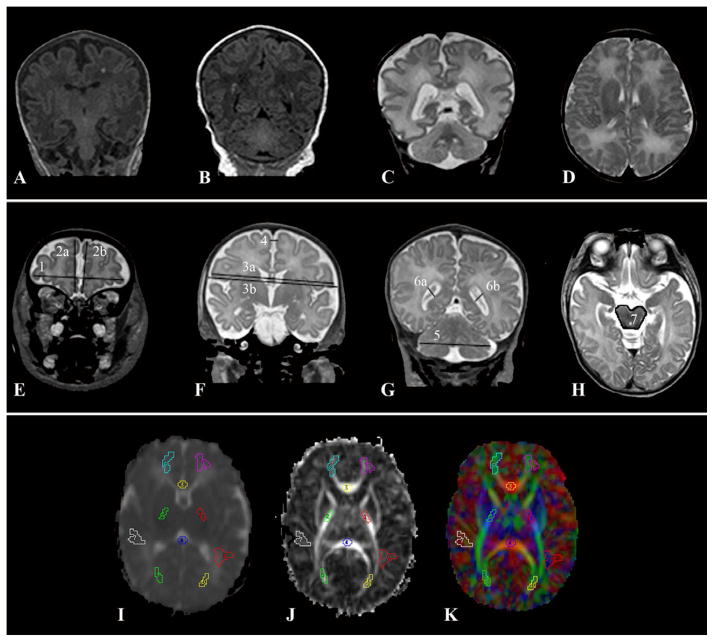
A standardized scoring system was utilized to evaluate ten different variables within the white and gray matter for brain injury and maturation.15,16 Two independent raters (CO, TI) assessed the white matter for focal white matter signal abnormality, diffuse excessive high signal intensity (DEHSI), hemorrhage, ventricular size, white matter volume, and myelination in the posterior limb of the internal capsule (PLIC). Gray matter was assessed for amount of extra-axial space, maturation of gyrification, abnormality in the deep nuclear gray matter, and other abnormality. A score of 1–4 was assigned to each variable, with 1 being normal, 2 mild abnormality, 3 moderate abnormality, and 4 severe abnormality (Figure 1). For focal white matter signal abnormality, one or two focal lesions was considered mild, three-five lesions was considered moderate, and greater than five lesions was considered severe.
Figure 1.
A–D: Qualitative Scoring Abnormalities A. T1-weighted image with abnormalities that include focal signal abnormality, delayed myelination in PLIC, increased extra-axial space, delayed gyrification. B. T1-weighted image with bilateral focal signal abnormalities, delayed gyrification. C. T2-weighed image with ventriculomegaly, DEHSI, increased extra-axial space, moderate-severe delay in gyrification. D. T2-weighted image with DEHSI. E–H: Subset of Brain metrics. 1) Bifrontal diameter 2a) Right frontal height 2b) Left frontal height 3a) Brain biparietal diameter 3b) Bone Biparietal Diameter 4) Interhemispheric distance 5) Transverse cerebellar diameter 6a) Right ventricular diameter 6b) Left ventricular diameter 7) Brainstem area. I–K: Diffusion Imaging. I. Mean diffusivity J. Fractional Anisotropy K. RGB color plot. Regions of interest are the same for each image (from top to bottom): left and right frontal white matter, genu of the corpus collosum, left and right posterior limb of the internal capsule, splenium of the corpus collosum, left and right subcortical white matter, left and right optic radiations
Brain Metrics
Simple brain metrics were measured on T2-weighted images as described previously.13 Two independent raters (JL, CO) measured 19 parameters of nine tissue distances (right and left frontal height, right and left frontal length, bifrontal diameter, bone biparietal diameter, brain biparietal diameter, transverse cerebellar diameter, and brainstem area) and ten fluid distances (third ventricle, interhemispheric distance, right and left extra-axial fluid space, right and left cranio-caudal interopercular distance, right and left antero-posterior interopercular distance, and right and left ventricular diameter) across five slices in the coronal or axial view (Figure 1). Simple brain metrics were also performed on thirty-six healthy term control infants.
Diffusion Imaging
The diffusion images were registered to a term control neonatal atlas and reconstructed to 2×2×2 mm3. Parametric maps were generated for mean diffusivity (MD), relative anisotropy (RA), fractional anisotropy (FA), and eigenvalues (λ1, λ2, and λ3). Measures of axial (λ1) and radial ((λ2+λ3)/2) diffusivity were recorded. Diffusion encodings corrupted by subject motion were automatically removed and a residual map was created to determine variance in the reconstructed data. DTI parameter sampling was performed using Analyze 9.0 (Mayo Clinic, Rochester, MN) and regions of interest were placed in seven white matter regions: genu and splenium of the corpus collosum, posterior limb of the internal capsule, frontal white matter, subcortical white matter, optic radiation, and centrum semiovale (Figure 1). Inter-rater reliability testing on nine infants provided an intra-class correlation coefficient of 0.98 (95% confidence interval 0.97 – 0.98).
Statistics
Statistical analysis was performed using SPSS version 17.0 (Chicago, Illinois) and SAS version 9.2 (Cary, North Carolina). Comparisons between CHD infants and control infants were done using a t-test for continuous variables and chi square test or fisher’s exact test for categorical variables. Comparisons across groups of CHD infants were performed using an ANOVA with Bonferroni correction for continuous variables and chi square analysis for categorical variables. ANCOVA was performed to control for covariates, with brain metrics as the dependent variable and postmenstrual age at the time of MRI, birth weight, and gender as the covariates. Brain diameters were also analyzed with a mixed model approach to assess for regional variations. The variables of group, region, and the group by region interaction were fixed effects, and infant within group was a random effect. The brainstem area was analyzed with ANOVA on group. Pearson’s correlations were done for diffusion values, focal signal abnormality, and metrics; and then partial correlations were performed on significant correlations to control for postmenstrual age at the time of MRI.
Results
Patient Population
There were 71 infants consented and enrolled in the study. One infant was excluded for a previously undiagnosed genetic abnormality (45XY, der (13; 14) (q10; q10)) and three were excluded for poor quality MRI, resulting in a total of 67 subjects available for analysis.
Ten infants were classified as SV, of which nine infants had pulmonary atresia (six isolated, two with tetrology of fallot, and one with Ebstein’s anomaly) and one infant had complex heterotaxy, total anomalous pulmonary venous drainage, and atrioventricular canal. Sixteen infants were classified as SVA, with 14 infants having hypoplastic left heart syndrome (HLHS) and two infants having more complex lesions. Twenty-seven infants were classified as 2V, with the majority (n = 24) having transposition of the great arteries (TGA), of whom only four infants had a ventricular septal defect. Two infants had double outlet right ventricle and one had total anomalous pulmonary venous drainage. Fourteen infants were classified as 2VA, one with truncus arteriosus and interrupted aortic arch; five with coarctation; four with interrupted aortic arch; and four with hypoplastic aortic arches. SVA infants were more likely to be diagnosed antenatally (p<.01). There were no differences in any other patient characteristics across CHD diagnostic groupings (Table 1 – online supplement).
Table 1.
Patient Characteristics of CHD Infants
| All CHD (n = 67) | SV (n = 10) | SVA (n = 16) | 2V (n = 27) | 2VA (n = 14) | |
|---|---|---|---|---|---|
| Male sex n (%) | 38 (57) | 3 (30) | 10 (62) | 15 (56) | 10 (72) |
| Ethnicity: n (%) | |||||
| European | 37 (55) | 3 (30) | 11 (69) | 13 (48) | 10 (72) |
| Maori | 18 (27) | 5 (50) | 2 (12) | 10 (37) | 1 (7) |
| Pacific Island | 8 (12) | 0 (0) | 3 (19) | 3 (11) | 2 (14) |
| Asian | 4 (6) | 2 (20) | 0 (0) | 1 (4) | 1 (7) |
| Antenatal Diagnosis: n (%) | 41 (61) | 6 (60) | 16 (100) | 13 (48) | 6 (43) |
| Cesarean Delivery: n (%) | 13 (19) | 2 (20) | 2 (13) | 6 (22) | 3 (21) |
| GA* at Birth – wks Mean (range) | 39 (36–42) | 38 (36–40) | 39 (36–41) | 40 (37–42) | 39 (36–41) |
| Birth Weight – kg Mean (range) | 3.3 (2.1–4.7) | 3.2 (2.3–4.7) | 3.3 (2.7–4.5) | 3.4 (2.6–4.2) | 3.1 (2.1–3.8) |
| SGA† n (%) | 7 (11) | 1 (10) | 2 (12.6) | 1 (4) | 3 (21.3) |
| HC‡ at Birth – cm Mean (range) | 34.9 (31.0–39.0) | 33.7 (31.9–37.0) | 35.0 (31.0–37.0) | 35.0 (32.0–38.0) | 35.1 (31.5–39.0) |
| Apgar Score 1 min Mean (range) | 8 (2–10) | 7 (2–9) | 8 (2–9) | 8 (6–9) | 8 (5–10) |
| Apgar Score 5 min Mean (range) | 9 (7–10) | 9 (7–10) | 9 (9–10) | 9 (7–10) | 9 (8–10) |
| Age at MRI – days Mean (range) | 8 (2–23) | 7 (3–20) | 6 (2–17) | 9 (5–23) | 8 (2–19) |
| Weight at MRI – kg Mean (range) | 3.3 (2.0–4.9) | 3.2 (2.3–4.9) | 3.3 (2.6–4.7) | 3.5 (2.7–4.2) | 3.1 (2.0–3.8) |
GA = gestational age,
SGA = small for gestational age,
HC = head circumference
Infants with CHD did not differ in gestational age, gender, birth weight, or weight at the time of MRI compared to term born infants without CHD. Infants with CHD underwent MRI scanning at an earlier postnatal age compared to control infants (CHD 8 days vs. control 15 days, p <.001).
MRI Qualitative Scoring
Focal white matter signal abnormalities were present in 42% (n=28) and DEHSI was present in 96% (n=64) of infants, of which most were mild abnormalities. Of infants with focal white matter signal abnormalities, 75% (21/28) had mild abnormality, 14% had moderate abnormality (4/28), and 11% had severe abnormality (3/28). Hemorrhage occurred in two infants, both of which were small intraventricular hemorrhages (Table 2). Increased extra-axial fluid space was present in 51% of infants (n=34), and delayed gyrification was present in 64% (n=43) (Table 3). No infants had infarcts pre-operatively. Three infants had abnormalities classified as “other”: one infant with sinus venous thrombosis, one infant with isolated temporal lobe diffusion restriction that was not typical in its distribution for focal infarction, and one infant with cerebellar hemorrhage. There was no difference between the cardiac groups for the individual scoring variables. None of the MRIs were performed based on clinical neurologic concerns, and infants with abnormalities on MRI did not display any obvious symptomatology.
Table 2.
Qualitative Scoring of White Matter Variables.
| White Matter Variable, n | SV (n=10) | SVA (n=16) | 2V (n=27) | 2VA (n=14) | Total (n=67) |
|---|---|---|---|---|---|
| Focal Signal
| |||||
| Normal | 5 | 10 | 17 | 7 | 39 |
| Mild | 2 | 5 | 8 | 6 | 21 |
| Moderate | 2 | 1 | 1 | 0 | 4 |
| Severe | 1 | 0 | 1 | 1 | 3 |
| DEHSI* | |||||
| Normal | 1 | 1 | 0 | 1 | 3 |
| Mild | 6 | 11 | 19 | 9 | 45 |
| Moderate | 3 | 4 | 8 | 3 | 18 |
| Severe | 0 | 0 | 0 | 1 | 1 |
| Hemorrhage
| |||||
| Normal | 9 | 16 | 26 | 14 | 65 |
| Mild | 1 | 0 | 1 | 0 | 2 |
| Moderate | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Ventricular Size
| |||||
| Normal | 5 | 7 | 13 | 4 | 29 |
| Mild | 2 | 9 | 13 | 9 | 33 |
| Moderate | 3 | 0 | 1 | 1 | 5 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Volume
| |||||
| Normal | 6 | 10 | 9 | 8 | 33 |
| Mild | 3 | 6 | 18 | 6 | 33 |
| Moderate | 1 | 0 | 0 | 0 | 1 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Myelination in PLIC† | |||||
| Normal | 3 | 11 | 16 | 6 | 36 |
| Mild | 6 | 4 | 11 | 7 | 28 |
| Moderate | 1 | 1 | 0 | 1 | 3 |
| Severe | 0 | 0 | 0 | 0 | 0 |
DEHSI = diffuse excessive high signal intensity,
PLIC = posterior limb of the internal capsule
Table 3.
Qualitative Scoring of Gray Matter Variables.
| Gray Matter Variable, n | SV (n=10) | SVA (n=16) | 2V (n=27) | 2VA (n=14) | Total (n=67) |
|---|---|---|---|---|---|
| Extra-axial Space
| |||||
| Normal | 5 | 8 | 13 | 7 | 33 |
| Mild | 5 | 7 | 14 | 6 | 32 |
| Moderate | 0 | 1 | 0 | 1 | 2 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Maturation of Gyrification
| |||||
| Normal | 4 | 6 | 11 | 3 | 24 |
| Mild | 4 | 9 | 16 | 10 | 39 |
| Moderate | 2 | 1 | 0 | 1 | 4 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Deep Nuclear Gray Matter
| |||||
| Normal | 7 | 10 | 24 | 12 | 53 |
| Mild | 3 | 5 | 3 | 2 | 13 |
| Moderate | 0 | 1 | 0 | 0 | 1 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Other Abnormality
| |||||
| Normal | 9 | 16 | 26 | 13 | 64 |
| Mild | 1 | 0 | 1 | 0 | 2 |
| Moderate | 0 | 0 | 0 | 1 | 1 |
| Severe | 0 | 0 | 0 | 0 | 0 |
Brain Metrics
After controlling for birth weight, postmenstrual age at scan, and gender, CHD infants displayed smaller brain tissue measures than control infants in the frontal lobe, parietal lobe, cerebellum, and brainstem (Table 4). CHD infants had smaller measures of the left extra-axial fluid space (p<.001), but larger fluid measures in the right (p<.01) and left (p<.001) cranio-caudal interoperculum.
Table 4.
Comparison of Pre-operative Brain Metrics in Controls and Infants with CHD.
| Measurement (mean in cm) | Control (n = 36) | SV (n=10) | SVA (n=16) | 2V (n=27) | 2VA (n=14) | All CHD (n = 67) | Mean Difference* (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Right Frontal Height | 4.98 | 3.96 | 4.08 | 3.89 | 4.11 | 3.99 | −0.99 (−1.24 to −0.75) | <.001 |
| Left Frontal Height | 4.93 | 3.89 | 4.07 | 3.85 | 4.03 | 3.95 | −0.99 (−1.23 to −0.74) | <.001 |
| Bifrontal Diameter | 7.25 | 6.12 | 6.39 | 6.18 | 6.43 | 6.27 | −0.97 (−1.18 to −0.76) | <.001 |
| Bone Biparietal Diameter | 8.93 | 8.28 | 8.61 | 8.56 | 8.57 | 8.53 | −0.40 (−0.58 to −0.22) | <.001 |
| Brain Biparietal Diameter | 8.53 | 7.77 | 8.12 | 8.06 | 8.16 | 8.05 | −0.48 (−0.67 to −0.29) | <.001 |
| Transverse Cerebellar Diameter | 5.56 | 5.18 | 5.20 | 5.33 | 5.26 | 5.26 | −0.30 (−0.42 to −0.19) | <.001 |
| Brainstem Area | 2.74 | 2.05 | 2.20 | 2.40 | 2.22 | 2.26 | −0.48 (−0.62 to −0.33) | <.001 |
Mean difference represents the difference between control infants and all CHD infants
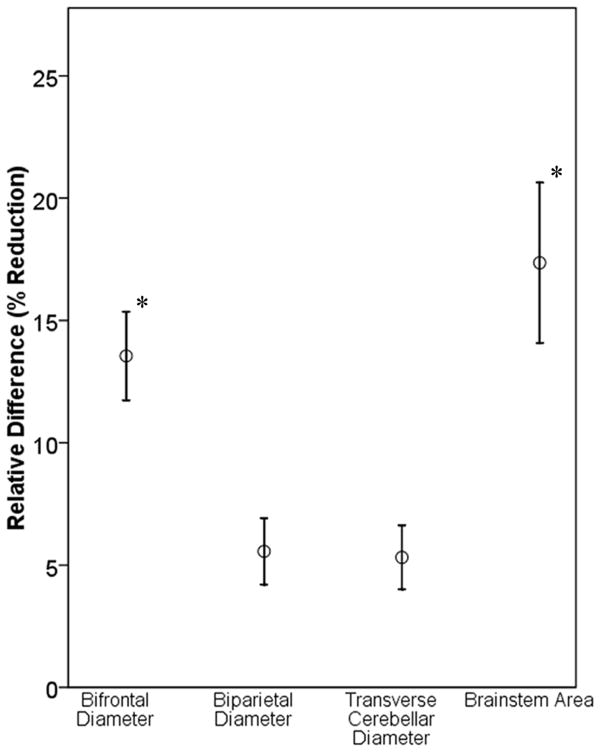
To evaluate for regional differences in brain size, the relative difference was calculated for the bifrontal diameter, biparietal diameter, transverse cerebellar diameter, and brainstem area in CHD infants, with reference to control infants. Although CHD infants had smaller measures in all of these regions, the greatest effect was seen in the frontal lobe (p<.001) and brainstem (p<.001) (Figure 2). There was a strong correlation between the frontal and parietal (r = 0.73, p<.001), frontal and cerebellar (r = 0.47, p<.001), and parietal and cerebellar (r = 0.54, p<.001) measures. In contrast, the brainstem area had weaker correlations with the parietal (r = 0.27, p <.05) and cerebellar (r = 0.29, p<.05) measures and no correlation to the frontal measure (r = 0.02, p = NS).
Figure 2.
Relative Difference in Regional Brain Metrics. *p<.001 compared to brain biparietal diameter and transverse cerebellar diameter
When cardiac groups were compared, SV infants had a smaller brainstem area than 2V infants (p<.05). However, after controlling for birth weight, postmenstrual age at scan, and gender, there were no differences in brain metrics between cardiac groups (Table 4).
Relationship between injury and brain maturation and growth
The presence of focal white matter signal abnormalities did not relate to the presence of increased extra-axial space (p=0.70), delayed gyrification (p=0.30), or myelination in the PLIC (p=0.98). In addition, focal white matter signal abnormalities did not relate to brain metrics in the bifrontal diameter (p=0.53), biparietal diameter (p=0.97), transverse cerebellar diameter (p=0.95), or brainstem area (p=0.51).
Diffusion Imaging
Of the 67 infants included in the study, 26 had 20-direction diffusion data acquired, of which seven were excluded due to poor quality, leaving 19 infants’ data sets for analysis. Clinical characteristics of infants with DTI data did not differ from characteristics of all CHD infants. There was a strong correlation between diffusion values from the right and left hemispheres, therefore, further analysis was only performed on measures from the left hemisphere. As no data were available utilizing this DTI acquisition in term born control infants, we cannot evaluate the impact of CHD on DTI. However, within the CHD cohort, we evaluated the relationship of focal white matter signal abnormality and brain growth with DTI microstructural measures. After controlling for postmenstrual age at the time of scan, there were no relationships between focal signal abnormality and diffusion measures. Analysis of the four regional brain metrics described above revealed that smaller brain measures in the frontal lobe and parietal lobe correlated with higher MD and radial diffusivity and lower FA values, which were most consistent in the PLIC (Table 5: online supplement). Analysis between cardiac groups was not undertaken for diffusion measures due to small sample size.
Table 5.
Correlations of Brain Metrics and Diffusion Values.
| Unadjusted r | P value | Adjusted r | P value | |
|---|---|---|---|---|
| Bifrontal Diameter | ||||
| PLIC*: MD | −0.74 | <.001 | −0.70 | <.01 |
| PLIC: RD | −0.72 | <.001 | −0.68 | <.01 |
| PLIC: FA | 0.57 | <.05 | 0.54 | <.05 |
| Brain Biparietal Diameter | ||||
| PLIC: MD | −0.68 | <.01 | −0.64 | <.01 |
| PLIC: RD | −0.70 | <.01 | −0.66 | <.01 |
| PLIC: FA | 0.59 | <.01 | 0.56 | <.05 |
| Transverse Cerebellar Diameter | ||||
| PLIC: MD | −0.52 | <.05 | 0.02 | NS |
| PLIC: RD | −0.59 | <.01 | −0.28 | NS |
| PLIC: FA | 0.55 | <.05 | 0.43 | NS |
| Optic Radiation: MD (level of CSO†) | −0.52 | <.05 | −0.41 | NS |
| Optic Radiation: RD (level of PLIC) | −0.50 | <.05 | −0.28 | NS |
| Optic Radiation: RD (level of CSO) | −0.54 | <.05 | −0.42 | NS |
| Corticospinal Tract: FA | 0.55 | <.05 | 0.28 | NS |
| Brainstem Area | ||||
| PLIC: MD | −0.53 | <.05 | −0.30 | NS |
| PLIC: RD | −0.59 | <.05 | −0.45 | NS |
| PLIC: FA | 0.55 | <.05 | 0.51 | NS |
| Corticospinal Tract: FA | 0.51 | <.05 | 0.35 | NS |
Pearson’s correlation coefficients were adjusted for postmenstrual age at the time of MRI.
PLIC = posterior limb of internal capsule,
CSO = centrum semiovale
Discussion
This study confirms and extends previous reports demonstrating that infants with CHD have high rates of focal white matter lesions and less mature brains pre-operatively. Importantly, our study is the first to demonstrate pre-operative regional variation in impaired growth, with the frontal lobes and brainstem being the more severely affected areas. In addition, we confirm the lack of relationship between white matter injury and any measure of delayed brain growth or maturity suggesting that influences on impaired brain growth occur independently of focal white matter injury.
Pre-operative cerebral abnormalities are common in infants with CHD, including focal white matter injury, periventricular leukomalacia, and infarct.3–6 We identified a high rate of pre-operative focal white matter lesions, occurring in 42% of our cohort. It is important to note that our previously published data demonstrated white matter injury in 27% of infants; however this included our cohort and infants from another center (Royal Children’s Hospital, Melbourne, Australia) and excluded infants with 2VA physiology.3 In the current cohort, we also evaluated DEHSI and found this form of signal abnormality in 96% of CHD infants. The presence of DEHSI appears to relate to diffusion measures in preterm infants17, but the visual assessment of this signal abnormality has low inter- and intra-observer agreement.18 In addition, the neurodevelopmental significance of DEHSI remains unclear. Thus, we limited our definition of white matter injury to focal signal abnormalities rather than including DEHSI.
Delay in cerebral development on MRI has been classified using a variety of techniques in infants with CHD. Using a maturation scoring system, term infants with CHD have been shown to have a 4 week delay in development.7 Maturational delay in brain microstructure, with an increase in average diffusivity and a decrease in FA, has also been shown at term in CHD infants and found to be unrelated to visible injury.6 This delay in brain development appears to evolve in the third trimester of pregnancy suggesting slowed growth as early as the fetal setting.11
In our study, extra-axial space and maturation of gyrification were used to qualitatively evaluate growth and demonstrated abnormalities in 51% and 64% of our infants, respectively. We also evaluated growth with simple brain metrics and demonstrated a smaller brain size in infants with CHD. Focal white matter signal abnormality did not correlate with impaired brain growth, suggesting that the reduction in brain size occurred independently of visible qualitative abnormalities. There was a relationship between frontal and parietal measures and diffusion measures, suggesting that delayed maturation at the microstructural level in these supratentorial regions accompanies poor growth.
Our data report new findings in relation to the regional nature of alterations in brain size pre-operatively, with the frontal lobe and brainstem having the greatest alterations. There are limited data on the regional effects in CHD. A post-operative study at 15 months of age showed reduction in gray matter volume, most prominently in the frontal lobe, and a decrease in frontal lobe white matter.12 Our data are consistent with this study suggesting regional vulnerability in the frontal lobe.12 In addition, we also found a major difference in the brainstem area. Importantly, these findings were present pre-operatively. The consequences of these regional alterations remain unclear.
With regards to the regional vulnerability of the frontal lobes, one may hypothesize that such vulnerability is related to differences in the rates of brain development, with the frontal lobe being an area of relative immaturity at term equivalent postmenstrual age. Synaptogenesis occurs from the third trimester throughout the first two years of life and is followed by pruning (or synapse elimination), both of which are most protracted in the prefrontal cortex.19 Peak FA values on diffusion imaging occur later in the frontal lobe than other regions and remain higher at term gestation, suggesting greater immaturity within this region at term, with a more protracted developmental trajectory.20 Volumetric studies have supported these frontal lobe findings, showing peak volumes of frontal lobe gray matter occurring around puberty.21 This immaturity and delayed trajectory may predispose this region to injury.22 Nonetheless, the impact of impairment in frontal growth in infants with CHD at term equivalent on later expansion of the frontal lobe and its function are unknown.
An additional mechanism of vulnerability to the frontal lobe regions may relate to selective alterations in the anterior cerebral circulation in infants with CHD. Reduced pulsatility index in the middle cerebral artery is a marker of cerebral blood flow redistribution and is associated with adverse perinatal outcome and abnormal neurobehavior in small for gestational age infants.23 Infants with CHD have been shown to have a decrease in the cerebral-to-placental resistance ratio, which has been associated with smaller head circumference at birth.24 However, regional blood flow measures have not been undertaken in infants with CHD and are worthy of future investigation.
The etiology of the sensitivity of the brainstem to growth alterations remains unclear. In an effort to better delineate whether brainstem size reflected delayed growth in other brain regions, we examined correlations between the brainstem and other measures. Brainstem size had no correlation to the size of the frontal lobe, and was only very weakly correlated with the size of the parietal lobe and cerebellum, suggesting that there may be independent mechanisms influencing the development of the brainstem. To further support evidence of different mechanisms of growth impairment within the supra- and infratentorial regions in infants with CHD, we found differential correlations between smaller brain measures and altered white matter diffusion. Disturbances in white matter microstructure related to cerebral growth in the frontal and parietal regions, but not to cerebellar or brainstem growth. This emphasizes the importance of regional investigations of cerebral development.
We did not find any relationship between white matter injury and delayed brain growth or maturity. This finding appeared to be consistent across all assessments of growth and maturity – qualitative scoring, quantitative brain metrics, and diffusion measures. Miller et al described similar findings with evidence of delayed maturation by diffusion and spectroscopy that was unrelated to brain injury.6 However, an association between cerebral immaturity and white matter injury has been described in other data.25
It is currently unclear how these pre-operative findings impact clinical and neurodevelopmental outcome. Alterations in frontal lobe volume in infants with CHD at 15 months of age appear to relate to motor performance. 12 However, there are no data reporting the relationship between pre-operative brain MRI findings (injury or maturation) to neurological outcome, making this an area worthy of future investigation. The ability to fully characterize the nature and timing of cerebral abnormalities and the long-term impact of these lesions may provide insight into practice changes or potential neuroprotective interventions.
The primary limitation of this study is the lack of term born control infants with diffusion imaging. Because our control infants had different MRI acquisitions, we could not reliably compare diffusion values. There may also be regional or lesion specific differences in diffusion that we were not able to delineate due to small sample size.
In conclusion, our findings confirm that pre-operative cerebral abnormalities, including delayed maturation and white matter injury, are common in infants with CHD. Our additional new findings include regional vulnerability with more pronounced abnormalities in the frontal lobe and brainstem. This may be secondary to the regional differences in normal brain development and/or regional alterations in cerebral circulation. However, further studies are needed to investigate the mechanisms and timing of these abnormalities and how they relate to clinical and neurological outcomes.
Acknowledgments
Role of Funding
This research was supported by grants from the National Heart Foundation of New Zealand, the Green Lane Research and Education Fund, the Auckland Medical Research Fund, the Doris Duke Charitable Foundation, the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HD043010, the National Institutes of Health K23 HD053212, and award number P30HD062171 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development.
Footnotes
Disclosures/Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 2.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153:55–60. doi: 10.1016/j.jpeds.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807–11. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–14. [PubMed] [Google Scholar]
- 5.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 6.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 7.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. discussion 36–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 9.Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15:543–52. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 10.Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Matsui M, Matsuzawa J, et al. Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2009;137:146–53. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30:125–31. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy RR, McGaurn SA, Wernovsky G, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–57. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 15.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–9. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 16.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 17.Cheong JL, Thompson DK, Wang HX, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30:623–8. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart AR, Smith MF, Rigby AS, Wallis LI, Whitby EH. Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol. 2010;40:1390–6. doi: 10.1007/s00247-010-1633-7. [DOI] [PubMed] [Google Scholar]
- 19.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi R, Gupta RK, Husain N, et al. Region-specific maturation of cerebral cortex in human fetal brain: diffusion tensor imaging and histology. Neuroradiology. 2009;51:567–76. doi: 10.1007/s00234-009-0533-8. [DOI] [PubMed] [Google Scholar]
- 21.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 22.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological psychology. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Martinez R, Figueras F, Oros D, et al. Cerebral blood perfusion and neurobehavioral performance in full-term small-for-gestational-age fetuses. Am J Obstet Gynecol. 2009;201:474, e1–7. doi: 10.1016/j.ajog.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–43. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 25.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. The Journal of thoracic and cardiovascular surgery. 2010;139:543–56. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]