Abstract
Background
Prior studies have presented contradictory results after analyzing associations between donor and recipient sex on survival after heart transplantation and causes of death such as acute rejection (AR) and cardiac allograft vasculopathy (CAV). We used the ISHLT Registry, the largest repository of heart transplant outcomes worldwide, to comprehensively address these questions.
Methods
We studied 60,584 adult recipients of heart transplants performed between 1990 and 2008. Outcomes of interest were overall survival, death-censored allograft survival, AR, and CAV, which were studied using regression models. To assess whether donor: recipient sex mismatch affected outcomes, the experience of male recipients with female versus male donors was compared to that of female recipients with female versus male donors through inclusion of an interaction term between donor and recipient sex.
Results
Significant differences were observed between male and female recipients in overall survival and death-censored allograft survival for female versus male donors. Male recipients of female allografts had a 10% increase in adjusted mortality relative to male recipients of male allografts, while female recipients of female allografts had a 10% decrease in adjusted mortality relative to female recipients of male allografts (p<0.0001). Findings were similar for death-censored allograft survival. There were no significant differences in the effect of donor sex on AR or CAV between male and female recipients.
Conclusions
Analysis of the ISHLT data set has demonstrated a strong association between donor: recipient sex-mismatch and reduced survival after heart transplantation.
INTRODUCTION
Orthotopic heart transplantation (OHT) has evolved into the treatment of choice for patients with end-stage heart disease who continue to have symptoms despite maximal medical therapy. However, outcomes after heart transplantation remain constrained by sub-optimal long-term survival, as well as by the development of acute rejection (AR) and cardiac allograft vasculopathy (CAV). These adverse events have motivated the investigation of donor- and recipient-specific characteristics that influence post-transplant outcomes.
There are many reasons to believe that donor and recipient sex may play an important role in outcomes after OHT. The relationship between sex hormones and immunological processes has been extensively documented, but is not well understood. Several early studies identified female donor sex as an independent predictor of recipient mortality after OHT.(1–4) Further investigations, however, highlighted the importance of donor: recipient sex mismatch, with demonstration of reduced short- and long-term survival in male recipients of female allografts.(5–8) More recently, an analysis of the United Network for Organ Sharing database examined the impact of donor: recipient sex matching on post-transplant survival, and demonstrated that men who received male allografts had the highest cumulative 5-year survival compared to other donor: recipient sex combinations.(9)
With respect to other post-transplant outcomes, several single-center studies have reported a higher incidence of AR in female OHT recipients.(10, 11) Other reports have suggested that donor: recipient sex mismatch increases the number of rejection events after OHT.(7) These studies, however, are all hindered by their small sample size (N=150–366). Finally, CAV, an obliterative vascular disease involving the allograft coronary arteries,(12) is the leading cause of death beyond the first year after OHT.(13, 14) Although CAV is thought to be partially immune-mediated, there is a paucity of data examining the role of sex-based differences in development of CAV. Again, single-center reports have suggested associations between donor and recipient sex mismatch and the development of CAV (15–17) but their results are conflicting.
Given the limitations of prior work on this topic, we analyzed the ISHLT Registry to examine the influence of donor and recipient sex on heart transplant outcomes over a twenty year period. Unlike prior studies, we compare the experience of male recipients with sex-matched versus mismatched allografts to that of female recipients with sex-matched versus mismatched allografts through use of an interaction term between donor and recipient sex. This approach formally addresses whether donor: recipient sex mismatch is associated with the outcomes of interest.
METHODS
Data Source
We used registry data provided by the ISHLT, which collects data on the worldwide thoracic organ transplant experience. No patient or center identifiers were included in this analysis; institutional review board approval was therefore not required by our center. The data set is comprised of heart transplants performed between January 1990 and December 2008, with follow-up through September 2010.
Study Design
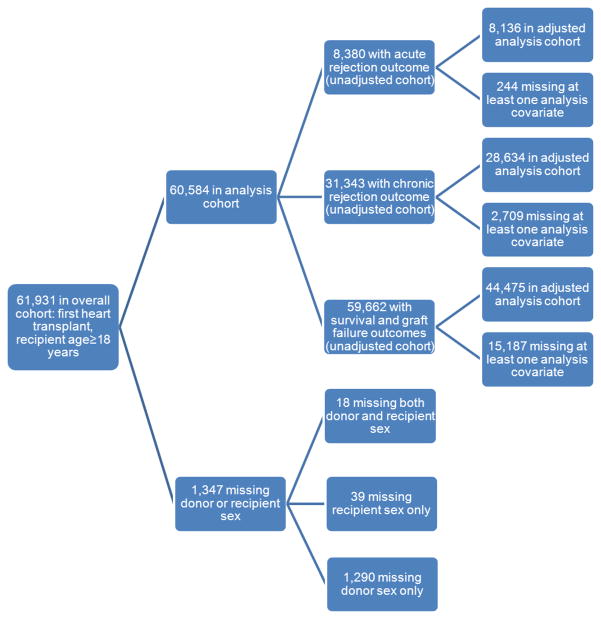
We retrospectively examined a cohort of adults (≥ 18 years) who received their first heart transplant between 1990 and 2008. Of the 61,931 subjects in the overall cohort, 1,347 were excluded due to missing data on donor or recipient sex, yielding a final cohort size of 60,584 (Figure 1).
Figure 1.
ISHLT cohort construction and missing data
Study Variables and Outcomes
The transplant data set contained 297 variables pertaining to the pre-operative, intraoperative, and post-operative period until hospital discharge after the transplant surgery. Follow-up data sets included 54 variables related to post-transplant outcomes and events. We selected variables for analysis based on clinical relevance and results from prior studies. These included donor variables, recipient variables, and transplant-specific variables. The endpoints examined were (1) time to death, (2) time to death-censored allograft failure, (3) AR (treated or not) during the first two years post-transplant, and (4) development of CAV.
Statistical Analysis
Outcome definitions
(1) Overall survival is derived as time from transplantation until death, where subjects who did not die during their observed study period were censored at their last follow up, (2) Death-censored allograft survival is defined as time from transplantation to occurrence of allograft failure, where subjects were censored at either their death (if it was unrelated to allograft failure) or at the time of last follow up, (3) AR was defined as acute rejection (treated or not) occurring within two years of transplantation. AR was assigned to be missing if no rejection status was ever reported, and (4) CAV was defined as coronary artery disease ever occurring during follow-up. CAV was assigned to be missing if CAV status was never reported during follow up.
Analytic tools
Regression techniques were employed to assess whether there was an association between donor: recipient sex mismatch and outcomes of interest. For time to death and time to death-censored allograft failure, Cox proportional hazards models that account for censoring were employed. The former yields odds ratios and the latter yields hazard ratios that describe the differential risk in outcome by donor and recipient sex status. For time-to-event data, Kaplan-Meier estimates were generated to graphically depict survival curves corresponding to four donor: recipient sex combinations. For the binary outcomes (AR and CAV) logistic regression techniques were used. All analyses were performed with SAS software, version 9.2 (copyright 2011 SAS Institute Inc).
Missing Data
For all analyses, a complete case analysis was performed, i.e. patients missing at least one variable in the model (outcome or covariate) were excluded from the analysis. For several variables, implausible values were observed. This phenomenon most likely stems from the multinational nature of the ISHLT database resulting in the use of various measurement scales. No assumptions could be made about the measurement system used, however, for a particular value. For these variables, values that were determined to be outside a plausible range were set to missing. An exception was with pulmonary vascular resistance, where the ranges of plausible values assuming two common scales did not overlap and hence values on one scale (between 80 and 560 dynes-sec/cm5) were re-calibrated to the other (divided by 80 to yield Wood Units).
Potential Confounders
We adjusted for recipient-, donor-, and transplant-related characteristics. These included donor and recipient age; donor cause of death; recipient diagnosis (indication for transplantation); and transplant era. Transplant era was defined by year of transplant in four categories: 1990–1994, 1995–1999, 2000–2004, and 2005–2008. To further explore donor: recipient weight mismatch as a potential confounder, we included weight as a covariate in the models in three ways (1) donor and recipient weight, (2) difference between donor and recipient weight, and (3) ratio of recipient weight to donor weight. We were unable to adjust for other potentially relevant covariates (such as graft ischemic time, recipient waitlist prioritization status, and recipient co-morbidities) due to the large amount of missing data for each variable.
Models
To assess whether there is an effect of donor: recipient sex mismatch on heart transplant outcomes, it is of interest to evaluate whether the experience of a female recipient with a female (versus male) allograft differs from that of a male recipient with a female (versus male) allograft. This is equivalent to assessing whether there is an interaction effect between donor and recipient sex. Thus all models include recipient and donor sex as main effects as well as a product term of the two. Unadjusted models included only main effects and the interaction term; adjusted models included these terms as well as the covariates listed above.
RESULTS
Study Cohort
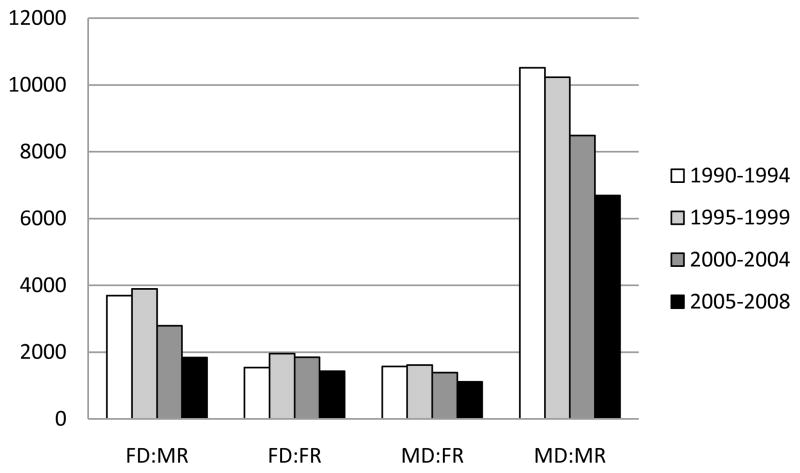
We examined 60,584 adults receiving their first heart transplant. Seventy nine percent of the heart transplant recipients were male. The mean recipient age was 51 ± 12 years, and the main indications for transplantation were dilated cardiomyopathy (45%) and coronary artery disease (44%). The median follow-up time was 4.1 years (interquartile range, 8.2 years) and a total of 24,531 recipients died during this follow-up period. Categorization of recipients by donor sex resulted in four groups: male recipient-male donor (n=35,927), male recipient-female donor (n=12,215), female recipient-male donor (n=5,680), and female recipient-female donor (n=6,762). The distribution of donor: recipient sex groups by transplant era, divided in 5 year intervals, are presented in Figure 2.
Figure 2.
Distribution of heart transplants performed during the study period, stratified by donor and recipient sex and transplant era. FD: female donor, MD: male donor, FR: female recipient, MR: male recipient.
Baseline Characteristics
There were significant differences between male and female recipients at baseline (Table 1). Specifically, male recipients were older and heavier. They had a higher incidence of hypertension and diabetes, higher serum creatinine, and were more likely to have coronary artery disease as the indication for transplantation. Moreover, male recipients were more likely to be hospitalized and require hemodynamic support prior to transplant. Female recipients had higher pulmonary vascular resistance and PRA, and a slightly longer allograft ischemic time. Donors of cardiac allografts to male recipients tended to be older and have a higher incidence of head trauma as the cause of death, compared to donors of allografts to female recipients.
Table 1.
Characteristics of Study Cohort, Stratified by Donor and Recipient Sex
| Variable | Female Recipient | Male Recipient | All | p-value | ||
|---|---|---|---|---|---|---|
| Female Donor | Male Donor | Female Donor | Male Donor | |||
| Total N | 6,762 (11%) | 5,680 (9%) | 12,215 (20%) | 35,927 (59%) | 60,584 | |
| Treatment Era | <0.0001 | |||||
| 1990–1994 | 1,536 (23%) | 1,572 (28%) | 3,694 (30%) | 10,518 (29%) | 17,320 | |
| 1995–1999 | 1,954 (29%) | 1,609 (28%) | 3,894 (32%) | 10,233 (29%) | 17,690 | |
| 2000–2004 | 1,848 (27%) | 1,386 (24%) | 2,788 (23%) | 8,487 (24%) | 14,509 | |
| 2005–2008 | 1,424 (21%) | 1,113 (20%) | 1,839 (15%) | 6,689 (19%) | 11,065 | |
| Recipient Demographics | ||||||
| Age (years) | 49 ± 12 | 48 ± 13 | 52 ± 11 | 52 ± 11 | 51 ± 12 | <0.0001 |
| Weight (kg) | 64 ± 13 | 68 ± 14 | 75 ± 13 | 82 ± 14 | 77 ± 15 | <0.0001 |
| Pre-Transplant Status | ||||||
| Hospitalized | 2,028 (47%) | 2,422 (56%) | 4,110 (55%) | 12,781 (55%) | 21,341 | <0.0001 |
| On inotropes | 1,676 (41%) | 1,874 (44%) | 3,116 (44%) | 9,664 (43%) | 16,330 | 0.0018 |
| On life support | 2,018 (50%) | 2,263 (55%) | 3,776 (56%) | 12,460 (58%) | 20,517 | <0.0001 |
| Recipient Comorbidities | ||||||
| Hypertension | 1,075 (31%) | 1,092 (33%) | 1,976 (36%) | 6,854 (39%) | 10,997 | <0.0001 |
| Diabetes | 557 (16%) | 543 (17%) | 969 (19%) | 3,685 (22%) | 5,754 | <0.0001 |
| Serum creatinine (mg/dl) | 1.12 ± 0.51 | 1.14 ± 0.49 | 1.35 ± 0.55 | 1.36 ± 0.52 | 1.31 ± 0.53 | <0.0001 |
| Mean pulmonary artery pressure (mmHg) | 29 ± 10 | 30 ± 10 | 30 ± 10 | 31 ± 11 | 30 ± 11 | <0.0001 |
| Pulmonary vascular resistance (WU) | 2.8 ± 1.3 | 2.8 ± 1.3 | 2.6 ± 1.3 | 2.6 ± 1.2 | 2.6 ± 1.3 | <0.0001 |
| Panel of reactive antibodies (%) | 6.7 ± 8.5 | 6.6 ± 18.2 | 2.9 ± 11.6 | 2.8 ± 10.7 | 3.7 ± 13.1 | <0.0001 |
| Diagnosis | <0.0001 | |||||
| Coronary artery disease | 1,617 (24%) | 1,356 (24%) | 5,949 (50%) | 17,427 (49%) | 26,349 | |
| Cardiomyopathy | 3,855 (58%) | 3,347 (60%) | 4,827 (40%) | 14,830 (42%) | 26,859 | |
| Other | 1,167 (18%) | 900 (16%) | 1,210 (10%) | 3,062 (9%) | 6,339 | |
| Donor characteristics | ||||||
| Age (years) | 35 ± 14 | 28 ± 12 | 37 ± 13 | 31 ± 12 | 33 ± 13 | <0.0001 |
| Weight (kg) | 65 ± 14 | 72 ± 14 | 71 ± 15 | 80 ± 14 | 76 ± 15 | <0.0001 |
| Cause of death | <0.0001 | |||||
| Anoxia | 439 (7%) | 307 (6%) | 676 (6%) | 1,375 (4%) | 2,797 | |
| Stroke | 2,842 (46%) | 944 (18%) | 5,351 (47%) | 6,793 (21%) | 15,930 | |
| Head trauma | 1,920 (31%) | 3,080 (58%) | 3,058 (27%) | 17,926 (55%) | 25,984 | |
| CNS tumor | 70 (1%) | 48 (1%) | 148 (1%) | 257 (1%) | 523 | |
| Other | 911 (15%) | 931 (18%) | 2,055 (18%) | 6,498 (20%) | 10,395 | |
| Graft ischemic time (hours) | 2.6 ± 1.5 | 2.7 ± 1.3 | 2.5 ± 1.5 | 2.6 ± 1.4 | 2.6 ± 1.4 | <0.0001 |
Heart transplant outcomes
The results of our multivariate models are presented below. In summary, significant differences were observed between male and female recipients in overall survival and death-censored allograft survival for female versus male donors. Male recipients of female allografts had increased mortality relative to male recipients of male allografts, while female recipients of female allografts had reduced mortality relative to female recipients of male allografts. There were no significant differences in the effect of donor sex on AR or CAV between male and female recipients; however, these analyses were limited by the large amount of missing data on these outcomes, as well as differences between transplant centers in assessing for and reporting the presence of AR and CAV. We have presented the data for models adjusting for donor and recipient weight, among the other covariates mentioned previously. Results did not differ significantly from the models adjusting for donor: recipient weight difference and the ratio of recipient: donor weight (data not shown).
Death and Death-Censored Allograft Failure
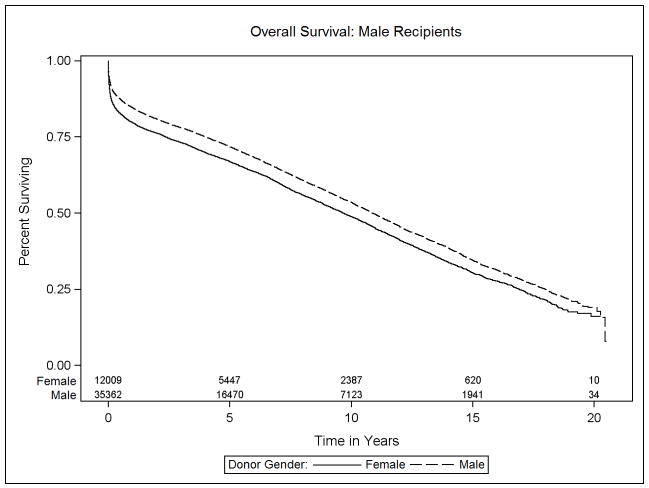
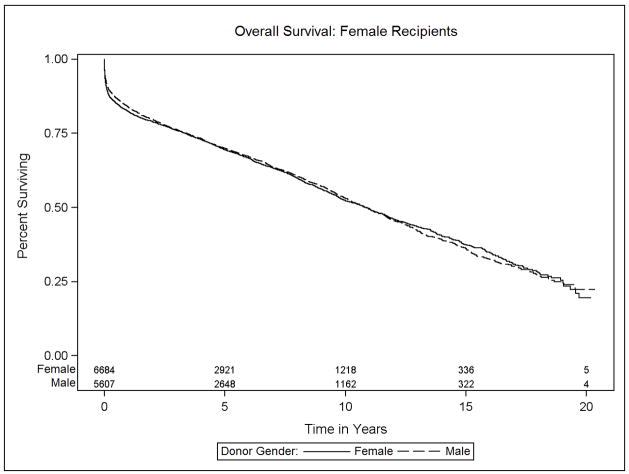
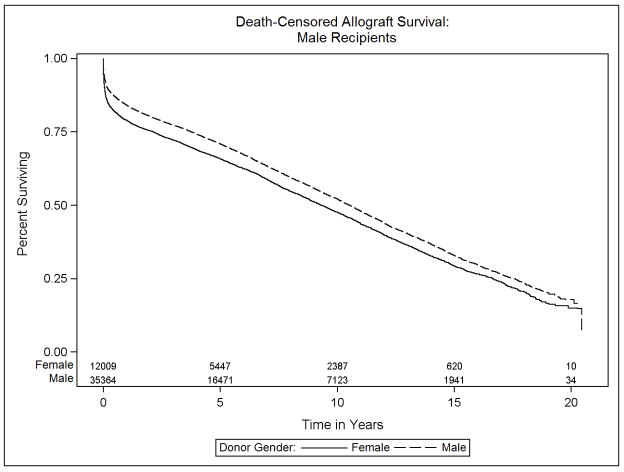
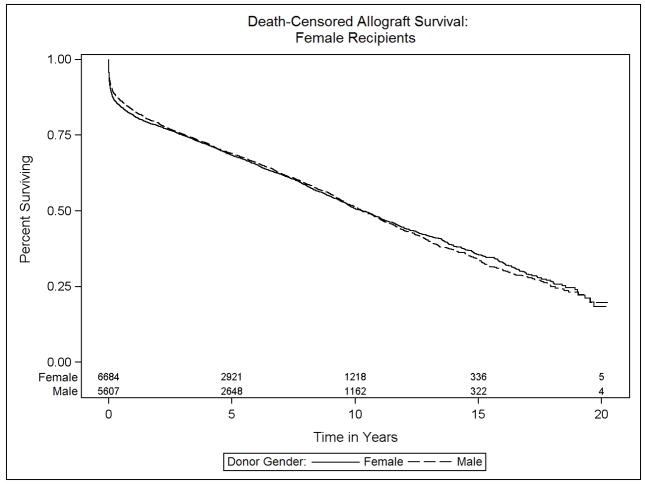
Data on transplant recipient death and death-censored allograft failure was available on 98.5% (59,662 of 60,584 recipients) of donor: recipient pairs in this study. The median survival was 10.6 years (interquartile range, 14.4 years). The effect of donor sex differed significantly between male and female recipients (p<0.001). Specifically, among male recipients, receipt of a female relative to male allograft resulted in a 10% increase in the risk of mortality (HR 1.10, 95% CI 1.04–1.17). Female recipients, in contrast, experienced a 10% survival advantage after receipt of a female versus male allograft (HR 0.90, 95% CI 0.83–0.98). Figures 3a and 3b, Table 2).
Figure 3.
Kaplan-Meier estimates of overall survival for heart transplant recipients, stratified by donor and recipient sex: (a) Male recipients, (b) Female recipients
Table 2.
Death--Unadjusted and Adjusted Cox Proportional Hazards Models
| Model | Size | Recipient | Donor | HR | 95% CI | Interaction p-value* |
|---|---|---|---|---|---|---|
| Unadjusted | 59,662 | Male | Female vs Male | 1.18 | 1.14–1.21 | |
| Female | Female vs Male | 1.01 | 0.96–1.07 | <0.001 | ||
| Adjusted | 24,136 | Male | Female vs Male | 1.10 | 1.04–1.17 | |
| Female | Female vs Male | 0.90 | 0.83–0.98 | <0.001 |
p-value to compare male and female recipients’ experience in receiving a sex-matched vs mismatched allograft
We then examined the endpoint of death-censored allograft failure in order to focus on allograft failure events leading to death or re-transplantation. Similar to overall survival, male and female recipients differed in their experience with respect to donor sex p=0.0003, Figures 4a and 4b, Table 3). Male recipients of female allografts had a 9% increase in the risk of adjusted death-censored allograft failure relative to male recipients of male allografts (HR 1.09, 95% CI 1.03–1.15). Conversely, examination of female recipients revealed a slight decrease in the relative hazard of death-censored allograft failure between those receiving same versus opposite sex donor organs (HR 0.91, 95% CI 0.83–0.99).
Figure 4.
Kaplan-Meier estimates of death-censored allograft survival for heart transplant recipients, stratified by donor and recipient sex: (a) Male recipients, (b) Female recipients
Table 3.
Death-Censored Allograft Failure--Unadjusted and Adjusted Cox Proportional Hazards Models
| Model | Size | Recipient | Donor | HR | 95% CI | Interaction p-value* |
|---|---|---|---|---|---|---|
| Unadjusted | 59,664 | Male | Female vs Male | 1.17 | 1.14–1.21 | |
| Female | Female vs Male | 1.01 | 0.96–1.07 | <0.001 | ||
| Adjusted | 24,136 | Male | Female vs Male | 1.09 | 1.03–1.15 | |
| Female | Female vs Male | 0.91 | 0.83–0.99 | 0.0003 |
p-value to compare male and female recipients’ experience in receiving a sex-matched vs mismatched allograft
Acute Rejection and Cardiac Allograft Vasculopathy
To further investigate possible causes for the differential survival seen between sex-matched versus mismatched heart transplants, we examined two common post-transplant complications: AR and CAV. These analyses were limited by the large amount of missing data on these outcomes in the ISHLT Registry. Specifically, data on acute rejection was available on 14% of recipients (8,380/60,584) and data on CAV was available for 52% of recipients (31,343/60,584). Furthermore, methods for diagnosis and voluntary reporting of these post-transplant complications varied between centers. Thus, these results are suggestive but not definitive, and should be interpreted with caution.
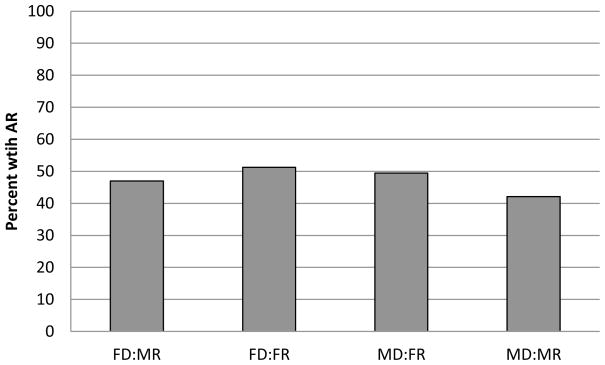
The incidence of acute rejection during the first two years post-transplant was 45% (Figure 5). Although men who received female versus male allografts had a 22% increase in the odds of acute rejection, and females who received female versus male allografts had no increase in the odds of rejection, the effect of donor sex did not differ significantly by recipient sex (p=0.27). Models that adjust for potential confounders yielded comparable descriptions of these relations (Table 4).
Figure 5.
Incidence of acute rejection by two years post-transplant, stratified by donor and recipient sex
Table 4.
Acute Rejection--Unadjusted and Adjusted Logistic Regression Models
| Model | Size | Recipient | Donor | OR | 95% CI | Interaction p-value* |
|---|---|---|---|---|---|---|
| Unadjusted | 8,380 | Male | Female vs Male | 1.22 | 1.07–1.38 | |
| Female | Female vs Male | 1.21 | 1.05–1.39 | 0.27 | ||
| Adjusted | 7,638 | Male | Female vs Male | 1.21 | 1.05–1.39 | |
| Female | Female vs Male | 1.06 | 0.87–1.28 | 0.25 |
p-value to compare male and female recipients’ experience in receiving a sex-matched vs mismatched allograft
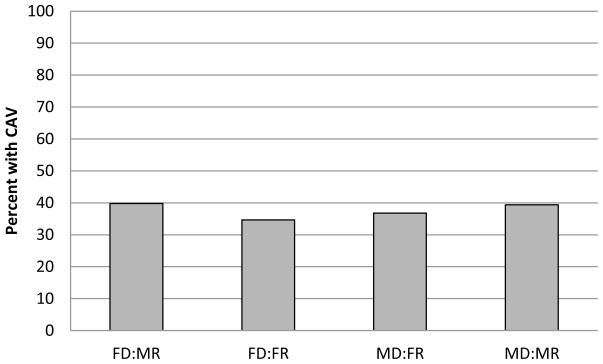
The overall incidence of CAV development, at any time post-transplant, was 39% (Figure 4). Receipt of a female allograft was associated with a lower incidence of CAV. Specifically, male recipients of a female versus male allograft had a 19% lower odds of developing CAV (OR 0.81, 95% CI 0.74–0.88). Similarly, female recipients of a female versus male allograft had 18% lower odds of developing CAV (multivariate OR 0.82, 95% CI 0.72–0.93) (Table 5), where the effect of donor sex on CAV did not differ by recipient sex (p=0.9).
Table 5.
Cardiac Allograft Vasculopathy--Unadjusted and Adjusted Logistic Regression Models
| Model | Size | Recipient | Donor | OR | 95% CI | Interaction p-value* |
|---|---|---|---|---|---|---|
| Unadjusted | 31,343 | Male | Female vs Male | 1.02 | 0.96–1.08 | |
| Female | Female vs Male | 0.91 | 0.83–1.01 | 0.06 | ||
| Adjusted | 20,935 | Male | Female vs Male | 0.81 | 0.74–0.88 | |
| Female | Female vs Male | 0.82 | 0.72–0.93 | 0.9 |
p-value to compare male and female recipients’ experience in receiving a sex-matched vs mismatched allograft
DISCUSSION
We have leveraged the advantages provided by the ISHLT Registry, the largest existing data repository of heart transplant outcomes worldwide, to examine differences in post-transplant survival based on donor and recipient sex. We then explored the survival differences identified by examining differences in AR and CAV in sex-matched versus mismatched transplants, although these analyses were limited by missing data and inconsistent data quality. In doing so, we have built upon previous studies in which these endpoints were studied individually.
We first examined differences in survival between our four donor: recipient sex strata, and demonstrated that patients who undergo sex-matched heart transplantation have improved survival compared to those with sex-mismatched allografts. This observation is especially profound for male recipients of female allografts, who consistently demonstrate the lowest survival in the short- and long-term. To specifically examine the relationship between sex-differences and allograft loss after heart transplantation, we studied the endpoint of death-censored allograft failure, which includes allograft loss leading to death or retransplantation, and excludes deaths due to causes other than allograft failure, such as infection and malignancy. As with overall survival, this analysis also showed inferior outcomes in sex-mismatched transplants, with the poorest outcomes for male recipients of female allografts.
Previous studies have demonstrated inferior survival after heart transplantation in male recipients of female allografts. These range from single-center studies of 174(7) to 869(5) heart transplants, to multi-center registry studies including 18,000(9) to 25,000(8) heart transplant procedures. By utilizing the ISHLT Registry, we were able to confirm these previous findings with survival data on close to 60,000 heart transplant recipients worldwide.
Several theories have been proposed to explain the increased mortality seen after sex-mismatched heart transplants. By examining differences in development of AR and CAV in this large patient population, we were able to examine major post-transplant complications that may have impacted survival. We did not detect significant differences in AR or CAV between male and female heart transplant recipients, when comparing sex-matched versus mismatched transplants, suggesting that these complications did not account for the survival differences observed. However, our analyses had limitations. In the case of AR, transplant centers have varying practices with respect to endomyocardial biopsies and other routine screening techniques. Furthermore, voluntary reporting of this post-transplant complication to the Registry was low (14%). In the case of CAV, it is worth noting that the Registry does not have a uniform definition or grading system, such that some centers may have diagnosed CAV based on angiography and others by intravascular ultrasound. Furthermore, data on CAV severity was not available.
While keeping these limitations in mind, we found significantly lower odds of CAV development in recipients of female donor organs. This finding contrasts with prior single-center studies showing a higher incidence of CAV in recipients of female donor hearts.(11, 16–18) The reason behind this discrepancy is unclear, but we suspect that our findings may reflect a higher prevalence of coronary atherosclerosis in male donor organs. Previous studies have shown that the presence of donor atherosclerosis is associated with the development of angiographic CAV after heart transplantation.(19) Furthermore, “baseline” coronary angiography with intravascular ultrasound imaging at one month post-transplant has demonstrated greater coronary artery maximal intimal thickness in male allografts.(20) Thus, recipients of female allografts may have less CAV due to less pre-existing donor disease. On the other hand, it is certainly possible that post-transplant factors may result in less coronary artery endothelial injury in female allografts. Regardless, we did not detect any difference in CAV development when comparing male and female recipients’ experience in receiving a sex-matched versus mismatched allograft.
Other theories for differences in mortality between donor: recipient sex strata refer to genetic, hormonal,(21) and immunologic(22, 23) factors. Furthermore, sex differences in susceptibility to ischemia-reperfusion injury have also been proposed.(8) All of these theories, however, remain speculative and require further investigation. Nevertheless, this study provides further evidence for a significant survival advantage for recipients of sex-matched heart transplants. This data lends support to sex matching, when feasible, bearing in mind the complex nature of the matching process that must account for recipient acuity (waitlist status), donor and recipient blood type, and the presence of pre-formed anti-HLA antibodies in the transplant recipient. This survival advantage persisted after adjusting for donor: recipient weight differences and may therefore support liberalization of our current strategy for size matching.
This study is limited by its retrospective nature, as we do not have control over the data quality. Reporting to the ISHLT registry is voluntary and there is a large amount of incomplete follow-up and missing data. This limited our ability to include potential confounders in the multivariable models, as doing so would have significantly reduced the sample size and power of our analyses. Variable definitions and inconsistencies in reporting also affected data quality. Furthermore, uniform definitions were lacking for many variables, including the study endpoints. We now recognize differences between acute cellular and antibody-mediated rejection based on histologic appearance; however, AR in this data set likely encompasses both processes. In addition, there was no standard definition for diagnosis of CAV, as mentioned previously. This, combined with a lack of data on CAV severity, limits our ability to further explore these outcomes. We also acknowledge that center-specific differences may affect heart transplant outcomes—the very large and international experience represented in this database hopefully mitigates that effect.
CONCLUSIONS
By using the ISHLT Registry, we have demonstrated a strong association between sex-matched allografts and survival. In particular, male recipients of female allografts have reduced overall survival and death-censored graft survival, while female recipients of female allografts confer an advantage with respect to these outcomes. These results thereby advance our knowledge of sex-related differences in heart transplantation.
Figure 6.
Prevalence of cardiac allograft vasculopathy by last follow-up visit, stratified by donor and recipient sex
Footnotes
DISCLOSURES: This work was funded by grants from the International Society for Heart and Lung Transplantation and the National Institutes of Health (K23HL091143). There are no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryan CF, Mitchell SI, Borkon AM, et al. Influence of donor gender on patient mortality after heart transplantation. Transplant Proc. 1996;28:149–51. [PubMed] [Google Scholar]
- 2.McCarthy JF, McCarthy PM, Massad MG, et al. Risk factors for death after heart transplantation: does a single-center experience correlate with multicenter registries? Ann Thorac Surg. 1998;65:1574–8. doi: 10.1016/s0003-4975(98)00138-6. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 3.Solomon NA, McGiven JR, Alison PM, et al. Changing donor and recipient demographics in a heart transplantation program: influence on early outcome. Ann Thorac Surg. 2004;77:2096–102. doi: 10.1016/j.athoracsur.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 4.Tsai FC, Marelli D, Bresson J, et al. Recent trends in early outcome of adult patients after heart transplantation: a single-institution review of 251 transplants using standard donor organs. Am J Transplant. 2002;2:539–45. doi: 10.1034/j.1600-6143.2002.20608.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khaldi A, Oyer PE, Robbins RC. Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant. 2006;25:461–8. doi: 10.1016/j.healun.2005.11.456. [DOI] [PubMed] [Google Scholar]
- 6.De Santo LS, Marra C, De Feo M, Amarelli C, Romano G, Cotrufo M. The impact of gender on heart transplantation outcomes: a single center experience. Ital Heart J. 2002;3:419–23. [PubMed] [Google Scholar]
- 7.Prendergast TW, Furukawa S, Beyer AJ, 3rd, Browne BJ, Eisen HJ, Jeevanandam V. The role of gender in heart transplantation. Ann Thorac Surg. 1998;65:88–94. doi: 10.1016/s0003-4975(97)01105-3. [DOI] [PubMed] [Google Scholar]
- 8.Zeier M, Dohler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–6. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ES, Allen JG, Patel ND, et al. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail. 2009;2:401–8. doi: 10.1161/CIRCHEARTFAILURE.108.844183. [DOI] [PubMed] [Google Scholar]
- 10.Esmore D, Keogh A, Spratt P, Jones B, Chang V. Heart transplantation in females. J Heart Lung Transplant. 1991;10:335–41. [PubMed] [Google Scholar]
- 11.Sharples LD, Caine N, Mullins P, et al. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991;52:244–52. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23:S187–93. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo MR, Naftel DC, Pritzker MR, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998;17:744–53. [PubMed] [Google Scholar]
- 14.Gao SZ, Schroeder JS, Hunt S, Stinson EB. Retransplantation for severe accelerated coronary artery disease in heart transplant recipients. Am J Cardiol. 1988;62:876–81. doi: 10.1016/0002-9149(88)90885-5. [DOI] [PubMed] [Google Scholar]
- 15.Caforio AL, Tona F, Fortina AB, et al. Immune and nonimmune predictors of cardiac allograft vasculopathy onset and severity: multivariate risk factor analysis and role of immunosuppression. Am J Transplant. 2004;4:962–70. doi: 10.1111/j.1600-6143.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 16.Erinc K, Yamani MH, Starling RC, et al. The influence of donor gender on allograft vasculopathy: evidence from intravascular ultrasound. Transplant Proc. 2004;36:3129–31. doi: 10.1016/j.transproceed.2004.10.072. [DOI] [PubMed] [Google Scholar]
- 17.Mehra MR, Stapleton DD, Ventura HO, et al. Influence of donor and recipient gender on cardiac allograft vasculopathy. An intravascular ultrasound study. Circulation. 1994;90:II78–82. [PubMed] [Google Scholar]
- 18.Yamani MH, Erinc SK, McNeill A, et al. The impact of donor gender on cardiac peri-transplantation ischemia injury. J Heart Lung Transplant. 2005;24:1741–4. doi: 10.1016/j.healun.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Tanaka K, Anzai H, et al. Influence of pre-existing donor atherosclerosis on the development of cardiac allograft vasculopathy and outcomes in heart transplant recipients. J Am Coll Cardiol. 2006;47:2470–6. doi: 10.1016/j.jacc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 20.Tuzcu EM, Hobbs RE, Rincon G, et al. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation. Insights from intravascular ultrasound. Circulation. 1995;91:1706–13. doi: 10.1161/01.cir.91.6.1706. [DOI] [PubMed] [Google Scholar]
- 21.Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–79. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 22.Panajotopoulos N, Ianhez LE, Neumann J, Sabbaga E, Kalil J. Immunological tolerance in human transplantation. The possible existence of a maternal effect. Transplantation. 1990;50:443–5. doi: 10.1097/00007890-199009000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Shibue T, Kondo K, Iwaki Y, Terasaki PI. Effect of sex on kidney transplants. Clin Transpl. 1987:351–60. [PubMed] [Google Scholar]