Abstract
The ERK 1/2 protein require a dual phosphorylation at conserved threonine and tyrosine residues to be fully activated under normal physiological conditions. Thus, ERK1/2 kinase activity is often defined by the quantity of phosphorylated kinase. However, this may not accurately represent its true activity under certain pathological conditions. We investigated whether ERK1/2 kinase activity is proportional to its phosphorylation state in a rat focal ischemia model with and without rapid ischemic preconditioning. We showed that phosphorylated-ERK1/2 protein levels were increased 2.6±0.07 fold, and ERK1/2 kinase activity was increased 10.6±1.9 fold in animals receiving ischemic preconditioning alone without test ischemia compared with sham group (P<0.05, n=6/group), suggesting that phosphorylated-ERK1/2 protein levels represent its kinase activity under these conditions. However, preconditioning plus test ischemia robustly blocked ERK1/2 kinase activity, while it increased phosphorylated-ERK1/2 protein levels beyond those receiving test ischemia alone, suggesting that phosphorylated-ERK1/2 protein levels were not representative of actual kinase activity in this pathological condition. In conclusion, protein phosphorylation levels of ERK1/2 do not always correspond to kinase activity, thus, measuring the true kinase activity is essential.
Keywords: ischemic preconditioning, kinase activity, MAPK, ERK1/2, focal ischemia, stroke
1
Mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) 1/2 is involved in neuronal injury induced by stroke (Sawe et al. 2008). ERK1/2 is critical for the characteristic cell proliferation, differentiation and programmed cell death observed in brain injury (Roux and Blenis 2004), therefore, it is a potential target for stroke treatment. However, how ERK1/2 affects brain injury is controversial; it has been shown to both protect and worsen neuronal injury after stroke (Sawe et al. 2008). Understanding the underlying mechanisms of ERK1/2 activity and its role in brain injury after stroke will be important when developing ERK1/2 related stroke treatment.
ERK1/2 kinase activity is characterized by its ability to phosphorylate substrates, including nuclear transcriptional factors, other protein kinases, membrane receptors, and cytoskeletal proteins (Robbins et al. 1993). To be fully active, ERK1/2 must undergo a dual phosphoryation at conserved threonine (Thr) and tyrosine (Tyr) residues (Turjanski et al. 2009). This dual phosphorylation results in conformational changes, domain rotation and remodeling in the ERK1/2 kinase protein, which allows substrates to bind and undergo phosphorylation by ERK1/2 kinase (Turjanski et al. 2009). ERK1/2 must be phosphorylated to be active; therefore, many studies have used protein levels of phosohorylated-ERK1/2 (p-ERK1/2) as a marker for activity. How substrates are recruited to the active site of ERK1/2 and how they are phosphorylated by ERK1/2 is still unclear (Turjanski et al. 2009). In addition, the observation that ERK1/2 is phosphorylated when active is derived from studies using normal physiological conditions and mostly in vitro systems (Boulton et al. 1991). Whether ERK1/2 phosphorylation is fully active in the presence of pathological conditions, such as severe cerebral ischemia, is unclear. In this study, we investigated whether p-ERK1/2 protein levels in a rat focal ischemia model coincide with its kinase activity in the presence or absence of rapid ischemic preconditioning (RIPC). We measured p-ERK1/2 levels by Western blot and detected kinase activity using an in vitro kinase assay, in which kinase activity is measured by its ability to phosphorylate one of its substrates, the Elk-1 protein. Our goal was to improve our understanding of the relationship between p-ERK1/2 protein levels and activity, and to provide insights into the underlying intrinsic protective mechanisms of ischemic preconditioning.
2. Experimental Procedures
2.1. Focal Cerebral Ischemia and preconditioning
Experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care (Protocol #: APLAC 12642), and experiments were conducted in accordance with the guidelines of Animal Use and Care of the National Institutes of Health and Stanford University. All efforts were made to minimize the number of animals used and their suffering. Focal cerebral ischemia was generated as described previously (Zhao et al. 2005). Male 270–350 g Sprague-Dawley rats (Charles River, Wilmington, MA) were used in all experiments. Rats were anesthetized with 5% isoflurane and maintained with 2–3% isoflurane through a face mask. Blood pressure, heart rate and respiratory rate were monitored throughout surgery and kept within physiological ranges. Body temperature was maintained at 37°C throughout surgery. During experiments PO2 was maintained from 100–130 mmHg and PCO2 from 40–60 mmHg. A 2-cm vertical scalp incision was made midway between the left eye and ear. The temporalis muscle was bisected and a 2-mm burr hole was made at the junction of the zygomatic arch and squamous bone. The distal MCA was exposed and cauterized above the rhinal fissure at the cross of lateral vein and MCA. The procedure was carefully performed with minimal injury to the cortex from cauterization. CCAs remain occluded for 30 minutes and clips were removed. Rapid ischemic preconditioning was performed 1hour prior to permanent MCA occlusion (MCAo) by transient MCAo by clip for 15 minutes.
Rats were sacrificed at 1, 5, or 24 hours after MCAo, and tissue corresponding to the ischemic core and penumbra was dissected and prepared for Western blot and kinase assay analysis. Samples from sham-operated rats were also prepared.
2.2.Tissue preparation and Western blotting
Brain tissue corresponding to the ischemic penumbra and core were dissected for Western blot analysis (Fig. 1). Ischemic penumbra was defined as the tissue saved by preconditioning 2 days post-stroke, and the corresponding region from the control ischemic brain was dissected for comparison. Whole cell protein was extracted from fresh brain tissue, and Western blot was performed as described with modification ( Zhao et al. 2005). Samples of 12 µg protein each were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4–15% Ready Gel (BIO-RAD Laboratories, CA) for 1.5 hours. Protein bands were transferred from the gel to polyvinylidinene fluoride (Millipore, Bedford, MA) membranes for 1 hour.
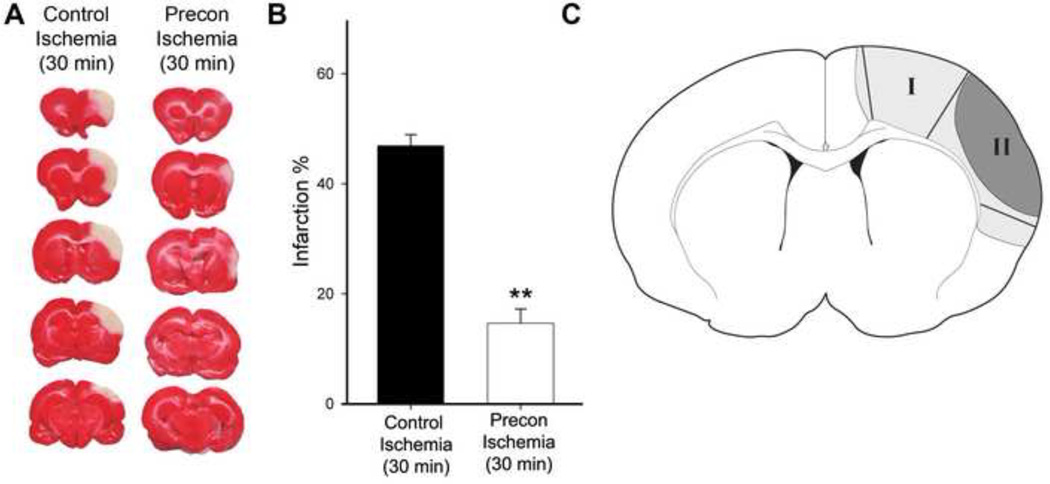
Fig. 1. Rapid preconditioning inhibited infarct size.
A. Representative infarction with TTC staining. Ischemic brains were harvested 2 d after stroke for TTC staining, sectioned into 5 blocks, and stained with TTC solution. B. Bar graphs (the right column) representing the average infarction size normalized to the non-ischemic cortex. N=7/group. ** vs control ischemia (30 min), P<0.01. C. Diagram showing the ischemic tissues corresponding to ischemic penumbra and core that were dissected for analysis. Ischemic penumbra (region I) is defined as the ischemic region spared by preconditioning; the ischemic core (region II) refers to the ischemic area that develops into infarction in animals receiving preconditioning and test ischemia. In the test ischemic group, a line defining the ischemic border was drawn 1.5 mm lateral to the midline. From this line, an approximately 2mm wide area of ischemic tissue was dissected as region I. The remaining ischemic tissue after dissection of region I was dissected as the ischemic core. The corresponding regions in the preconditioning animals were dissected as ischemic penumbra and core.
To determine phosphorylation or total protein levels of ERK1/2, primary antibodies to ERK1/2 (Cell Signaling Technology, MA cat. 9101) were diluted to 1:1000 in blocking solution and incubated with membranes overnight at 4°C with gentle agitation. Next, membranes were incubated 1 hour with horseradish peroxidase-conjugated secondary anti-rabbit antibody (1:1000, Cell Signaling Technology, MA), and for 5 minutes with ECL-Plus-substrate (GE Healthcare, Sunnyvale, CA). Membranes were scanned using Typhoon trio (GE Healthcare, Sunnyvale, CA).
To detect β-actin, membranes were incubated overnight at 4°C with gentle agitation in a mixture of primary antibodies to β-actin (1:20000, rabbit, Bethyl Laboratories, Inc., TX, cat. A300–491A). Secondary antibodies (1:5000, Alexa Fluor®488 donkey anti-rabbit, Invitrogen, CA) to detect β-actin were then incubated with membranes for 1 hour at room temperature. Protein bands of β-actin were scanned by the Typhoon trio.
The optical densities of all protein bands were analyzed using ImageQuant 5.2 software (Molecular Dynamics, Inc). The same samples from rats with sham surgery were used to compare to control animals and postconditioning animals, and all samples were run on the same gel.
2.3. ERK1/2 kinase assay
The p44/p42 MAP Kinase Assay Kit (Cell Signaling Technology, MA) was used to assess ERK1/2 kinase activity. The prepared tissues were assayed according to the manufacturer’s instructions. Cell lysates were incubated with beads for co-immunoprecipitation with ERK1/2, and the beads were then incubated with Elk-1, the substrate protein for the ERK1/2 kinase assay. Kinase activity was revealed by the amount of Elk-1 phosphorylated (p-Elk-1) by ERK1/2; p-Elk-1 was detected by Western blot.
2.4. Statistics
All results were presented as mean ± S.E.M. Statistical analyses were performed by ANOVA followed by the Student-Newman-Keuls post hoc test.
3. Results
3.1.RIPC reduced infarct size
We have previously shown that RIPC robustly reduced infarct size measured at 2 days post-stroke. The results from these experiments for 2 groups (Fig. 1) illustrating that rapid preconditioning inhibited ischemic damage are shown.
3.2. RIPC alone enhanced both ERK1/2 phosphorylation and kinase activity
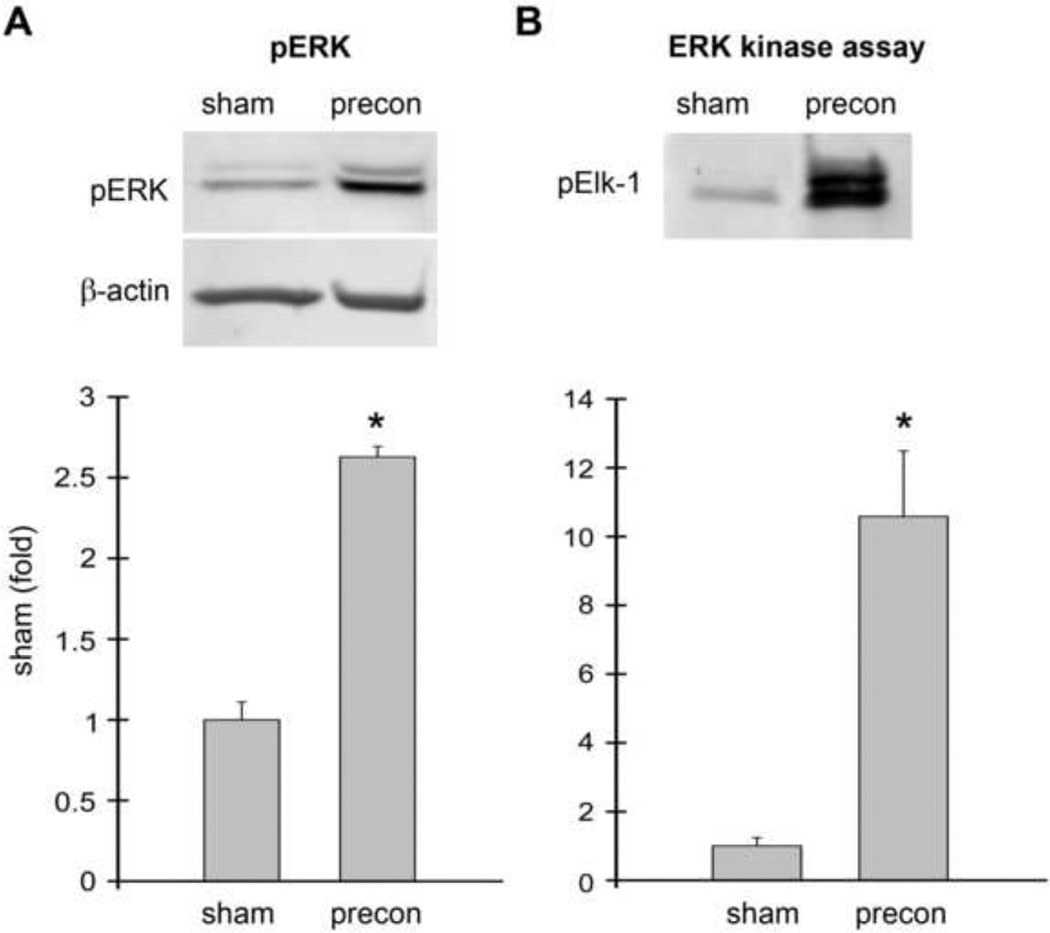
Because stroke was induced 1 hour after RIPC, RIPC itself may have a significant effect on the brain when ischemia occurs. Therefore, we investigated how RIPC affects p-ERK1/2 and kinase activity 1 hour after RIPC. Results by Western blot suggest that p-ERK1/2 levels were significantly increased about 2.5 fold in the RIPC group compared to the sham group (Fig. 2). The in vitro kinase assay results also showed that p-Elk-1 protein levels when phosphorylated by activated ERK1/2 were significantly increased more than 10-fold in RIPC brains. These results suggest that ERK1/2 kinase activity was consistent with p-ERK1/2 protein levels in animals receiving RIPC alone.
Fig. 2. ERK1/2 phosphorylation and activity was increased in rats receiving ischemic preconditioning alone without stroke.
Ischemic preconditioning was performed 1 h prior to permanent MCAo by transient MCAo with a micro clip for 15 min. Rat brains were harvested 1 h after preconditioning; no test ischemia was induced. The brain region corresponding to ischemic penumbra (region I defined in Fig.1) was dissected for analysis. Rat brains were processed for Western blot and ERK1/2 kinase assay. A. Western blot showing RIPC increased p-ERK1/2 levels (top). Two protein bands were observed. The top band is pERK1 (44 kDa), and the lower band is pERK2 (42kDa). The bar graph shows the average optical densities of the protein bands (bottom). B. RIPC also increased ERK1/2 activity as assessed by in vitro kinase assay compared to sham. Activated protein kinase phosphorylates its substrates, thus levels of a phophorylated protein substrate can be used as a marker for protein kinase activity. In this experiment, ERK kinase activity was determined by the amount of Elk-1 phosphorylated by ERK1/2; p-Elk-1 was detected by Western blot. * vs sham, P<0.05. N=3–6/group.
3.3. p-ERK1/2 levels were increased and ERK1/2 kinase activity was decreased in rats receiving both RIPC and test ischemia
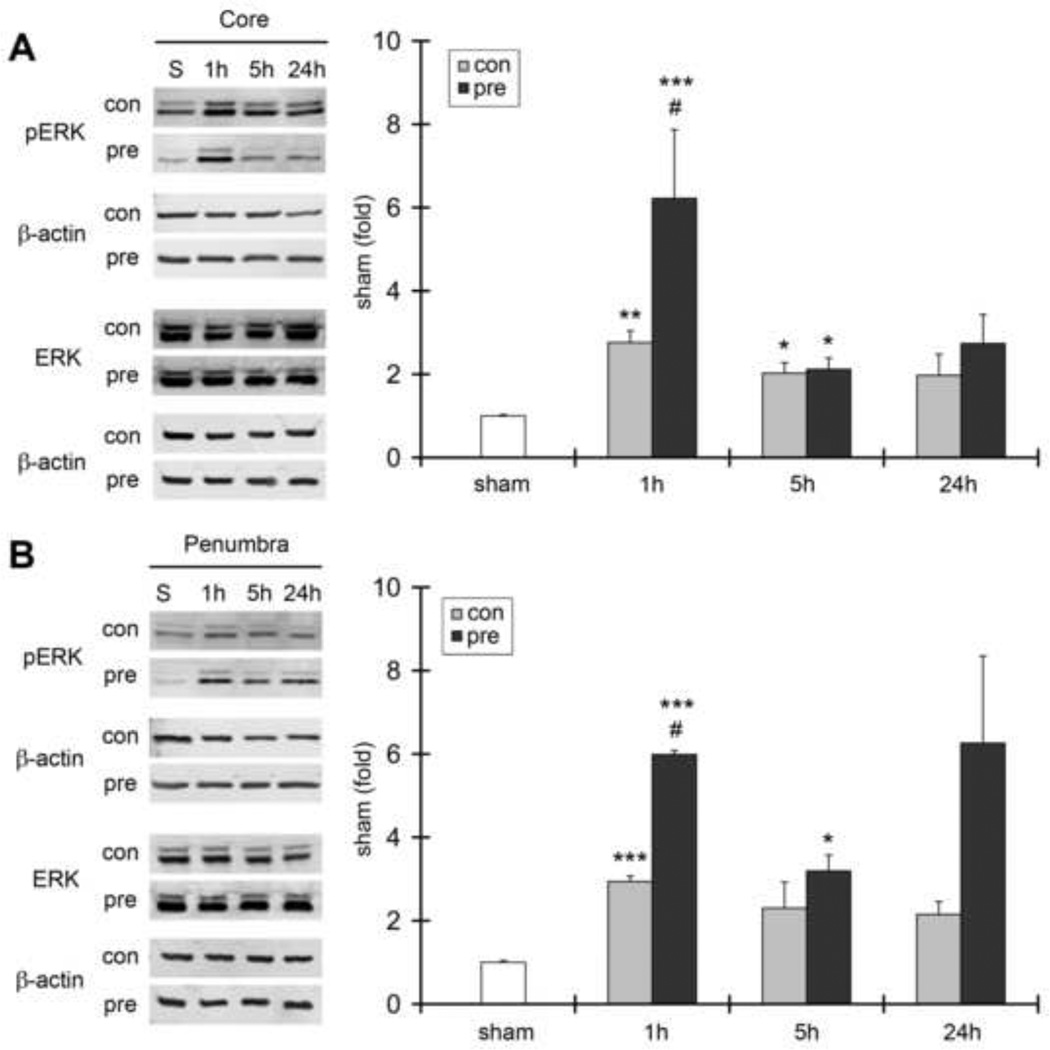
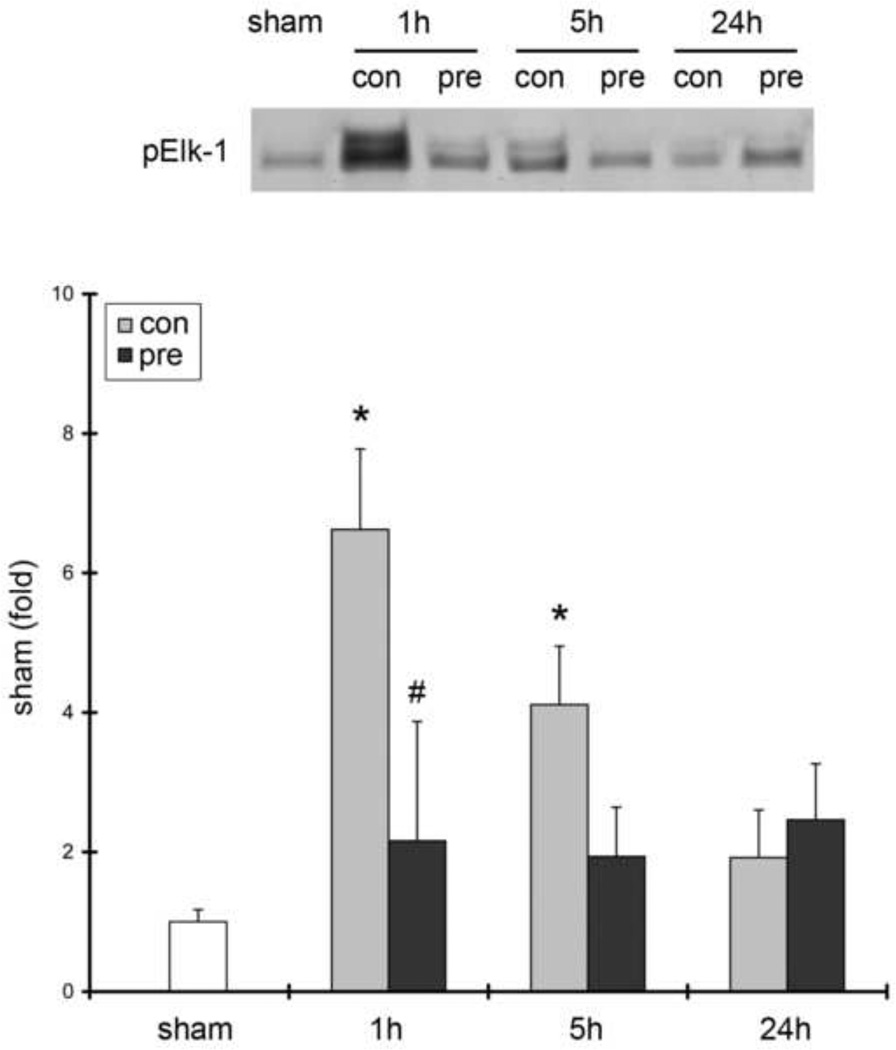
We next investigated how RIPC affects both p-ERK1/2 protein levels and ERK1/2 kinase activity in test ischemia with or without RIPC (Fig. 3). We observed increased p-ERK1/2 protein levels after reperfusion in both the ischemic core and penumbra at 1, 5, and 24 hours post-stroke onset. RIPC further increased p-ERK1/2 protein levels at 1 hour after stroke. In vitro kinase assay results showed that ERK1/2 activity in the penumbra, as measured by p-Elk-1 protein levels, was increased after test ischemia, suggesting that increases in ERK1/2 phosphorylation are accompanied by increases in ERK1/2 kinase activity (Fig. 4). Nevertheless, RIPC robustly blocked ERK1/2 kinase activity at 1 hour after stroke, even though it increased p-ERK1/2 protein levels at this time point. Our results suggest that increases in p-ERK1/2 levels are not proportional to increases in kinase activity.
Fig. 3. The effects of stroke and ischemic preconditioning on p-ERK1/2 and ERK1/2 activity.
A. Representative protein bands of p-ERK1/2 and ERK1/2 in the ischemic core 1, 5 and 24 h after stroke. The bar graphs show the mean value of p-Erk1/2 optical densities. No significant changes in ERK1/2 protein bands were detected after stroke, and RIPC did not affect its expression (statistical results are not shown). β-actin was probed to show even protein loading. pERK1/2 was increased after stroke, and RIPC further increased its levels at 1 h after stroke in the ischemic core. B. Representative protein bands of p-Erk1/2 and ERK1/2 in the ischemic penumbra. The bar graphs represented the mean values of protein densities, which were normalized to the values of the sham group. N=3–6/group *, **, *** vs sham, P<0.05. 0.01. 0.001, respectively. # vs control (test) ischemia, P<0.05.
Fig. 4. The effects of stroke on ERK1/2 kinase activity assessed by in vitro kinase assay.
Ischemic tissues corresponding to penumbra were dissected from animals 1, 5 and 24 h after stroke with or without RIPC. Cell lysates were prepared, co-immunoprecipitation with p-ERK1/2 antibodies was performed, and extracted p-ERK proteins were incubated with Elk-1 protein. p-Elk-1 was measured by Western blot. The amount of p-Elk-1 represents ERK1/2 kinase activity, which was significantly increased at 1 and 5 h after stroke, which preconditioning blocked. N=4–6/group. * vs sham, P<0.05; # vs control, P<0.05.
4. Discussion
We have demonstrated the first evidence that in brains treated with RIPC alone without test ischemia both p-ERK1/2 levels and ERK1/2 kinase activities were increased. In brains subjected to both RIPC and focal ischemia, however, RIPC robustly promoted p-ERK1/2 levels yet inhibited ERK1/2 kinase activity in the ischemic penumbra, as assessed by in vitro kinase assay. This suggests that the level p-ERK1/2 protein may not be an adequate marker to assess its kinase activity under certain pathological conditions. However, we did not perform a protein kinase assay for tissues of the ischemic core. Whether protein phosphorylation of ERK1/2 is consistent with its kinase activity needs further study. Nevertheless, since ischemic preconditioning did not spare brain tissues in the ischemic core, we deem that their correlation is not critical to clarify the protective mechanisms of ischemic preconditioning.
ERK1/2 proteins were identified more than 20 years ago (Boulton et al. 1991). In the early years, ERK1/2 activity was usually determined by its ability to phosphorylate one of its substrates, major basic protein (MBP), using radioisotope-labeled ATP ([γ-32P]ATP) (Robbins et al. 1993). Levels of phosphorylated MBP were analyzed by electrophoresis, and the corresponding protein bands were excised and counted by liquid scintillation to assess kinase activity (Robbins et al. 1993). Thereafter, ERK1/2 was found to undergo a dual phosphorylation at conserved threonine and tyrosine residues (Her et al. 1993; Zheng and Guan 1993; Derijard et al. 1995). Thus, in the following studies, which include cerebral ischemia, protein levels of phosphorylated ERK1/2 were used as a marker for kinase activity: (Ozawa et al. 1999; Sugino et al. 2000; Li et al. 2001; Friguls et al. 2002; Lennmyr et al. 2002; Yoo et al. 2005; Zhang et al. 2007; Shioda et al. 2009). In recent years, non-radioisotope methods were developed to measure ERK1/2 kinase activity. In our study, we prepared a solution of ERK1/2 extracted from brain tissues, added Elk-1, a substrate of ERK1/2, and measured the amount of p-Elk-1 by Western blot. We used this method to detect ERK1/2 activity under various conditions in animals with RIPC alone, test ischemia, and test ischemia plus RIPC. Our results were reliable and reproducible in each experimental setting and elucidated valuable information regarding the relationship between ERK1/2 phosphorylation and kinase activity.
We found that p-ERK1/2 protein levels and ERK1/2 kinase activity do not necessarily correlate in certain ischemic pathological conditions. In animals with RIPC and no test ischemia, p-ERK1/2 levels were increased 2.5-fold while kinase activity was increased more than 10-fold. This suggests that increased p-ERK1/2 levels do reflect kinase activity, although these increases were disproportionate. In addition, animals that received test ischemia without RIPC showed both increased p-ERK1/2 levels and kinase activities, consistent with animals receiving RIPC alone.
However, animals that received test ischemia with RIPC displayed significantly increased p-ERK1/2 levels compared to animals with test ischemia alone, yet kinase activities were robustly inhibited. In this case, therefore, using p-ERK1/2 levels as a marker for ERK1/2 activity would be misleading.
The underlying mechanisms responsible for the mismatch between p-ERK1/2 levels and kinase activity are unknown. Although dual phosphorylation of ERK1/2 proteins is required for them to be fully active, it is unclear whether this is sufficient. Multiple steps are involved in the activation of ERK1/2, including domain rotation and remodeling, and conformational changes that lead to substrate recruitment and phosphorylation (Turjanski et al. 2009). Under certain pathological conditions p-ERK1/2 may not be able to exert its full effects, such as in our study where ischemic preconditioning may block the ability of phosphorylated ERK1/2 to be fully active. Moreover, we cannot exclude limitations of the protein kinase assay itself, in which only one substrate of ERK1/2 was used. More substrates and a more universal method for measuring ERK1/2 kinase activity are required to confirm our conclusion.
The role of ERK1/2 in cerebral ischemia has been extensively studied. Whether it is beneficial or detrimental remains controversial. As we previously reviewed (Sawe et al. 2008), most studies agree that p-ERK1/2 protein levels increase after ischemia/reperfusion, however, depending on the experimental context this can either enlarge infarct size or inhibit brain injury. While some studies suggest that p-ERK1/2 promotes inflammation and oxidative stress (Stanciu et al. 2000; Wang et al. 2001; Friguls et al. 2002; Noshita et al. 2002; Wang et al. 2004; Xu et al. 2006), others argue that neuroprotectants, such as growth factors and ischemic preconditioning, increase p-ERK1/2 levels (Kitagawa et al. 1990; Barbacid 1994; Kaplan and Miller 2000; Kirino 2002; Burda et al. 2003; Kilic et al. 2006; Kim et al. 2011). Nevertheless, these studies used p-ERK1/2 levels as the sole marker of its activity. Our current study shows that discrepancies exist between p-ERK1/2 protein levels and its kinase activity under certain pathological conditions. The lack of kinase assay analysis in previous studies may have led to the misinterpretation of ERK1/2 kinase activity and results that remain controversial.
In conclusion, we demonstrated that p-ERK1/2 protein levels and kinase activity are not necessarily equivalent. Assaying p-ERK1/2 kinase activity is essential to understanding its role in ischemic injury after stroke.
Highlights.
-
➢
Preconditioning alone increased both ERK1/2 phosphorylation and kinase activity.
-
➢
Preconditioning plus ischemia increased ERK1/2 phosphorylation but reduced its kinase activity.
-
➢
ERK1/2 phosphorylation does not always represent its kinase activity.
Acknowledgements
The authors thank Elizabeth Hoyte for preparing the figures, and Cindy H. Samos for editing the manuscript. This study was supported by R01 NS27292-03 (GKS/HZ), AHA grant in aid (HZ), and NIH grants 1R21NS057750-01A2 (HZ) and 1R01NS 064136-01 (HZ).
Abbreviations
- MAPK
Mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- p-ERK1/2
phosohorylated-ERK1/2
- RIPC
rapid ischemic preconditioning
- CCAs
common carotid arteries
- MCAo
middle cerebral artery occlusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict disclosure: The authors have no confliction to disclose.
Authorship credit: Tetsuya Takahashi performed the experiments, analyzed and presented the data; Heng Zhao designed and drafted the manuscript; Gary K. Steinberg critically revised the final manuscript.
References
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Burda J, Hrehorovska M, Bonilla LG, Danielisova V, Cizkova D, Burda R, Nemethova M, Fando JL, Salinas M. Role of protein synthesis in the ischemic tolerance acquisition induced by transient forebrain ischemia in the rat. Neurochem Res. 2003;28:1213–1219. doi: 10.1023/a:1024232513106. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Friguls B, Petegnief V, Justicia C, Pallas M, Planas AM. Activation of ERK and Akt signaling in focal cerebral ischemia: modulation by TGF-alpha and involvement of NMDA receptor. Neurobiol Dis. 2002;11:443–456. doi: 10.1006/nbdi.2002.0553. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang H, Steinberg G, Zhao H. The Akt Pathway Is Involved in Rapid Ischemic Tolerance in Focal Ischemia in Rats. Translational Stroke Research. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her JH, Lakhani S, Zu K, Vila J, Dent P, Sturgill TW, Weber MJ. Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem J. 1993;296(Pt 1):25–31. doi: 10.1042/bj2960025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. Faseb J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Raval AP, Hirsch N, Perez-Pinzon MA. Ischemic Preconditioning Mediates Cyclooxygenase-2 Expression Via Nuclear Factor-Kappa B Activation in Mixed Cortical Neuronal Cultures. Transl Stroke Res. 2011;1:40–47. doi: 10.1007/s12975-009-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Karlsson S, Gerwins P, Ata KA, Terent A. Activation of mitogen-activated protein kinases in experimental cerebral ischemia. Acta Neurol Scand. 2002;106:333–340. doi: 10.1034/j.1600-0404.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- Li PA, He QP, Yi-Bing O, Hu BR, Siesjo BK. Phosphorylation of extracellular signal-regulated kinase after transient cerebral ischemia in hyperglycemic rats. Neurobiol Dis. 2001;8:127–135. doi: 10.1006/nbdi.2000.0363. [DOI] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–7930. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H, Shioda S, Dohi K, Matsumoto H, Mizushima H, Zhou CJ, Funahashi H, Nakai Y, Nakajo S, Matsumoto K. Delayed neuronal cell death in the rat hippocampus is mediated by the mitogen-activated protein kinase signal transduction pathway. Neurosci Lett. 1999;262:57–60. doi: 10.1016/s0304-3940(99)00034-8. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Zhen E, Cheng M, Xu S, Vanderbilt CA, Ebert D, Garcia C, Dang A, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1, 2, and 3. J Am Soc Nephrol. 1993;4:1104–1110. doi: 10.1681/ASN.V451104. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- Shioda N, Han F, Fukunaga K. Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–387. doi: 10.1016/S0074-7742(09)85026-5. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski AG, Hummer G, Gutkind JS. How mitogen-activated protein kinases recognize and phosphorylate their targets: A QM/MM study. J Am Chem Soc. 2009;131:6141–6148. doi: 10.1021/ja8071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu L, Venkatachalam S, Trzaskos JM, Friedman SM, Feuerstein GZ, Wang X. Differential regulation of IL-1beta and TNF-alpha RNA expression by MEK1 inhibitor after focal cerebral ischemia in mice. Biochem Biophys Res Commun. 2001;286:869–874. doi: 10.1006/bbrc.2001.5482. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK 1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66. doi: 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Choi JW, Han BH, Kim WK, Kim HC, Ko KH. Role of MAPK/ERK1/2 in the glucose deprivation-induced death in immunostimulated astroglia. Neurosci Lett. 2005;376:171–176. doi: 10.1016/j.neulet.2004.11.077. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Wei EQ, Li L, Qiao WL, Wang L, Zhang JF. Extracellular signal-regulated kinase pathways may mediate the protective effect of electrical stimulation of the paraventricular nucleus against ischaemia-reperfusion injury of the gastric mucosa. Clin Exp Pharmacol Physiol. 2007;34:742–752. doi: 10.1111/j.1440-1681.2007.04652.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. J Biol Chem. 1993;268:16116–16119. [PubMed] [Google Scholar]