Abstract
Chronic psychological stressis a risk factor formultiple diseases of aging. Accelerated cellular aging as indexed by short telomere length has emerged as a potential common biological mechanism linking various forms of psychological stress and diseases of aging. Stress appraisals determine the degree and type of biological stress responses and altered stress appraisals may be a common psychological mechanism linking psychological stress and diseases of aging. However, no previous studies have examined the relationship between stress appraisals and telomere length. We exposed chronically stressed female caregivers and non-caregiving controls (N= 50; M age = 62.14±6.10) to a standardized acute laboratory stressor and measured their anticipatory and retrospective threat and challenge appraisals of the stressor. We hypothesized that threat and challenge appraisals would be associated with shorter and longer telomere length respectively, and that chronic care giving stress would influence telomere length through altered stress appraisals. Higher anticipatory threat appraisals were associated with shorter age-adjusted telomere length (β = −.32, p = .03), but challenge appraisals and retrospective threat appraisals showed no independent association with telomere length. Caregivers reported significantly higher anticipatory (β = −.36, p = .006)and retrospective (β = −.29, p = .03) threat appraisals than controls, but similar challenge appraisals. Although there was no significant main effect of caregiver status on telomere length, care giving had a significant indirect effect on telomere length through anticipatory threat appraisals. Exaggerated anticipatory threat appraisals may be a common and modifiable psychological mechanism of psychological stress effects on cellular aging.
Keywords: cellular aging, challenge, chronic stress, stress appraisals, threat, telomere length
A large body of epidemiological evidence indicates that exposure to psychological stress increases risk for disease and early mortality (Boscarino, 1997; Dube et al., 2009; Ohlin et al., 2004; Schulz and Beach, 1999; Taylor et al., 2007). There are two striking but underappreciated features of the link between psychological stress and physical disease. The first is that many different forms of stress exposure, ranging from caregiving to low socioeconomic status to trauma exposure, increase risk for disease. The second is that psychological stress increases risk for many different types of physical disease, ranging from cardiovascular, autoimmune and neurodegenerative disorders to cancer. That associations exist between different types of stress exposures and different diseases suggests that common biological and psychological mechanisms may link stress exposure and disease risk.
The types of diseases that are more common in samples exposed to psychological stress provide clues about possible common biological mechanisms underlying the association between psychological stress and disease. Individuals who experience chronic or traumatic stress tend to be at increased risk for earlier development of diseases that typically emerge later in life, the chronic diseases of aging such as cardiovascular, autoimmune and neurodegenerative disorders and cancer (Dube et al., 2009; Ohlin et al., 2004; Schulz and Beach, 1999; Taylor et al., 2007). A potential common etiological factor across these diseases is “cellular aging” or the changes that occur in cells over time across the lifespan, eventually leading them to become functionally impaired and unable to proliferate (Blackburn, 2000; Chan and Blackburn, 2004; Sahin et al., 2011).
While cellular aging is multifaceted and difficult to precisely quantify, telomeres provide one index. Telomeres are DNA-protein complexes that cap the ends of chromosomes and protect against damage to the DNA that encodes genetic information. Telomeres shorten with somatic cell division and with exposure to oxidative stress, and evidence supports leukocyte telomere length as both a marker and potential mechanism of cellular aging (Blackburn, 2000; Sahin et al., 2011). Across studies, short leukocyte telomere length is associated with significantly higher risk for cardiovascular, autoimmune and neurodegenerative disorders and cancer (Cawthon et al., 2003; Goronzy et al., 2006; Willeit et al., 2010). Moreover, in line with the hypothesis that cellular aging is a potential common mechanism linking psychological stress and disease, short telomere length has been observed in individuals exposed to various forms of psychological stress (Cherkas et al., 2006; Damjanovic et al., 2007; Epel et al., 2004; Kiecolt-Glaser et al., 2011; O'Donovan et al., 2011a).
Individuals differ in their psychological and biological responses to stress. In particular, an individual’s appraisal or assessment of a specific psychological stressor determines their cognitive-emotional response, which in turn influences their biological response and hence the extent to which the stressor has the potential to “get under the skin” to influence physical health (Blascovich and Mendes, 2010). According to the classic Lazarus and Folkman “Transactional Model of Stress and Coping”, individuals who perceive that a stressor exceeds their resources to cope effectively will appraise the situation as threatening and exhibit a response characterized by threat-related cognitions and emotions such as worry and fear. In contrast, individuals who perceive that they have adequate resources to cope with a stressor will appraise it as challenging and exhibit a response characterized by challenge-related cognitions and emotions such as perceived control and excitement (Lazarus and Folkman, 1984). Subsequent research has shown that biological reactions to stressors appraised as threatening differ from those appraised as challenging, and that the former are associated with more harmful physiological reactions than are the latter (Blascovich and Mendes, 2010).
The degree to which individuals appraise stressors as threatening or challenging may reflect current and prior experiences of stress in addition to specific objective characteristics of the stressor (e.g., how dangerous the situation actually is) and specific characteristics of the individual (e.g., personality). For example, exposure to traumatic stress confers heightened sensitivity to threatening stimuli (Blackburn, 2000; Felmingham et al., 2010; Pine, 2003), and the chronic stress of growing up in a socioeconomically disadvantaged family has been associated with more threatening interpretations of ambiguous social situations in adolescents (Chen et al., 2004). We propose that other forms of psychological stress, particularly chronic stress, may also influence stress appraisals and heighten sensitivity to threatening stimuli. In particular, we propose that everyday stressors will be appraised as more threatening and less challenging against a backdrop of chronic stress because chronic stress depletes psychological and biological resources that are needed to deal with life stress. Higher threat and lower challenge appraisals of daily stressors would in turn lead to repeated and prolonged activation of harmful biological stress responses, which could promote cellular aging in chronically stressed individuals. However, at present, little is known about the relationship between stress appraisals and telomere length.
In the present study, we combine analyses at the levels of acute and chronic stress by examining threat and challenge appraisals of an acute laboratory stressorin chronically stressed caregivers and non-caregiving control participants in whom we also measured leukocyte telomere length. We hypothesized that: 1) higher threat appraisals and lower challenge appraisals will be associated with shorter telomere length; 2) caregivers will perceive an acute laboratory stressor as more threatening and less challenging than controls; 3) caregivers will have shorter telomere length than controls; and 4) there will be an indirect effect of caregiver status on telomere length through altered stress appraisals.
Methods
Participants
We recruited two groups of healthy postmenopausal women, caregivers and controls, to provide a clear contrast between groups with and without chronic stress. The group exposed to chronic stress included women who were providing at least 4 hours per day of care, every day, to a relative with dementia and who reported high levels of perceived stress as indexed by a score of at least 12 on the 10-item Perceived Stress Scale, which represents the population mean in women (Cohen et al., 1983). The second group of women consisted of non-caregiving controls with similar mean age and body mass index (BMI) as the caregiver group, and low levels of perceived stress. For inclusion in the study, all participants had to be non-smoking and without major or unstable medical conditions or confounding medications as assessed by self-report and by physical exam by the study physician. Additionally, all participants had to pass a chemistry screening for normal glucose, electrolytes, thyroid, liver and kidney function. In all, sixty-three participants (n = 34 caregivers; n = 29 controls) were recruited through flyers and posters in the community, as well as from elderly service providers in the San Francisco Bay Area, California. Of these, 51 women participated in the acute stress task and had data on telomere length. One of these 51 participants was then excluded because of being an outlier with telomere length greater than 2.5 standard deviations from the mean telomere length of the sample. Characteristics of the full sample of 63 participants in the larger study and of the subsample of 50 participants in this study are described in Table 1. There were no significant differences between the participants and non-participants on any of the demographic variables.
Table 1.
Sample characteristics
| Variable | Full Sample n = 63 |
Subsample n = 50 |
|---|---|---|
| Caregivers | 34 (54) | 27 (54) |
| Duration of caregiving: Median [IQR] |
5 – 200 47 [34, 62] |
5 – 137 48 [36, 60] |
| Age: Range (Mean±SD) |
51 – 79 (61.87±6.51) |
51 – 79 (62.14±6.10) |
| BMI: Range (Mean±SD) |
17.7 – 42.8 (26.57±5.41) |
17.7 – 37.5 (26.18±4.96) |
| Education No advanced degree Advanced degree |
46 (73) 17 (27) |
38 (76) 12 (24) |
| Household Income Below $50k/year $50k - $200k/year Missing |
15 (24) 40 (63) 8(13) |
12 (24) 33 (66) 5 (10) |
| Marital Status Married/living with partner Divorced/separated/widowed Never married |
38 (60) 15 (24) 10 (16) |
31 (62) 11 (22) 8 (16) |
| Ethnicity White Asian Black Hispanic/Latina Other |
52 (83) 6 (10) 3 (5) 1 (2) 1 (2) |
44 (88) 5 (10) 1 (2) 0 0 |
| Telomere Length: Range (Mean±SD) |
0.34 – 1.68 (0.93±0.30) |
0.34 – 1.61 (0.94±0.27) |
Notes. Figures refer to n (%) unless otherwise specified. Duration of caregiving refers to time since caregiving began in months.
Procedures
During their first visit to the laboratory, participants fasted for 10 hours and then donated blood, from the non-dominant arm between 0800 and 1000 hrs for telomere length measurement. During this visit participants also provided demographic information and completed questionnaire measures of perceived stress and neuroticism. At a second visit one week later, they ate a standardized lunch at 1200 hrs and then had a one-hour resting baseline period while listening to relaxing music. Following this, participants were exposed to a modified form of the Trier Social Stress Test (TSST) between 1400 and 1700 hrs, in which they were asked to give a speech about their personal strengths and weaknesses and to perform a difficult serial subtraction math task aloud (Kirschbaum et al., 1993). The phases of the modified TSST included four five-minute stressful periods, including: 1) introduction to two trained evaluative, non-responsive audience members for description of tasks; 2) a quiet period for speech preparation and completion of a questionnaire assessing anticipatory threat and challenge appraisals; 3) delivery of speech; and 4) completion of math task. All tasks were performed in front of an evaluative audience who maintained neutral facial expressions and tone of voice throughout. Participants then immediately completed a questionnaire assessing retrospective threat and challenge appraisals. At the end of the session, participants were fully debriefed. All procedures were fully approved by the University of California San Francisco Committee on Human Subjects Research and written informed consent was obtained from all participants.
Measures
Threat and challenge appraisals
A self-report questionnaire completed before the TSST assessed anticipatory threat and challenge appraisals, and an analogous questionnaire completed after the TSST assessed retrospective appraisals. The questions were based on previous work (Mendes et al., 2007b). The anticipatory threat appraisal scale included six items pertaining to cognitive (e.g., the upcoming tasks will be very demanding) and emotional (e.g., how anxious do you feel about the upcoming tasks?) appraisals of the upcoming task. The anticipatory challenge appraisal scale included eight items pertaining to cognitive (e.g., I have control over how I will do on the upcoming task) and emotional (e.g., how excited do you feel about the upcoming tasks?) appraisals, posed in the future tense. The retrospective appraisal scales included parallel items asking about the participant’s experience of the TSST (e.g., the tasks were very demanding), posed in the past tense. Items were responded to on a Likert scale ranging from −4 (strongly disagree) to +4 (strongly agree). Cognitive and emotional subscales for anticipatory and retrospective threat appraisals and anticipatory and retrospective challenge appraisals were converted into z scores and summed to create four scales that showed acceptable reliability as indexed by Cronbach’s alpha: anticipatory threat appraisals (α = .80); retrospective threat appraisals (α = .75); anticipatory challenge appraisals (α = .87); and retrospective challenge appraisals (α = .75).
Neuroticism
The Big Five Inventory was used to assess neuroticism (John et al., 1991). This scale comprises eight items that assess emotional stability or neuroticism (e.g., “I see myself as someone who worries a lot”). Participants rate their agreement with the items on a 5-point Likert scale ranging from 1 (“disagree strongly”) to 5 (“agree strongly”). Internal consistency for the scale was acceptable (α = .84).
Perceived stress
The 10-item Perceived Stress Scale was used to assess psychological stress experienced during the last month, including the extent to which situations were experienced as unpredictable, uncontrollable and overwhelming (Cohen et al., 1983). Participants rated the extent to which they felt or thought a particular way in the previous month on a 5-point Likert scale ranging from 0 (“never”) to 4 (“very often”). Internal consistency was high (α = .93).
Education
Participants’ educational attainment was assessed with a single item on highest level of education by degree.
Body mass index (BMI)
Weight and height were measured by trained research assistants, and BMI was calculated as weight (kg) divided by height in meters squared (m2).
Leukocyte telomere length
Blood samples were collected in 10-ml heparin tubes (Becton–Dickinson, Franklin Lakes, NJ). Leukocytes were isolated and frozen at −80°C. DNA was extracted from leukocytes by the University of California San Francisco DNA bank. Genomic DNA isolation was performed using a standardized and quality-controlled PureGene DNA isolation system (Gentra Systems, Minneapolis). DNA was analyzed for telomere length using quantitative polymerase chain reaction (qPCR) (Cawthon, 2002) with modifications as described in (Lin et al., 2010).
Data analysis
To examine if acute stress appraisals were associated with age-adjusted telomere length, we used linear regression models including age in the first step and appraisals in the second step of the model. In follow-up analyses, we adjusted for potential confounding and mediating factors by including BMI, education, perceived stress and neuroticism with age in the first step of separate linear regression models with appraisals in the second step. To examine if there was a relationship between caregiver status and telomere length through altered stress appraisals, we used a series of linear regression modelsto assess the extent to which: 1) caregiving status was associated with stress appraisals (a pathway); 2) stress appraisals were associated with telomere length (b pathway); and 3) caregiver status was associated with telomere length without (c pathway) and with (c'pathway) stress appraisals in the model. Because of our small sample size, we used the vce (bootstrap) command in STATA Version 11 to estimate p values and confidence intervals (CI) based on 1,000 resamples for all linear regression models. Results are presented as standardized regression coefficients (β) with p values and 95% CIs. However, the overall pattern of results was similar with and without bootstrapping.
To examine the indirect path representing the change in telomere length when caregiver status is kept constant and stress appraisals are changed to the level they would be at if caregiver status changed by one unit (abpathway), we used bias-corrected bootstrapping procedure macros in SPSS (Lockhart et al., 2011; Preacher and Hayes, 2004, 2008). These procedures allowed us to estimate the indirect effect ab as well as 95% confidence intervals for the population value of ab based on the value of ab in 20,000 resamples of our data with replacement (Preacher and Hayes, 2004, 2008). The traditional and most widely used mediation analysis technique is the causal steps approach, which specifies a series of requirements needed in support of a mediation model (Baron and Kenny, 1989). This approach involves no formal significance test of indirect effects (ab pathway) and our study is underpowered for the widely used Sobel test of indirect effects (Fritz and Mackinnon, 2007). Bias-corrected bootstrapping approaches to mediation analysis based on asymmetric distributions provide more accurate estimates of mediation effects (Lockhart et al., 2011; Preacher and Hayes, 2004, 2008). This approach is also in line with recent methodological advances in mediation analysis, which emphasizethat an indirect effect can occur even if the relationship between the independent and dependent variables is not statistically significant (Hayes, 2009; Mackinnon and Fairchild, 2009). We used Pearson’s correlations and Student’s t-tests to examine associations between continuous variables and group differences in continuous variables respectively. Means and standard deviations (SD) are provided for continuous variable as appropriate. Apart from the bootstrapped linear regression models that were conducted with STATA 11, all other statistical analyses were performed with SPSS 18.0.
Results
Acute Stress Appraisals and Telomere Length
Higher anticipatory threat appraisals were significantly associated with shorter telomere length (β = −.32, p = .03, 95% CI [−.19, −.01]). However, none of retrospective threat appraisals, (β = −.17, p = .36, 95% CI [−.17, .78]), anticipatory challenge appraisals (β = .15,p = .38, 95% CI [−.06, .16]), or retrospective challenge appraisals (β = .05, p = .77, 95% CI [−.17, 2.09]) was significantly associated with telomere length.
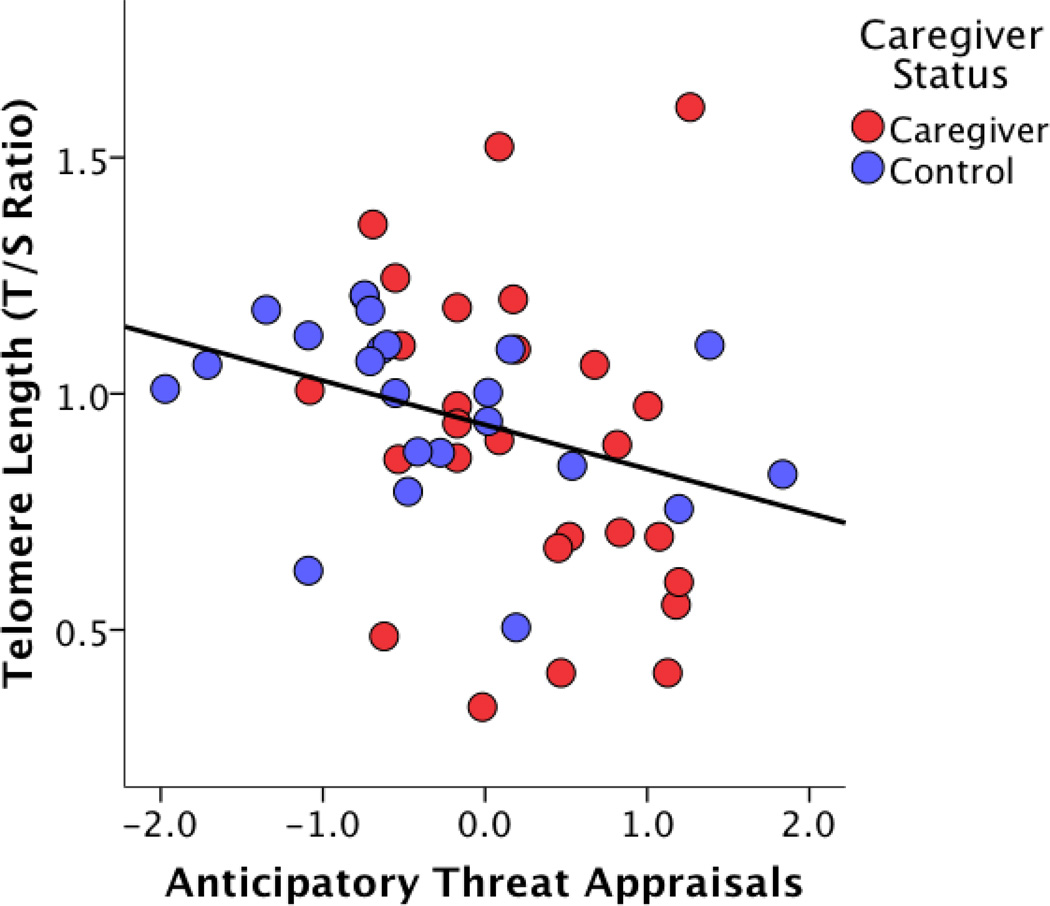
Participants reporting higher anticipatory threat appraisals had higher levels of perceived stress (r = .34, p = .02), higher retrospective threat appraisals (r = .44, p = .002) and lower anticipatory challenge appraisals (r = −.43, p< .001). However, there were no significant associations between anticipatory threat appraisals and BMI, neuroticism, or retrospective challenge appraisals and no significant differences in anticipatory threat appraisals between participants with and without an advanced degree. Moreover, none of the covariates were significantly associated with telomere length in this sample. Follow-up analyses separately adjusting for perceived stress, anticipatory challenge appraisals, or retrospective threat appraisals in addition to age indicated that the relationship between anticipatory threat appraisals and telomere length was independent of these potential covariates (all p< .05)(Figure 1).
Figure 1.
Significant relationship between anticipatory threat appraisals and telomere length in a sample of caregivers (red dots) and controls (blue dots). Higher anticipatory threat appraisals were associated with significantly shorter telomere length (β = −.32, p = .03).
Altered Stress Appraisals: A Mediator of the Chronic Stress – Telomere Length Relationship?
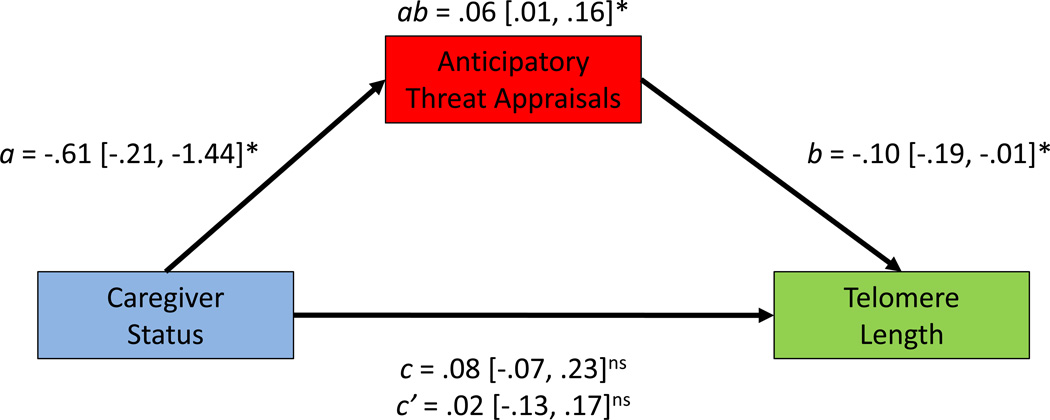
Levels of perceived stress were significantly higher in caregivers (Mean= 19.78, SD= 4.97) compared with controls (Mean = 9.18, SD = 4.87), t(47) = 7.49, p< .001. Moreover, chronically stressed caregivers anticipated that the upcoming tasks would be more threatening (β = −.36, p = .006, 95% CI [−1.04, −.18]) than did non-caregivers. After completing the tasks, the chronically stressed women rated them as more threatening than did controls (β = −.29,p = .03, 95% CI [−.90, −.04]). In contrast, there were no differences between chronically stressed women and controls on either anticipatory (β = .15, p = .27, 95% CI [−.21, .72])or retrospective (β = .10, p = .45, 95% CI [−.28, .63]) appraisals of challenge. Combined, the significant relationships between caregiver status and anticipatory threat appraisals and between anticipatory threat appraisals and telomere length are consistent with an indirect effect of caregiver status on telomere length operating through anticipatory threat appraisals (Figure 2).
Figure 2.
Illustration of theoretical direct and indirect effects of caregiver status on telomere length. Numbers represent unstandardized coefficients from linear regression with 95% confidence intervals in brackets. Path a represents the significant difference between caregivers and controls in anticipatory threat appraisals. Path b represents the significant relationship between anticipatory threat appraisals and telomere length. Path c represents the non-significant difference between caregivers and controls in telomere length, and path c' represents the non-significant difference between caregivers and controls including anticipatory threat appraisals in the model. Path ab represents the significant indirect effect of caregiver status on telomere length through anticipatory threat appraisals. Asterisks indicate statistical significance of the path coefficients, *p< .05, ns = non-significant.
In order to examine if there was indeed a significant indirect effect of caregiver status on telomere length through anticipatory threat appraisals, we employed procedures as described in the data analysis section to estimate a, b, c, c' and ab as illustrated in Figure 2. First, we confirmed the finding that caregivers had higher anticipatory threat appraisals than controls (a = −.61, β = −.36, p = .008, 95% CI [.21, 1.44]). Second, linear regression confirmed the age-adjusted association between anticipatory threat appraisals and shorter telomere length when including caregiver status in the model (b = −.10, β = − .31, p = .04, 95% CI [−.19, −.01]). Third, however, although caregivers had shorter age-adjusted telomere length (Mean T/S ratio = .90, SD = .33) than controls (Mean T/S ratio = .98, SD = .19), linear regression indicated that the groups were not significantly different on telomere length (c = .08, β = .14, p = .32, 95% CI [−.07, .23]). However, the effect size for this non-significant relationship between caregiver status and telomere length was markedly decreased with anticipatory threat appraisals in the model (c' = .02, β = .04, p = .79, 95% CI [−.13, .17]).
Bias corrected 95% confidence intervals indicated a statistically significant indirect effect of caregiver status on telomere length through anticipatory threat appraisals (absample = .06, abbootstrap = .06, 95% CI [.01, .16]). In follow-up linear regression analyses, we found that anticipatory threat appraisals were significantly associated with telomere length in caregivers (tβ = −.36, p = .03, 95% CI [−.34, −.02]) but not in controls (β = −.22, p = .28, 95% CI [−.12, .04]). Thus, although the relationship between anticipatory threat appraisals and telomere length was in the same direction in both caregivers and controls, the association between higher anticipatory threat appraisals and shorter telomere length was statistically significant only in caregivers.
Discussion
The present study provides evidence of an association between higher threat appraisals and accelerated cellular aging as indexed by shorter age-adjusted telomere length. Women who experienced higher levels of threat in anticipation of an upcoming acute stressor had significantly shorter leukocyte telomere length than those who anticipated less threat. Moreover, participants exposed to chronic stress (i.e., caregivers) appraised the acute stress tasks as more threatening than did the low-stress non-caregiving controls and there was an indirect effect of caregiver status on telomere length through anticipatory threat appraisals. In particular, women experiencing chronic psychological stress appraised a standardized stressor as more threatening, and such high threat appraisals were in turn associated with shorter age-adjusted telomere length. These data extend a growing body of research showing associations between psychological stress and telomere length by suggesting exaggerated threat anticipation as a novel psychological pathway by which different forms of stress exposure might accelerate telomere shortening.
A growing body of clinical research indicates that conditions that foster exaggerated threat perception, such as childhood adversity and post-traumatic stress disorder, are associated with short telomere length (Kiecolt-Glaser et al., 2011; O'Donovan et al., 2011a; Tyrka et al., 2009). Our data extend this work by showing that greater anticipatory threat appraisals of a standardized stressor are associated with shorter telomere length in chronically stressed caregivers and that there is an indirect effect of caregiver status on telomere length through exaggerated anticipatory threat appraisals.
One might wonder how threat appraisals, which are measured as a state-like response to specific stimuli in the lab, could be linked to a long-term health indicator like telomere length. We view threat appraisals of the standardized acute stressor as a reflection of two factors – dispositional threat sensitivity and the acute state responses to the specific tasks at hand. The dispositional threat sensitivity component is relatively stable and likely reflects variance attributable to genetic factors that have relevance for threat-related information processing, including the serotonin transporter gene (Munafo et al., 2008). However, threat sensitivity is also likely to be shaped by life experiences through epigenetic modifications, changes in brain structure and function generated through neural plasticity, and shaping of cognitive-behavioral reactions (e.g., Ellis and Boyce, 2008). Given that telomere shortening takes place over long periods of time, we believe that the relatively stable threat sensitivity component of threat appraisals is likely to be driving the association between threat appraisals and telomere length. Our proposed model is that psychological stress exposure leads to exaggerated threat sensitivity, which drives more frequent and prolonged threat perception in daily life, which in turn promotes activation of the biological stress responses that can drive telomere shortening. Thus, we view threat sensitivity as a potential mediator of the relationship between chronic stress and telomere length.
An alternative model, which is also consistent with our findings, is that anticipatory threat appraisals moderate the relationship between chronic stress and telomere length; in other words, that only the combination of chronic stress exposure and high threat appraisals is associated with accelerated telomere shortening. This model would suggest that only individuals with a high degree of threat sensitivity – as indexed by their high threat appraisals of the standardized stressor – who are also chronically exposed to high levels of stress would show accelerated telomere shortening. However, the relationship between higher anticipatory threat appraisals and shorter telomere length was significant and robust in the full combined sample even when controlling for caregiver status. Nonetheless, this and other explanations of our findings remain possible and additional research is required to compare potential models. Such research could productively examine the rate of telomere length change over time in individuals with a high degree of threat sensitivity who are either confronting a chronic life stressor such as caregiving or not confronting any major life stressors. If exposure to chronic stress is associated with increasing threat sensitivity and associated accelerations in the rate of telomere shortening over time, this would support our proposed model. If threat sensitivity remains entirely stable over time in both chronically stressed and non-chronically stressed individuals and only the chronically stressed participants with high levels of threat sensitivity show accelerated telomere shortening, this would support the alternative model. Regardless of the outcomes of such future studies, the two models and the present data overlap in the conclusion that anticipatory threat appraisals play a key role in the relationship between chronic stress and telomere length.
The present data indicate that anticipated threat is more strongly associated with shorter telomere length than threat reported retrospectively about experiences during a stressor. Previous work indicates that anticipatory threat elicits cardiovascular responses equivalent to those associated with actual exposure to threat, underlining the potential importance of anticipatory appraisals as determinants of biological stress reactivity (Waugh et al., 2010). Given the survival value associated with a strongly attuned threat-detection system, the ability to anticipate threats is a human adaptation that has considerable benefits, even if as our data suggestit also has costs (Neuberg et al., 2011; Stein and Nesse, 2011). Moreover, while the experience and recall of stressful events is constrained by perception and memory of those events, there are no such constraints on anticipated stressors leaving them more susceptible to the influence of cognitive biases. Finally, the anticipation of threat may be the most enduring form of psychological stress because people can perceive and worry about potential threats that are in the far-distant future.
Accumulating evidence indicates that exposure to real or imagined psychological threat activates multiple biological systems, including the sympathetic nervous system, the hypothalamic-pituitary adrenal (HPA) axis, and inflammatory response (Dickerson and Kemeny, 2004; Dickerson et al., 2008; Mendes et al., 2007a). The mechanisms by which greater biological stress reactivity could result in short telomere length have yet to be fully elucidated. However, repeated and prolonged activation of biological stress responses under conditions of chronically high levels of perceived threat may result in dysregulation of the HPA axis (Miller et al., 2007) and elevated inflammatory activity (Kiecolt-Glaser et al., 2003; O'Donovan et al., 2010). Elevated levels of circulating cortisol and inflammatory cytokines could in turn promote telomere shortening by reducing levels of telomerase, the enzyme that builds telomeres, and shortening telomere length via increased cell turnover and increased oxidative stress (Choi et al., 2008; Jaiswal et al., 2000; O'Donovan et al., 2011b;Slavich et al., 2010).
Challenge appraisals were neither significantly different between caregivers and controls, nor significantly associated with telomere length in our study. Previous work has also indicated stronger associations of negative compared with positive psychological factors with telomere length. In fact, while pessimism or the generalized tendency to expect negative outcomes in the future has been associated with short telomere length, optimism or the generalized tendency to expect positive outcomes in the future has not (O'Donovan et al., 2009). The biological responses associated with positive emotions and cognitions are not as well understood as those associated with negative emotions and cognitions, and there are no known direct pathways by which positive experiences could influence telomere length. However, positive psychological factors may have buffering effects on pathways activated by negative experiences or there may be as yet undiscovered pathways by which these factors may influence telomere maintenance.
Participants’ reactions to the standardized acute stressor we employed in this study provides a window into their reactions to everyday life stressors. Identifying an acute psychological response that is associated with short telomere length is important for several reasons. First, identifying psychological factors that underlie the relationship between chronic stress and short telomere length has the potential to tie together a large body of research showing that diverse forms of chronic psychological stress increase risk for diverse diseases of aging. Second, and perhaps most importantly, specific responses to acute psychological stressors may be amenable to intervention. Even if chronic stressors such as caregiving, financial strain and interpersonal difficulties are an inevitable part of life, cognitive-emotional responses to such events are not predetermined and are in fact amenable to intervention with cognitive-behavioral and other psychotherapeutic techniques (Beck and Clark, 1997). Targeting interventions at threat anticipation in the ambiguous and threatening situations of daily life may have the potential to slow down the rate of cellular aging, and thereby enhance health and increase longevity in chronically stressed populations.
Limitations
The present findings must be interpreted in light of several limitations. First, the results of our analyses must be interpreted with caution given our small sample size. Second, the cross-sectional design does not permit us to determine causal direction in the relations observed between chronic stress, threat appraisals, and telomere length. However, data from our laboratory indicates very high reliability in telomere length measurements across a one-week period (Lin et al., in preparation), and our design is strengthened by our focus on responses of a population of women experiencing chronic stress and by the employment of a standardized laboratory acute stress paradigm. The chronic stress of being a caregiver predates responses to the acute laboratory stressor, but we cannot confirm that other factors don’t contribute to both chronic stress and the acute stress response. Third, telomere length is only one potential index of the multifaceted and dynamic process of cellular aging. Future studies could include other emerging and established indices of cellular aging such as cell surface markers that are switched on or off in senescent cells (e.g., CD28, CD27, CD57, CD62L), levels of expression of tumor suppressor proteins (e.g., p53, p16) as well as functional measures of immune cell cytotoxicity and proliferative capacity (Beauséjour et al., 2003; Hong et al., 2011). Fourth, the measures of threat and challenge appraisal in this study may be specific to the social-evaluative stressor employed to elicit acute stress appraisals and may not generalize to other kinds of stress (Dickerson and Kemeny, 2004). Moreover, findings related to caregiving stress may not generalize to populations experiencing other forms of psychological stress. Finally, the indirect effect that we observed does not fall within the requirements of traditional mediation analyses because those analyses would require that caregivers and controls differ significantly on telomere length (Baron and Kenny, 1989). However, many current experts on methodology now support reporting of indirect effects where there is no significant relationship between the independent and dependent variables, but only a significant indirect effect of the independent variable on the dependent variable through the mediator (cf. Lockhart et al., 2011).
Conclusions
The present study is the first demonstration that psychological responses to the anticipation of an acute stressor are associated with leukocyte telomere length, an index and potential mechanism of cellular aging. Our results indicate that chronic stress may lead to higher threat appraisals in acutely stressful situations, and that such increased threat appraisals are associated with shorter telomere length. In contrast, chronic stress was not associated with altered challenge appraisals, and challenge appraisals were not independently associated with telomere length. Taken together, the data suggest two related conclusions. First, the tendency to perceive higher levels of threat in anticipation of daily life stressors may impact cellular aging. Secondly, higher anticipatory threat is a potential psychological mechanism by which diverse stressor exposures and states of clinical distress (e.g., PTSD, depression, anxiety) forms of psychological stress could accelerate cellular aging and increase risk for diseases of aging. In sum, acute online psychological responses to short-lived daily life stressors may have cumulative downstream effects on cellular aging and ultimately disease risk.
Research Highlight.
Chronic psychological stress may accelerate the rate of cellular aging by increasing anticipatory threat responses to daily life stressors.
Acknowledgements
The present research was made possible by grants from the Division of Behavioral and Social Research at the National Institute of Aging/National Institutes of Health R56 grant (ESE) and Bernard and Barbro Foundation (EHB) as well as by a Society in Science: Branco Weiss Fellowship (AOD). The Gladstone flow core and the Core Immunology Lab were supported by the UCSF-GIVI Center for AIDS Research P30AI027763. The CTSI CCRC and the Core Immunology Lab were supported by NIH/NCRR UCSF-CTSI grant number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of funders. Furthermore, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to thank the participants in this study for the time and energy that they devoted to this project as well as Peter Bacchetti, PhD, Josh Woolley, MD/PhD and George Slavich, PhD who provided helpful comments on a draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1989;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Clark DA. An information processing model of anxiety: automatic and strategic processes. Behav Res Ther. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, editors. The Handbook of Social Psychology. New York: Wiley; 2010. [Google Scholar]
- Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med. 1997;59:605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucl. Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Karmarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng N. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's Disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psych Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychol. 2008;27:116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Curr Direc Psychol Sci. 2008;17:183–187. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Nat Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41:246–251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- Hong S. Can we jog our way to a younger-looking immune system? Brain Behav Immun. 2011;25:1519–1520. doi: 10.1016/j.bbi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The Big Five Inventory--Versions 4a and 54. Berkeley: University of California, Berkeley, Institute of Personality and Social Research; 1991. [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer-Verlag; 1984. [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart G, MacKinnon DP, Ohlrich V. Mediation analysis in psychosomatic medicine research. Psychosom Med. 2011;73:29–43. doi: 10.1097/PSY.0b013e318200a54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: physiological responses during social interactions with expectancy-violating partners. J Pers Soc Psychol. 2007a;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Gray HM, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: physiological thriving during stressful intergroup encounters. Psychol Sci. 2007b;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter 5-HTTLPR genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberg SL, Kenrick DT, Schaller M. Human threat management systems: self-protection and disease avoidance. Neurosci Biobehav Rev. 2011;35:1042–1051. doi: 10.1016/j.neubiorev.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011a;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES for the Health Aging and Body Composition Study. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS ONE. 2011b;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlin B, Nilsson PM, Nilsson JA, Berglund G. Chronic psychosocial stress predicts long-term cardiovascular morbidity and mortality in middle-aged men. Eur Heart J. 2004;25:867–873. doi: 10.1016/j.ehj.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Pine DS. Developmental psychobiology and response to threats: relevance to trauma in children and adolescents. Biol Psychiatry. 2003;53:796–808. doi: 10.1016/s0006-3223(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Slavich GM, O'Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci Biobehav Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Nesse RM. Threat detection, precautionary responses, and anxiety disorders. Neurosci Biobehav Rev. 2011;35:1075–1079. doi: 10.1016/j.neubiorev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Taylor TR, Williams CD, Makambi KH, Mouton C, Harrell JP, Cozier Y, Palmer JR, Rosenberg L, Adams-Campbell LL. Racial discrimination and breast cancer incidence in US Black women: The Black Women's Health Study. Am. J. Epidemiol. 2007;166:46–54. doi: 10.1093/aje/kwm056. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2009;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Panage S, Mendes WB, Gotlib IH. Cardiovascular and affective recovery from anticipatory threat. Biol Psychol. 2010;84:169–175. doi: 10.1016/j.biopsycho.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]