Abstract
Enteroendocrine cells of the gastrointestinal (GI) tract play a central role in metabolism, digestion, satiety and lipid absorption, yet their development remains poorly understood. Here we show that Arx, a homeodomain-containing transcription factor, is required for the normal development of mouse and human enteroendocrine cells. Arx expression is detected in a subset of Neurogenin3 (Ngn3)-positive endocrine progenitors and is also found in a subset of hormone-producing cells. In mice, removal of Arx from the developing endoderm results in a decrease of enteroendocrine cell types including gastrin-, glucagon/GLP-1-, CCK-, secretin-producing cell populations and an increase of somatostatin-expressing cells. This phenotype is also observed in mice with endocrine-progenitor-specific Arx ablation suggesting that Arx is required in the progenitor for enteroendocrine cell development. In addition, depletion of human ARX in developing human intestinal tissue results in a profound deficit in expression of the enteroendocrine cell markers CCK, secretin and glucagon while expression of a pan-intestinal epithelial marker, CDX2, and other non-endocrine markers remained unchanged. Taken together, our findings uncover a novel and conserved role of Arx in mammalian endocrine cell development and provide a potential cause for the chronic diarrhea seen in both humans and mice carrying Arx mutations.
Keywords: Arx, transcription factor, glucagon, somatostatin, gastrin, CCK, secretin, serotonin, enteroendocrine cells, endocrine progenitors, specification, differentiation
INTRODUCTION
The mammalian gastrointestinal (GI) tract is lined with a single layer of epithelial cells that undergo continuous renewal throughout life. In the stomach and intestine, the self-renewing stem cells are the source of all epithelial cell types and are located in the neck of the gastric glands and in the crypts of intestine (Mills and Shivdasani, 2011; Simons and Clevers, 2011). Epithelial cell types in the stomach include mucin-secreting cells, pepsinogen-producing chief cells and acid-secreting parietal cells, whereas the intestine has absorptive enterocytes, mucus-secreting goblet cells, lysozyme-producing Paneth cells and secretory tuft cells (Gerbe et al., 2009; Gerbe et al., 2011; May and Kaestner, 2010). In addition, enteroendocrine cells secreting at least 15 different hormones are found interspersed along the epithelium of the digestive tract (May and Kaestner, 2010; Rindi et al., 2004).
Representing one of the complex endocrine systems in the body, the enteroendocrine system plays a central role in metabolism, digestion, satiety and lipid absorption (Beglinger and Degen, 2004; Drucker, 2007a; Drucker, 2007b; Gershon, 1968; Mellitzer and Gradwohl, 2011; Murphy and Bloom, 2006; Murphy et al., 2006; Rehfeld, 2004a; Rehfeld, 2004b). The importance of these cells is demonstrated by studies in which patients with malabsorptive diarrhea were found to have mutations in the NEUROGENIN3 gene, which resulted in undetectable enteroendocrine cells (Rubio-Cabezas et al., 2011; Wang et al., 2006). Despite the unequivocal importance of enteroendocrine cells in the regulation of diverse biological processes, there is a deficiency in our understanding of how the specific enteroendocrine subtypes are generated during GI development in mice and humans.
The mechanism by which enteroendocrine cells regulate the above biological processes is via an array of secreted hormones. In the intestine, cholecystokinin (CCK) is known to promote digestive enzyme release and gallbladder contraction, secretin is important to stimulate pancreatic enzyme release and biliary bicarbonate production, and glucagon-like peptide-1 (GLP-1) is involved in potentiating glucose-dependent insulin secretion and inhibits gastric emptying (Baggio and Drucker, 2007; Lloyd, 1994; Murphy and Bloom, 2006; Rehfeld, 2004b; Wren, 2008). In the stomach, hormones such as gastrin and somatostatin are important to stimulate and inhibit gastric acid release, respectively, while ghrelin is critical to control appetite (Schubert, 2010; Wren and Bloom, 2007; Wren et al., 2001).
Several transcriptional regulators have been shown in mice to regulate development of the enteroendocrine cells (May and Kaestner, 2010). While some factors are required for general endocrine cell differentiation (i.e. Neurogenin3; Ngn3, Math1), others are only necessary to specify enteroendocrine subtypes. Ngn3, a proendocrine transcription factor, is necessary for the formation of enteroendocrine progenitors in the GI tract, as Ngn3-deficient mice display a complete and a partial loss of enteroendocrine cells in the intestine and stomach, respectively, similar to what has been reported in humans (Jenny et al., 2002; Lee et al., 2002; Mellitzer et al., 2010; Wang et al., 2006). In addition, Math1 null mice show a complete deficiency in all secretory lineages including enteroendocrine cells, Paneth cells, and goblet cells in the intestine (Yang et al., 2001). In contrast, homeodomain transcription factors such as Pax4, Pax6, Nkx6.1, Nkx2.2, Nkx6.3, and Pdx1 are involved in the differentiation of specific enteroendocrine cell types (Choi et al., 2008; Guz et al., 1995; Jonsson et al., 1995; Jonsson et al., 1994; Larsson et al., 1996; Larsson et al., 1998; Offield et al., 1996). Taken together, these studies, largely in mice, demonstrate the importance of transcriptional regulation in enteroendocrine cell differentiation in the GI tract and provide the basic framework for our current understanding of how these specialized cells are formed during development. However, the mechanism of cell fate determination of such a diverse group of hormone producing cells from a single Ngn3+ progenitor population (Schonhoff et al., 2004) is largely unknown.
Here, we report a previously unappreciated role for Arx, a gene encoding a prd-homeodomain-containing transcription factor, during enteroendocrine cell development in mice and humans. We find that Arx is expressed in a subset of Ngn3+-endocrine progenitors and a subset of mature hormone producing-cells in the developing GI tract and that Arx is required for development of enteroendocrine population. Furthermore, the requirement of ARX was further examined in human proximal intestinal organoids generated from human embryonic stem (hES) cells (Spence et al., 2011), where ARX knockdown resulted in a significant decrease in the enteroendocrine cell markers CCK, secretin and glucagon.
Taken together, this study is the first to establish Arx as a novel and important factor during enteroendocrine cell differentiation in both mouse and human. Analysis performed in mice further shows that Arx functions in the Ngn3+ endocrine progenitors to promote gastrin, glucagon/GLP-1, CCK and secretin lineages, while simultaneously repressing the somatostatin cell lineage in the GI tract. Given that both Arx-deficient mice and patients with ARX mutations suffer chronic diarrhea (Itoh et al., 2010), these findings also provide strong evidence that the primary cause of diarrhea in these patients is likely due to alterations of specific enteroendocrine cell populations.
METHODS
Mice and tissue preparation
CD1 wild-type mice were used for characterizing Arx expression. Arx is X-linked and the derivation of the floxed conditional allele (ArxL/L), Foxa3-Cre, Ngn3-Cre and Villin-Cre mouse lines has been reported previously (el Marjou et al., 2004; Fulp et al., 2008; Lee et al., 2005; Schonhoff et al., 2004). These mice were maintained on a mixed background (CD1, 129 and C57BL/6) and were cared and handled according to the Children’s Hospital of Philadelphia’s Institutional Animal Care and Use Committee approved protocol. Timed pregnancies were determined based on the appearance of the vaginal plugs and were considered embryonic day (E) 0.5. Primers used for genotyping alleles used in this study were described previously (Fulp et al., 2008; Lee et al., 2005; Schonhoff et al., 2004).
Real-time PCR analysis
All tissue dissections were performed in cold 1X PBS, and the tail snips were used for genotyping. Total RNA was extracted in TRIZOL (Invitrogen) using the RNA Easy kit (Qiagen). Oligo-dT, Superscript plus other required reagents were used to synthesize cDNA. PCR reactions were set up using the Brilliant SYBR Green PCR Master Mix in the Stratagene Mx3005P Real-time PCR machine. All reactions were performed in triplicate with reference dye normalization. Primer sequences are available upon request.
Immunohistochemistry and histology
Tissues were fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin or optimal cutting temprature (OCT) freezing medium, and 8 μm sections collected. Slides were subjected to microwave antigen retrieval in 10 mmol/L sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% H2O2 in PBS for 15 minutes, and sections blocked with CAS-Block reagent (Invitrogen). The sections were incubated with primary antibodies overnight at 4°C and appropriate secondary antibodies for 2 hours at room temperature. Immunohistochemical detection was performed with the VECTASTAIN ABC kit (Vector Laboratories) and diaminobenzidine tetrahydrochloride (DAB) as the substrate. The primary antibodies used were: Glucagon (1:3000; Millipore), Somatostatin (1:3000; Santa Cruz), Arx (1:500; gift from Dr. Kanako Miyabayashi at Kyushu University), Pdx1 (1:200; Santa Cruz), Ghrelin (1:200; Santa Cruz), Isl-1 (1:50; Developmental Studies Hybridoma Bank 39. 4D5 and 40.2D6), Gastrin (1:200; Santa Cruz), 5-Hydroxytryptamine: 5-HT (Serotonin) (1:50000; ImmunoStar), Chromogranin A (1:3000; DiaSorin), Neurogenin 3 (1:500; Developmental Studies Hybridoma Bank), GLP-1(1:500; Abcam), CCK (1:100; Santa Cruz), DCAMKL1 (1:100; Abcam), Muc2 (1:750; Santa Cruz), Lysozyme (1:3000; Dako), Cdx2 (1:500; Biogenex). Sections were stained with Hematoxylin and Eosin (H&E), Alcian blue, and Oil-Red-O according to the standard protocols. Oil-Red-O staining was performed using frozen sections.
Hormone cell quantification
The hormone-positive cells from antrum and corpus of the stomach were counted, combined and normalized to the total epithelial area of the same or adjacent section for the analyses in ArxEndoderm and control mice. Separate regions (antrum and corpus) of the stomach were counted and normalized to the respective epithelial areas of the same or adjacent section for the analyses in ArxEndocrine and control mice. Hormone-positive cells from different regions of the intestine were counted and normalized to the respective epithelial area of the same or adjacent section. Epithelial area was measured with Aperio Image Analysis System.
Blood glucose measurement
Blood glucose was measured using an automatic glucometer (One Touch Ultra; LifeScan, Milpitas, CA).
hES-derived intestinal organoid culture
The human ES cell line WA09 was obtained from WiCell and maintained in feeder-free conditions on Matrigel (BD Biosciences) in mTesR1 medium (Stem Cell Technologies). Stable shRNA knockdown lines were generated by lentiviral-mediated transduction of single-cell suspensions with MISSION shRNA clones from Sigma-Aldrich (ARX shRNA: TRCN0000016333; non-targeting control shRNA: SHC002). After transduction, stable lines were selected with puromycin and maintained under selection throughout differentiation protocols. Human intestinal organoids were generated as previously described (Spence et al., 2011). Briefly, ES cells were plated in a 24-well plate and grown to near-confluency. To generate definitive endoderm, cells were treated with Activin A (100 ng/ml; R&D Systems) for three days in RPMI 1640 medium (Invitrogen) in the presence of 0%, 0.2%, and 2% dFBS (Thermo Scientific) on days 1, 2, and 3, respectively. Endoderm cells were then cultured in DMEM/F12 medium (Invitrogen) in the presence of 2% dFBS, Wnt3a (500 ng/ml; R&D Systems), and FGF4 (500 ng/ml; R&D Systems) for 3–4 days. Floating spheroids generated from the hindgut cultures were embedded in Matrigel (BD Biosciences) and cultured in DMEM/F12 medium supplemented with 10 μM HEPES, N2 Supplement (R&D Systems), B27 Supplement (Invitrogen), R-Spondin (500 ng/ml; R&D systems), Noggin (100 ng/ml; R&D Systems), and EGF (50 ng/ml; R&D Systems). Human intestinal organoids were passaged into fresh Matrigel every 14 days.
RESULTS
Arx is expressed in a subset of enteroendocrine cells in the GI epithelium
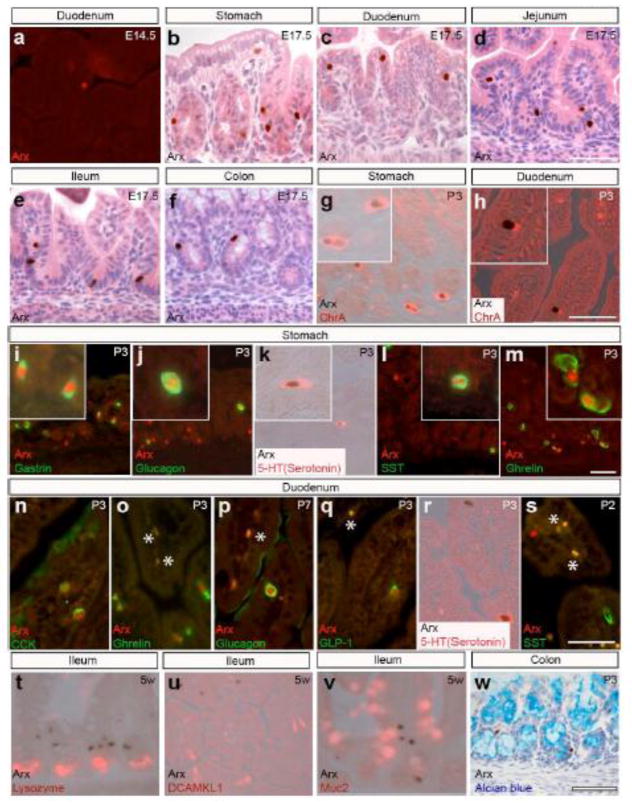
Immunostaining analysis was performed to characterize the temporal and spatial expression domains of Arx in the developing stomach and intestine of mice. While Arx expression was strong in the pancreas at embryonic day (E)13.5, its expression was not detectable in the stomach and intestine at this stage (data not shown). A day later, Arx expression was detected at a low frequency in the duodenum, but not in the stomach (Fig. 1a and data not shown). At E15.5, the GI epithelium begins to differentiate and form gastric units and nascent villi and shortly after this (~E17.5), Arx-expressing cells can be found along the crypt-villus axis in the entire developing digestive tract (Fig. 1b–f). We assessed whether Arx expression was restricted to enteroendocrine cells using chromogranin A (ChrA), an pan-endocrine cell marker, in postnatal day (P)3 stomach and duodenum, and found that Arx expression was restricted to a subset of the ChrA-positive cells (Fig. 1g and h). To evaluate which enteroendocrine subtypes express Arx, we analyzed Arx expression in specific gastrointestinal hormone-expressing cells. In the P3 stomach, Arx expression was localized to gastrin-, glucagon-, serotonin-, somatostatin-, and ghrelin-expressing cells (Fig. 1i–m). In the duodenum, Arx expression was found in enteroendocrine subpopulations including cholecystokinin- (CCK), ghrelin-, glucagon-, GLP-1-, and serotonin-positive cells (Fig. 1n–r). Interestingly, we did not detect co-localization of Arx with somatostatin in the duodenum (Fig. 1s). Lastly, Arx expression was not detected in the intestinal Paneth, tuft or goblet cells at all ages examined (Fig. 1t–w and data not shown). Taken together, these results indicate that Arx is restricted to a specific subset of enteroendocrine cells.
Figure 1.
Arx is expressed in the hormone producing cells scattered in the epithelium of the stomach and intestine. (a–f) Immunostaining analyses on E14.5 or E17.5 wild type stomach and intestine localize cells expressing Arx in the duodenal epithelium at E14.5 (a) and stomach (b), duodenum (c), jejunum (d), ileum (e) and colon (f) at E17.5. (g–h) Double immunostaining analysis on P3 wild type stomach (g) and duodenum (h) shows Arx is expressed in the chromograninA+ enteroendocrine cells. (i–s) Double immunostaining analysis on wild type stomach and duodenum localize Arx expression to gastrin (i; 12/25 cells), glucagon (j; 7/17 cells), 5-HT serotonin (k; 1/2 cells), somatostatin (l; 2/13 cells), ghrelin (m; 8/18 cells) producing cells in the stomach and CCK (n), ghrelin (o), glucagon (p), GLP-1 (q), 5-HT serotonin (r), but not somatostatin (s) producing cells in the duodenum. “*” denotes non-specific staining. Boxed areas in (i–m) show higher resolution of the images. Arx expression is not found in Paneth cells (t), tuft cells (u) and goblet cells (v–w). Scale bar: 50μm. Note: duodenum at P7 was used for glucagon/Arx co staining due to the low number of glucagon cells present in the duodenum of younger animals.
Arx expression is found in subsets of Pdx1+, Ngn3+ and Isl-1+ cells
To investigate when Arx might function during enteroendocrine development, we compared Arx expression with key transcriptional regulators (Pdx1, Ngn3, or Isl-1) that have been associated with specific developmental stages of enteroendocrine cell differentiation (Das and May, 2011; Guz et al., 1995; Jenny et al., 2002; Jonsson et al., 1995; Jonsson et al., 1994; Larsson et al., 1995; Lee et al., 2002; Mellitzer et al., 2010; Offield et al., 1996). At E17.5, very few Arx-positive cells co-expressing Pdx1 or Ngn3 were found in the stomach and duodenum (Supple. Fig. 1a–i and data not shown). In addition, a few cells co-expressing Arx and Isl-1 were detected in the duodenum (Supple. Fig. 1j–l). As Ngn3 marks endocrine progenitors and Isl-1 labels differentiated endocrine cells (Das and May, 2011; Jenny et al., 2002; Lee et al., 2002; Schonhoff et al., 2004), these findings suggest that Arx functions downstream of Ngn3 in the subset of undifferentiated endocrine progenitors and/or in the differentiated endocrine cells of the GI epithelium.
Endoderm-specific Arx-deficient mice exhibit growth retardation and chronic diarrhea
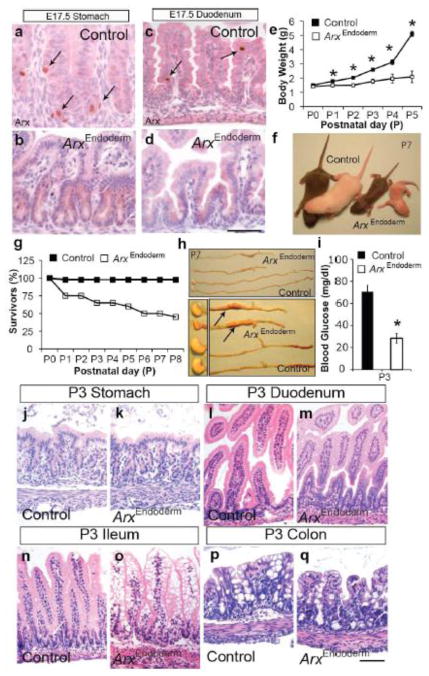
To examine the functional consequences of Arx deficiency in the epithelium of the GI tract, including stomach and intestine, we generated mice with Arx deletion in the developing endoderm (Foxa3-Cre; ArxL/L or Foxa3-Cre; ArxL/Y; referred to as ArxEndoderm mutant hereafter) by intercrossing Foxa3-Cre mice to Arx-floxed mice (Fulp et al., 2008; Lee et al., 2005). We assessed the efficiency of Arx removal in the developing stomach and intestine at the onset of enteroendocrine cell differentiation at E17.5. Compared to the control mice, ArxEndoderm mutants lacked all Arx-expressing cells at E17.5 in the stomach and duodenum (Fig. 2a–d and data not shown). Mutants were born at the expected Mendelian ratio and were outwardly indistinguishable from their control siblings at birth. However, in the next few days, ArxEndoderm mutants fell behind their control littermates in growth (Fig. 2e–f). By the end of the first week, all ArxEndoderm mutant animals had severe diarrhea and half of these mice died, the other half of the mutants survived past weaning and appeared grossly normal albeit reduced in size, and continued to have diarrhea (Fig. 2g). Early lethality was not due to lack of feeding as all mutant mice had milk in their stomach (Fig. 2h). Although the digestive tract was smaller and shorter in the ArxEndoderm mutants compared to the control littermates, these reductions were proportional to the length and body weight of the ArxEndoderm mutant mice (Fig. 2h and data not shown). In our macroscopic analyses of the ArxEndoderm mutants, we observed dilation in the ileum (arrows; Fig. 2h). Lastly, P3 ArxEndoderm mutant mice also displayed relative hypoglycemia (Fig. 2i), which can result from malnourishment due to chronic diarrhea (as discussed below) and/or due to islet glucagon cell deficiency in the pancreas (Collombat et al., 2003).
Figure 2.
ArxEndoderm mutant mice fail to thrive with abnormal ileal epithelium. (a–d) Immunostaining analysis on E17.5 control and ArxEndoderm mutant stomach and duodenum show efficient Arx removal. Arrows mark Arx expressing cells in control tissues (a, c). Scale bar: 50μm. (e) Daily body weight measurements of control and ArxEndoderm mutant mice from postnatal (P) day 0 to day 5. (f) P7 ArxEndoderm mutant mice appear smaller compared to their control littermates. (g) Survival graph shows approximately 50% of the ArxEndoderm mutant mice die during the first week of life. n=43 (control) and n=20 (ArxEndoderm mutant) mice were used at the beginning of the experiment. (h) ArxEndoderm mutants show dilated ileum (arrows). (i) Measurement of random fed glucose levels show P3 ArxEndoderm mutants suffer from relative hypoglycemia. (j–q) Hematoxylin/eosin staining of the P3 control and ArxEndoderm mutants reveal normal histology in the stomach (j–k), duodenum (l–m), and colon (p–q), but appearance of vacuoles in the ileum (n–o).
Abnormal architecture observed in the ileal epithelium of ArxEndoderm mutant mice
The impact of Arx removal on the overall histology of the GI epithelium was next assessed. Hematoxylin and eosin stained tissue sections from the control and ArxEndoderm mutant mice revealed normal architecture of the stomach, duodenum and colon; however, in the ileum we observed abnormal vacuoles in the enterocytes of ArxEndoderm mutant mice (Fig. 2j–q). Since Arx expression is not restricted to the ileum or found in enterocytes (data not shown), the histological changes seen in the ileum is likely secondary to the loss of Arx in enteroendocrine cell populations (see below for further discussion).
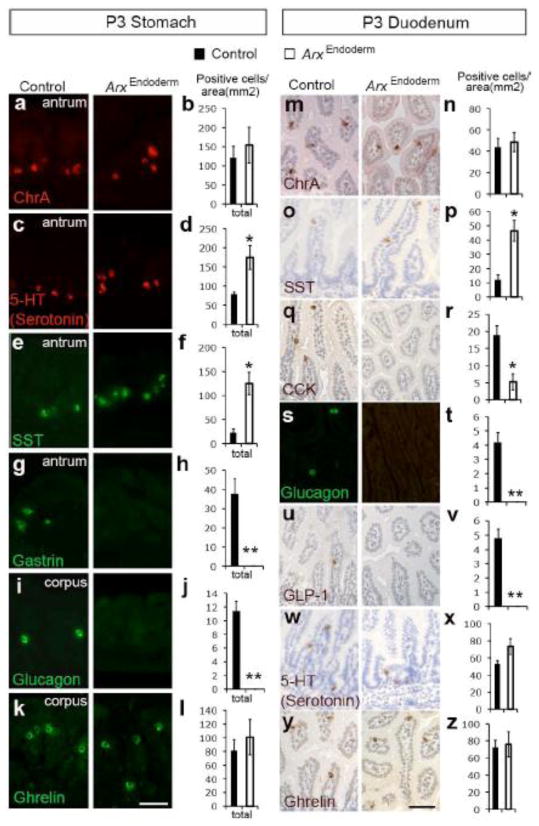
Gut hormones critical for digestion and motility are affected in ArxEndoderm mutant mice
As Arx is expressed in developing and postnatal enteroendocrine cells (Fig. 1 and Supple. Fig. 1), we investigated the impact of Arx deletion on formation of enteroendocrine cells using the pan-endocrine marker ChrA. While the total number of ChrA-positive enteroendocrine cells remained unchanged, the proportion of serotonin-and somatostatin-expressing cells was increased, and the frequency of gastrin- and glucagon-expressing cells was reduced in the P3 ArxEndoderm mutant stomach (Fig. 3a–j). Although Arx expression was detected in a subset of ghrelin-expressing cells (Fig. 1m), we did not detect a difference in the number of this cell type (Fig. 3k–l). Similar analyses were conducted in the P3 duodenum where we found a significant increase in the number of somatostatin-expressing cells (Fig. 3o–p) and a dramatic reduction in the number of CCK-, glucagon-, and GLP-1-expressing cells (Fig. 3q–v). The number of serotonin- and ghrelin-expressing cells was unchanged as was the total number of ChrA-positive enteroendocrine cells (Fig. 3m–n and w–z). In addition, similar changes in the enteroendocrine cell types were observed in the P3 jejunum and ileum of ArxEndoderm mutant mice (Supple. Fig. 2 and data not shown). Real-time PCR analysis confirmed changes in expression levels of these hormone genes and additionally observed a decrease in TPH-1 and secretin mRNA levels in the P3 ArxEndoderm stomach and duodenum (Supple. Fig. 3a–b). While deficiency of several gastrointestinal transcriptional regulators including Pax4, Pax6, Nkx6.1, Nkx2.2, Nkx6.3, and Pdx1 have been shown to play a role in the development of enteroendocrine populations that were affected in the ArxEndoderm mice, we did not detect any changes in the expression levels of any of these genes in the P3 ArxEndoderm stomach and duodenum (Supple. Fig. 3c–d).
Figure 3.
Changes in the numbers of serotonin, somatostatin, gastrin, glucagon, GLP-1 and CCK-expressing cells in the ArxEndoderm mutant mice. (a–l) Immunostaining for chromogranin A (a–b), 5-HT serotonin (c–d), somatostatin (e–f), gastrin (g–h), glucagon (i–j) and ghrelin (k–l) in P3 control and ArxEndoderm mutant stomach with cell count averages from several animals. Cell numbers from corpus and antrum were counted, combined, and normalized to the total surface area. (m–z) Immunostaining for chromogranin A (m–n), somatostatin (o–p), CCK (q–r), glucagon (s–t), GLP-1 (u–v), 5-HT serotonin (w–x) and ghrelin (y–z) in P3 control and ArxEndoderm mutant duodenum with cell count averages from different mice. Cell numbers from duodenum were quantified and normalized to the total surface area. Bars represent means ±SEM (n=4 for both groups. * p<0.05, ** p<0.001). Scale bar: 50μm. Note: Sections used for these experiments were not immediate adjacent sections; therefore, ChrA+ cell number will not equal to the summation of all individual hormones examined. Also, ChrA does not label all enteroendocrine cells (Mellitzer et al., 2010).
Arx is required in endocrine progenitors to specify cell fate choices
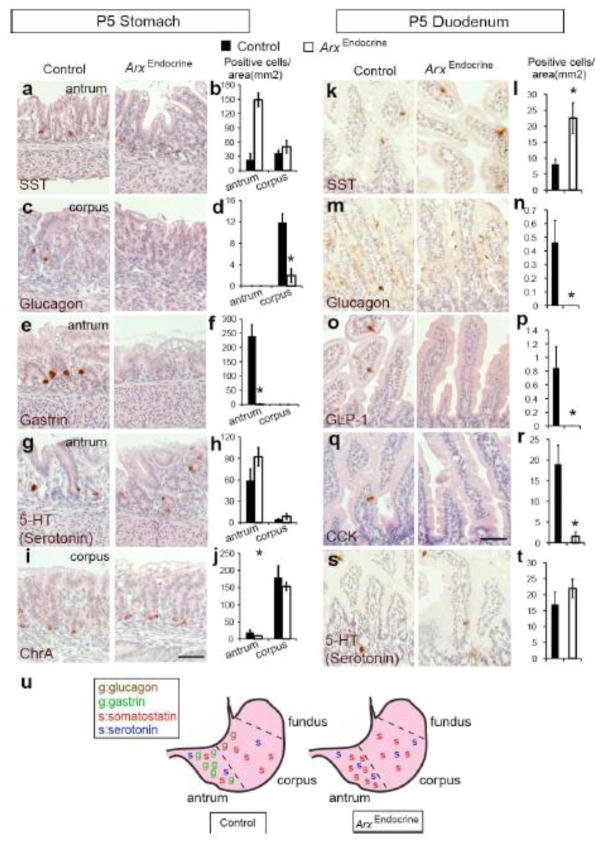
Arx is localized to a very few somatostatin cells in the stomach and is completely absent in these cells of the duodenum (Fig. 1l and s) and yet there is a dramatic increase in the number of this cell type in the ArxEndoderm mutant mice (Fig. 3g–f and o–p). There are several possible explanations for the increase of somatostatin cell population seen in the ArxEndoderm mutant mice, however one explanation is that Arx may play a cell fate decision role in the endocrine progenitors and that these cells become redirected into the somatostatin lineage when the Arx-dependent pathway is deleted. Alternatively, the increase in the somatostatin cell number could be due to fate switching from another enteroendocrine cell type upon Arx removal. To test the former possibility, we deleted Arx specifically in the Ngn3+ endocrine progenitors (ArxEndocrine mutant mice) by intercrossing Ngn3-Cre mice to Arx-floxed mice (Fulp et al., 2008; Mastracci et al., 2011; Schonhoff et al., 2004). As enteroendocrine populations are known to show stereotypical variation across different regions of the stomach, (Fig. 5u; left image; (Jensen et al., 2000; Rindi et al., 2004), we analyzed changes in enteroendocrine cell numbers specifically in the gastric corpus and antrum of P5 control and ArxEndocrine mutant mice. Similar to what we have observed in the ArxEndoderm mice, the number of somatostatin-expressing cells increased dramatically in the antrum, but not in the corpus (Fig. 4a–b). In contrast, glucagon- and gastrin- expressing cells were profoundly reduced in the corpus and antrum, respectively (Fig. 4c–f). We observed no significant increase in the numbers of serotonin-positive cells in the antrum or corpus; in contrast to what we had seen in the ArxEndoderm mutant stomach (Fig. 3c–d and Fig. 4g–h). Lastly, we did not detect changes in the number of ChrA-expressing cells in either the antrum or corpus of ArxEndocrine mutant mice (Fig. 4i–j). These data suggest that in the stomach Arx is required in the Ngn3+ endocrine progenitors to control cell fate choices for glucagon, gastrin and somatostatin, but not serotonin lineages. Alternatively, there may be an existence of endocrine precursor population that is positive for Arx, but devoid of Ngn3 expression that can also give rise to serotonin lineage in the stomach (see below for further discussion).
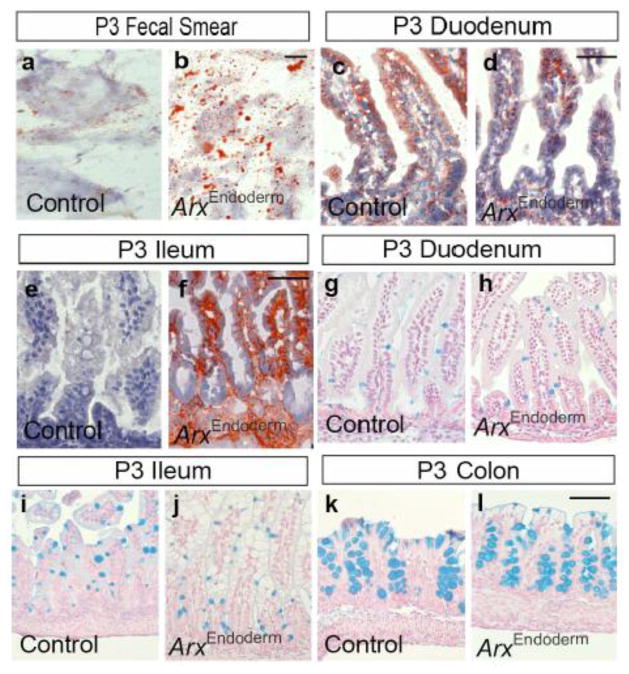
Figure 5.
Gastrointestinal epithelium appears normal with fat accumulation in the ileum of P3 ArxEndoderm mutants. (a–b) Oil-red-O staining of fecal smear. (c–f) Oil-red-O staining of the P3 duodenum and ileum demonstrates presence of increased fat accumulation only in the ileum of ArxEndoderm mutants. (g–l) Alcian blue staining of the duodenum, ileum and colon reveals no significant changes in the numbers of goblet cells between ArxEndoderm mutant and control mice. Scale bar: 50μm.
Figure 4.
Arx is required in the endocrine progenitors to control cell fate decisions. Immunostaining analysis on P5 control and ArxEndocrine mutant stomach and duodenum for somatostatin (a–b, k–l), glucagon (c–d, m–n), gastrin (e–f), GLP-1 (o–p), and CCK (q–r), 5-HT serotonin (g–h, s–t) and ChrA (i–j), For the stomach, cell numbers were quantified and normalized to the epithelial area separately for antrum and corpus. For the duodenum, total cell numbers were counted and normalized to the total epithelial area. Bars represent means ±SEM. (n=4, * p<0.05, ** p<0.001). Scale bar: 50μm. (u) The drawing summaries the observed regional changes of enteroendocrine populations in the stomach. Note: Sections used for these experiments were not immediate adjacent sections; therefore, ChrA+ cell number will not equal to the summation of all individual hormones examined. Also, ChrA does not label all enteroendocrine cells (Mellitzer et al., 2010).
In the duodenum of ArxEndocrine animals, there was an increase in the numbers of somatostatin-expressing cells and a reduced number of glucagon-, GLP-1, and CCK-expressing cells, while the number of serotonin-expressing cells remained unchanged (Fig. 4k–t). The striking similarities in the changes of intestinal enteroendocrine cell populations between ArxEndoderm and ArxEndocrine mice strongly suggest that Arx is largely acting in endocrine progenitors to control specification of distinct subtypes of enteroendocrine lineages (Fig. 4u).
GI dysfunction and alterations in enteroendocrine cell populations are observed in ArxIntestine mutant mice
Arx also plays a critical role in islet alpha cell development in the pancreas (Collombat et al., 2003) and pancreatic dysfunction has been postulated to contribute to the diarrhea seen in patients with ARX mutations (Itoh et al., 2010). To determine whether the ArxEndoderm mutant intestinal phenotypes are independent of the known role of Arx in pancreas development, we generated intestine-specific Arx-deficient mice (Villin-Cre; ArxL/L or Villin-Cre; ArxL/Y; referred to as ArxIntestine mutant hereafter) by intercrossing Villin-Cre transgenic mice to Arx-floxed mice (el Marjou et al., 2004; Fulp et al., 2008). We found that although ArxIntestine mutant mice have normal survival curves, they too suffered from chronic diarrhea with mildly reduced glucose levels compared to control mice at P3 (95±6.5mg/dl for controls and 58±8mg/dl for mutants). These findings demonstrate that intestinal Arx loss is the predominant cause for the diarrhea and hypoglycemia in ArxEndoderm mice and that loss of Arx in the pancreas of these mutant mice only contributes to the hypoglycemia phenotype.
Immunostaining of intestinal tissue from adult ArxIntestine mutant mice was performed which showed an increase in somatostatin-expressing cells and decreases in CCK, glucagon/GLP-1 cell populations in the duodenum (Supple. Fig 4). Furthermore, analysis of the ArxEndoderm mice that survived to adulthood showed that they continued to have soft stool and exhibited similar changes in the enteroendocrine populations in the stomach and duodenum (data not shown). These results suggest that although older mice continue to have chronic diarrhea likely due to enteroendocrine cell dysfunction, survival is not affected beyond early stages of their lives.
Lipid accumulation in the ileum of ArxEndoderm and ArxIntestine mutant mice
The cause of chronic diarrhea was next evaluated in Oil-red-O stained fecal samples from P3 ArxEndoderm and ArxIntestine mutant mice, which showed an increased in staining indicating potential defects in the intestinal fat absorption (Fig. 5a–b and Supple. Fig. 5a–b). To further investigate this defect, different regions of the intestine were stained with Oil-red-O in which excess lipid accumulation was evident only in the ileum of ArxEndoderm mutant mice (Fig. 5c–f and data not shown). These findings demonstrate that the abnormal vacuoles seen in the ileum of ArxEndoderm were in fact lipid droplets (Fig. 2n–o4). Excess lipid accumulation was also observed in the ileum of P3 ArxIntestine and ArxEndocrine mutants (Supple. Fig. 5c–f and data not shown). Taken together, these findings suggest that intestinal Arx deficiency impacts development of several enteorendocrine cell populations, whose functions include the regulation of lipid processing and/or metabolism in the neonatal intestine.
Lastly, since the goblet cell number was increased in the Ngn3-null mice at early postnatal stages and a possibility of goblet and enteroendocrine cells sharing part of their developmental program (Jenny et al., 2002; Jensen et al., 2000; Poulsom et al., 1993), we examined if there were changes in goblet cell population in our ArxEndoderm mutant mice. Alcian blue stainings were performed and quantified in the intestine of P3 control and ArxEndoderm mutant mice and showed no obvious changes in the numbers of goblet cells in the duodenum (2.25±0.16 for control and 2.39±0.32 for mutant; per villus), ileum (4.59±0.21 for control and 4.27±0.34 for mutant; per villus) or colon (10.22±0.37 for control and 10.54±1.2 for mutant; per gland) (Fig. 5g–l). These observations indicate that Arx removal and/or altered enteroendocrine cell populations in the ArxEndoderm mutant mice have no impact on the goblet cell development.
ARX knock down in human intestinal tissue also causes enteroendocrine cell deficiency
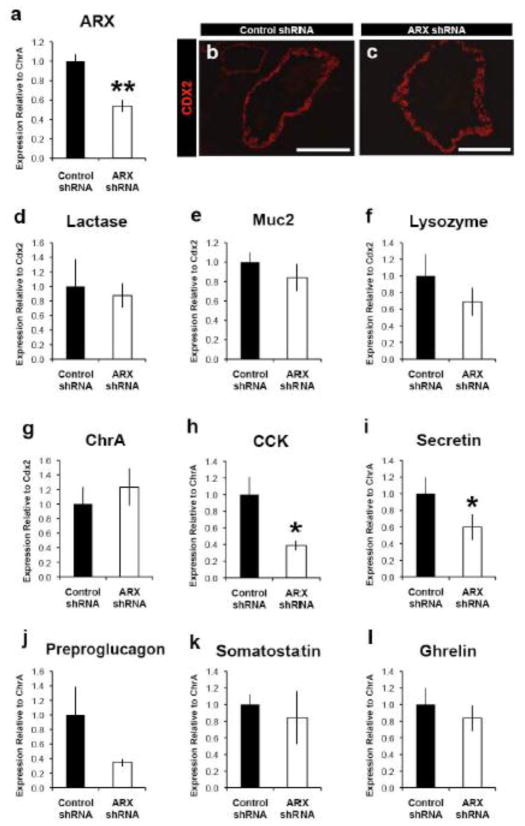
It has previously been reported that patients carrying ARX mutations suffer from diarrhea (Itoh et al., 2010), a phenotype that is also observed in Arx-deficient mice. To determine if this coincides with the loss of specific enteroendocrine cells as it does in mice, we investigated if ARX is required for normal human enteroendocrine cell development. We performed shRNA-mediated loss-of-function in human intestinal organoids (HIOs) derived from pluripotent stem cells via directed differentiation (Spence et al., 2011). To generate stable shRNA-ARX knockdown lines, human ES cells were transduced with lentivirus expressing ARX shRNA and differentiated into proximal intestinal organoids, which had between 50–60% reduction in ARX mRNA levels (Fig. 6a). HIOs were analyzed at day 68, at which time all intestinal cell types can be observed (Spence et al., 2011). Immunostaining analysis showed normal CDX2 expression in epithelium the ARX knockdown organoids as compared to control organoids, which indicates that ARX knockdown did not inhibit the formation of a CDX2-positive epithelium (Fig. 6b–c). Additionally, ARX knockdown did not affect expression levels of markers for enterocytes (Lactase), goblet cells (Muc2), or Paneth cells (Lysozyme) (Fig. 6d–f). Furthermore, there were no changes in the endocrine markers CHRA or Ghrelin. However, ARX knockdown did cause a 60–80% reduction in CCK, Secretin, and Preproglucagon mRNA levels (Fig. 6g–l) demonstrating that development of a subset of enteroendocrine cells is dependent on the presence of ARX in humans as it is in mice. Interestingly, Somatostatin gene expression was not increased in ARX depleted human organoids as it was in Arx-deficient mice (Fig. 3o–p, 4m–o, Supple Fig. 3b, Supple. Fig. 4a–b). These results suggest that human enteroendocrine cell development requires ARX and the GI dysfunction in patients with ARX mutations may be caused by altered numbers of enteroendocrine cells in the GI tract.
Figure 6.
Human enteroendocrine cell development requires ARX. Proximal intestinal tissue was generated from human embryonic stem cell lines and analyzed at 68 days after differentiation. Stable lines containing ARX shRNA had between 50–60% reduction in ARX mRNA (a) and a similar reduction in the proximal enteroendocrine cell markers CCK (h), Secretin (i), and Preprolucagon (j). There was no significant change in the pan-endocrine marker ChrA (g) or the enteroendocrine cell markers Somatostatin and Ghrelin (k,l). Overall, ARX knockdown did not affect intestinal development, as there were no differences observed in the pan-epithelial marker CDX2 (b,c) or differentiation of enterocytes (Lactase, d), goblet cells (MUC2, e), or paneth cells (Lysozyme, f). Data were analyzed using a Students t-test. *p<0.05. **p<0.01. Scale bars in b,c represent 200 μm.
DISCUSSION
The function of Arx has been identified and studied extensively in the brain and endocrine pancreas (Collombat et al., 2005; Collombat et al., 2007; Collombat et al., 2003; Colombo et al., 2007; Friocourt et al., 2008; Hancock et al., 2010; Kitamura et al., 2002; Marsh et al., 2009; Mastracci et al., 2011), yet its role in developing GI endocrine cells has not been characterized. Findings from our study not only contribute vastly to the body of knowledge on transcriptional control of enteroendocrine cell development in mice and humans, they also provide insights into the pathogenesis of GI symptoms observed in patients with ARX mutations.
Our analyses of mice deficient for Arx in the developing endoderm (ArxEndoderm) and in the intestine (ArxIntestine) show changes in the enteroendocrine populations, which are likely to result in hormone imbalance, leading to severe diarrhea. In addition, removing Arx in Ngn3+ endocrine progenitors (ArxEndocrine) further demonstrates that Arx is required for cell fate decisions in these cells. In fact, similar changes in the numbers of gastric somatostatin, gastrin and glucagon cells and intestinal somatostatin, CCK, glucagon/GLP1 cells were observed in the ArxEndocrine and ArxEndoderm mutants. Although our findings do not directly demonstrate that Arx is involved in favoring certain enteroendocrine lineages at the expense of the others within the endocrine progenitor population as demonstrated in the Arx-deficient pancreas (Collombat et al., 2005; Collombat et al., 2003), these observations are suggestive of such a possibility in the intestine based on the lack of Arx expression in the somatostatin cells and a profound increase of this cell type upon Arx removal in the endocrine progenitors.
The importance of enteroendocrine progenitors, mature enteroendocrine cells, and hormones secreted by these cells have been suggested to play a critical role in maintaining overall homeostasis of the intestinal crypt compartment (Mellitzer et al., 2010). In contrast to the altered cell homeostasis observed in the Ngn3-deficient adult intestine (Mellitzer et al., 2010), we did not detect any disorganization in the crypts in the neonatal or adult Arx-deficeint mice suggesting that the affected enteroendocrine populations in the Arx-deficient mice may not play a role in maintaining the crypt homeostasis. Furthermore, in contrast to an increase in the goblet cell number observed in the Ngn3 null mice (Jenny et al., 2002), we also did not detect changes in this cell type in the intestine of the ArxEndoderm mice suggesting that Arx is not involved in regulating part of the developmental program that might be shared by enteroendocrine and goblet cells as previously suggested (Jenny et al., 2002; Jensen et al., 2000; Poulsom et al., 1993).
While in Arx-deficient mice the gross architecture of the crypts and villi in the gastrointestinal epithelium appears normal with presence of all intestinal cell types, an abundance of lipid-filled enterocytes was detected in the ileum of ArxIntestine, ArxEndoderm and ArxEndocrine mutant mice at early postnatal stages. This phenotype is reminiscent of the intestinal phenotype found in the Pleomorphic adenoma gene-like 2 (PlagL2) deficient pups in which lipid-filled enterocytes were found in the jejunum (Van Dyck et al., 2007). Since PlagL2 is expressed in the enterocytes and Arx is only found in the enterendocrine cells, our findings suggest that enteroendocrine subpopulations affected in the Arx-deficient mice are likely hormone cell types that are involved in regulating lipid absorption and/or transport. Since no obvious morphological alterations or lipid-filled enterocytes were found in the proximal intestine of the Arx-deficient mice, the lipid transport system is likely overloaded and only affected profoundly in the distal region of the intestine similar to what was described in the distal jejunum of PlagL2 mutants at nursing ages (Van Dyck et al., 2007). Although we cannot identify the specific hormones that are responsible for regulating gut lipid metabolism, our findings suggest that combined changes in somatostatin, CCK, secretin and glucagon/GLP1 populations are likely impacting this process.
Interestingly, although similar hormone populations were affected in the adult Arx-deficient mice, we did not detect the presence of vacuoles in the ileal enterocytes in mice that were fed normal chow (data not shown) suggesting that there is an age- and diet-specific impact on hormone deficiency and lipid metabolism in the Arx mutants. As milk contains higher fat content than the solid food that the adult mice consume, it would be interesting to see whether placing adult Arx-deficient mice on a high fat diet would also lead to an increase in the number of lipid-filled intestinal enterocytes similar to what was observed in the nursing pups.
In contrast to the ArxEndoderm mutants which showed chronic diarrhea and reduced survival, ArxEndocrine mutant mice had a normal survival curve and did not suffer from diarrhea even though similar hormone populations, except gastric serotonin cells, were found to be affected profoundly in the stomach and intestine. It is important to keep in mind that there are at least 15 enteroendocrine subtypes present in the GI tract and Arx is selectively removed in the Ngn3+ endocrine progenitors in the ArxEndocrine mutants and in all epithelial cells in the ArxEndoderm mutants. Furthermore, it has been suggested that gastric serotonin cell development is controlled in Ngn3-dependent and independent fashions as this cell type is not completely missing in the stomach of Ngn3 null mice (Jenny et al., 2002; Lee et al., 2002). Consistent with this model and if Ngn3-Cre activity in the ArxEndocrine mutants was delayed and only present in Ngn3/Arx endocrine progenitors of the stomach (yellow cell; Fig. 7a), then the serotonin cell population would only be partially affected in the ArxEndocrine mutants and more profoundly impacted in the ArxEndoderm mutants. In fact, this is exactly what we observed in our immunostatining analyses for serotonin cells in the stomach of these two mutants. Additionally, some unexamined enteroendocrine cells in the stomach may also be selectively affected only in the ArxEndoderm mutants. Therefore, a global gene expression analysis (e.g. RNA-seq) will be most informative to thoroughly determine if additional genes associated with enteroendocrine subpopulations were uniquely affected in the ArxEndoderm mutants.
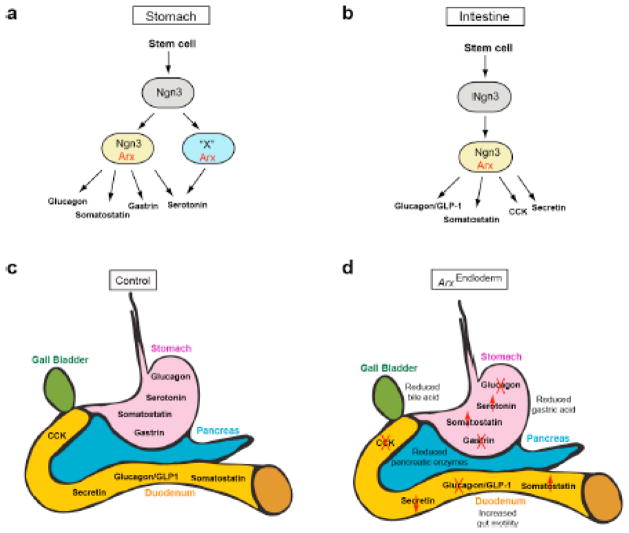
Figure 7. Models for the proposed role of Arx during enteroendocrine cell differentiation and the summary of the observed cell population changes and processes affected in ArxEndoderm mutant mice.
(a) Arx is expressed in the Ngn3+ progenitors (yellow) for the development of gastric glucagon, somatostatin and gastrin cells. However, serotonin cells can arise from either Arx/Ngn3 lineage (yellow) or another precursor expressing Arx and an unidentified factor X (blue). All these enteroendocrine subtypes are probably derive from Ngn3+ endocrine progenitors (grey). (b) Arx is expressed in the Ngn3+ endocrine progenitors (yellow) for the development of somatostatin, glucagon/GLP-1, CCK and secretin cells in the intestine. (c–d) ArxEndoderm mutant mice display increased gut motility resulting from a reduced number of gastrin-, CCK-, glucagon/GLP-1-, and secretin-expressing cells and an increase in somatostatin cells (d) relative to control littermates (c). Changes in these cell types result in a reduction of gastric acid, pancreatic enzymes, and bile acids.
The chronic diarrhea seen in the ArxIntestine and ArxEndoderm mice is also apparent in subset of patients with ARX mutations who not only suffer from a wide variety of neurologic conditions, including X-linked lissencephaly with abnormal genitalia (XLAG), but also have a reduced number of pancreatic endocrine and exocrine cells (Bienvenu et al., 2002; Gecz et al., 2006; Itoh et al., 2010; Kitamura et al., 2002; Stromme et al., 2002a; Stromme et al., 2002b). The shortened life span of these patients and mice with whole body Arx mutations has been attributed to profound neurologic dysfunction or the complete absence of glucagon-producing alpha-cells in the pancreas instead of endocrine cell dysfunction in the intestine (Collombat et al., 2003; Gecz et al., 2006; Hancock et al., 2010; Marsh et al., 2009). Our data suggest that gastrointestinal defects in patients with ARX mutations may also contribute to the overall degenerative condition in these patients.
It has been proposed that a deficiency in both pancreatic polypeptide and pancreatic exocrine enzymes contributes to the intractable diarrhea in ARX patients (Itoh et al., 2010). However, we did not detect a change in the number of pancreatic polypeptide-producing PP cells nor was there a reduced amount of pancreatic amylase staining in our ArxEndoderm mutant mice (data not shown). In addition, our analysis of the ArxIntestine mutants, which showed similar GI dysfunction further provides evidence that the diarrhea observed in the mice as well as the humans more likely stems from changes in the GI hormone levels that result in loss of regulated release of digestive enzymes from the exocrine pancreas.
Although animal models are ideal for identifying functions of genes of interest, the use of hES cell-derived human intestinal tissue has allowed us to identify both conserved and human-specific functions of ARX during intestinal endocrine cell differentiation. For example, ARX is required in both mice and humans for normal development of CCK, Secretin and Glucagon/GLP-1-expressing cells. In contrast, ARX is not required for development of human Somatostatin-expressing cells in the proximal intestine, similar to what has been shown in the human pancreas (Itoh et al., 2010). These findings not only provide valuable information on basic enteroendocrine cell development but also emphasize the importance of cell-based model systems to study human diseases as human tissues are often difficult to obtain for such studies.
Arx is acting as a key factor in the endocrine progenitors during enteroendocrine cell differentiation. Even though there are many more endocrine subtypes present in the GI epithelium as compared to the endocrine pancreas, Arx is also involved in promoting the glucagon/GLP-1 cell populations and repressing the somatostatin cell population in the intestine (Fig. 7b–d). Additionally Arx is also required for the development of CCK and secretin cells, two endocrine subtypes that are unique to the intestine (Fig. 7b–d). Interestingly, in the stomach, an increase in the somatostatin cell population was only detected in the antrum of ArxEndocrine mutant mice, a region that is devoid of glucagon cells and filled with gastrin cells. The decrease in the gastrin cell number and the increase in the somatostatin cell number were suggestive of a possibility that Arx might be involved in promoting gastrin and repressing somatostatin cell differentiation simultaneously in the antral stomach. The potential of these two enteroendocrine populations sharing a common precursor in the stomach is further supported by the presence of gastrin and somatostatin co-expressing cells in addition to the cells expressing either gastrin or somatostatin alone (Larsson et al., 1995). The development of the gastrin and somatostatin cells has also been suggested to be regulated by Islet-1 (Isl-1), a LIM-homeodomain transcription factor (Larsson et al., 1995). In addition, Isl-1 plays a key role in regulating Arx expression in the islet alpha cells as Isl-1 removal in the embryonic pancreas results in a decrease of Arx expression (Du et al., 2009; Liu et al., 2011). Based on these observations, we hypothesize that differentiation of gastrin and somatostatin cells is likely controlled by a combinatorial role of Isl-1 and Arx as expression of these two transcription factors overlaps in the stomach (unpublished data). Lastly, lineage tracing studies will be required in the future to determine the relationship between all the hormone populations that were affected in the Arx-deficient mice and if Arx promotes one enteroendocrine population at the expense of the other.
It has been shown that not all cells express equal levels of transcription factor as demonstrated in the pancreas for Pdx1 and Ngn3 (Fujitani et al., 2006; Nishimura et al., 2006; Villasenor et al., 2008; Wang et al., 2009). Our expression analyses show that Arx is only found in subsets of Ngn3+ endocrine progenitors and hormone-producing cells. One possible explanation is the potential presence of ArxHI and ArxLO cells that are at different stages or ages within the enteroendocrine population. In order to determine whether ArxHI and ArxLO cells truely exist in the GI epithelium, additional Arx antibodies with higher sensitivities or assessment of Arx expression via LacZ staining in the Arx+/LacZ mice (Collombat et al., 2003) will be required. Overall, our findings reveal a novel role for Arx in the endocrine progenitor during enteroendocrine development. Future studies on Arx in the GI tract will help determine whether Arx is also required at later stages to maintain the mature phenotypes of this population and to determine the molecular mechanisms by which Arx is involved in regulating this process including regulation of hormone gene expression.
CONCLUSION
Here we demonstrate that Arx is required in the endocrine progenitors to specify particular enteroendocrine cell fates. The enteroendocrine populations affected in the absence of Arx are critical in regulating digestion (gastrin, somatostatin, CCK and secretin) and gut motility (serotonin and GLP-1). Therefore, changes in the number of these cell types may affect overall physiology, including release of gastric acid, bile, and digestive enzymes, and rate of chyme transit in these mice (Fig. 7c–d; (Baggio and Drucker, 2007; Beglinger and Degen, 2004; Drucker, 2007a; Drucker, 2007b; Gershon, 1968; Lloyd, 1994; Mellitzer and Gradwohl, 2011; Murphy and Bloom, 2006; Murphy et al., 2006; Rehfeld, 2004a; Rehfeld, 2004b; Schubert, 2010; Wren, 2008; Wren and Bloom, 2007; Wren et al., 2001).
Our study also provides evidence that the lack of specific enteroendocrine cell types in the GI tract is likely the primary cause of GI dysfunction seen in the global ARX mutation patients. These findings will provide a thorough understanding for designing more efficient differentiation protocols toward generation of specific enteroendocrine types in vitro and generating effective treatments for patients with ARX mutations.
Supplementary Material
HIGHLIGHTS.
Arx is expressed in GI endocrine progenitors and in hormone producing cells.
Ablation of Arx in mice results in changes in enteroendocrine subtypes.
Arx acts as a cell fate determinant in endocrine progenitors.
Ileal lipid droplet accumulation observed in the Arx-deficient mice.
Human intestinal endocrine cell development requires ARX.
Acknowledgments
We are grateful to the members of the May lab for critical readings of the manuscript. We also want to thank Crystal Wilcox, Drs. Jeffrey Golden and Andrew Leiter for sharing their mice. We are also grateful to Dr. John Lynch for sharing his reagents and discussions for the study. We thank members of the Morphology Core in the Center for Molecular Studies in Digestive and Liver Disease (P30-DK050306). CLM is supported by NIH-DK078606, NIH-DK019525 and JDRF2-2007-703. JMW and KWM are supported by NIH-GM072915 and NIH-DK080823 and KWM is supported by the training grant NIH-HD046387. NAT is supported by the training grant NIH-T32-DK007066. We want to thank the Cincinnati Children’s Hospital Pluripotent Stem Cell Facility for technical support.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L. Fat in the intestine as a regulator of appetite--role of CCK. Physiol Behav. 2004;83:617–21. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Ben Jeema L, Zemni R, Vinet MC, Francis F, Couvert P, Gomot M, Moraine C, van Bokhoven H, Kalscheuer V, Frints S, Gecz J, Ohzaki K, Chaabouni H, Fryns JP, Desportes V, Beldjord C, Chelly J. ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet. 2002;11:981–91. doi: 10.1093/hmg/11.8.981. [DOI] [PubMed] [Google Scholar]
- Choi MY, Romer AI, Wang Y, Wu MP, Ito S, Leiter AB, Shivdasani RA. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol Cell Biol. 2008;28:3208–18. doi: 10.1128/MCB.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–80. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–70. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Collombat P, Colasante G, Bianchi M, Long J, Mansouri A, Rubenstein JL, Broccoli V. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007;27:4786–98. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, May CL. Expression analysis of the Islet-1 gene in the developing and adult gastrointestinal tract. Gene Expr Patterns. 2011;11:244–54. doi: 10.1016/j.gep.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007a;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Unraveling the complexities of gut endocrinology. Nat Clin Pract Endocrinol Metab. 2007b;3:317. doi: 10.1038/ncpendmet0461. [DOI] [PubMed] [Google Scholar]
- Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–69. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, Raguenes O, Chelly J, Ferec C, Nakajima K, Parnavelas JG. Cell-autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–66. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky PA, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 2008;17:3740–60. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gecz J, Cloosterman D, Partington M. ARX: a gene for all seasons. Curr Opin Genet Dev. 2006;16:308–16. doi: 10.1016/j.gde.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137:2179–80. doi: 10.1053/j.gastro.2009.06.072. author reply 2180–1. [DOI] [PubMed] [Google Scholar]
- Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–80. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Serotonin and the motility of the gastrointestinal tract. Gastroenterology. 1968;54:453–6. [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–8. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon Deficiency Reduces Hepatic Glucose Production and Improves Glucose Tolerance In Adult Mice. Mol Endocrinol. 2010;24:1605–1614. doi: 10.1210/me.2010-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Takizawa Y, Hanai S, Okazaki S, Miyata R, Inoue T, Akashi T, Hayashi M, Goto Y. Partial loss of pancreas endocrine and exocrine cells of human ARX-null mutation: consideration of pancreas differentiation. Differentiation. 2010;80:118–22. doi: 10.1016/j.diff.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21:6338–47. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Ahlgren U, Edlund T, Edlund H. IPF1, a homeodomain protein with a dual function in pancreas development. Int J Dev Biol. 1995;39:789–98. [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–84. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- Larsson LI, St-Onge L, Hougaard DM, Sosa-Pineda B, Gruss P. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–9. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Tingstedt JE, Madsen OD, Serup P, Hougaard DM. The LIM-homeodomain protein Isl-1 segregates with somatostatin but not with gastrin expression during differentiation of somatostatin/gastrin precursor cells. Endocrine. 1995;3:519–524. doi: 10.1007/BF02738827. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–97. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–95. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Liu J, Hunter CS, Du A, Ediger B, Walp E, Murray J, Stein R, May CL. Islet-1 Regulates Arx Transcription during Pancreatic Islet {alpha}-Cell Development. J Biol Chem. 2011;286:15352–60. doi: 10.1074/jbc.M111.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KC. Gut hormones in gastric function. Baillieres Clin Endocrinol Metab. 1994;8:111–36. doi: 10.1016/s0950-351x(05)80228-9. [DOI] [PubMed] [Google Scholar]
- Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, Brooks-Kayal A, Golden JA. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–76. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TL, Wilcox C, Panea C, Golden JA, May CL, Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–5. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120:1708–21. doi: 10.1172/JCI40794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Gradwohl G. Enteroendocrine cells and lipid absorption. Curr Opin Lipidol. 2011;22:171–5. doi: 10.1097/MOL.0b013e32834622a2. [DOI] [PubMed] [Google Scholar]
- Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–24. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–9. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27:719–27. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–39. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Chinery R, Sarraf C, Van Noorden S, Stamp GW, Lalani EN, Elia G, Wright NA. Trefoil peptide gene expression in small intestinal Crohn’s disease and dietary adaptation. J Clin Gastroenterol. 1993;17(Suppl 1):S78–91. doi: 10.1097/00004836-199312001-00016. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004a;36:735–41. doi: 10.1055/s-2004-826154. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Clinical endocrinology and metabolism. Cholecystokinin. Best Pract Res Clin Endocrinol Metab. 2004b;18:569–86. doi: 10.1016/j.beem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Jensen JN, Hodgson MI, Codner E, Ellard S, Serup P, Hattersley AT. Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes. 2011;60:1349–53. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–54. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2010;26:598–603. doi: 10.1097/MOG.0b013e32833f2010. [DOI] [PubMed] [Google Scholar]
- Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Scheffer IE, Gecz J. Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 2002a;24:266–8. doi: 10.1016/s0387-7604(02)00079-7. [DOI] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, Lutcherath V, Gedeon AK, Wallace RH, Scheffer IE, Turner G, Partington M, Frints SG, Fryns JP, Sutherland GR, Mulley JC, Gecz J. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002b;30:441–5. doi: 10.1038/ng862. [DOI] [PubMed] [Google Scholar]
- Van Dyck F, Braem CV, Chen Z, Declercq J, Deckers R, Kim BM, Ito S, Wu MK, Cohen DE, Dewerchin M, Derua R, Waelkens E, Fiette L, Roebroek A, Schuit F, Van de Ven WJ, Shivdasani RA. Loss of the PlagL2 transcription factor affects lacteal uptake of chylomicrons. Cell Metab. 2007;6:406–13. doi: 10.1016/j.cmet.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–9. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martin MG. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–80. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A. 2009;106:9715–20. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM. Gut and hormones and obesity. Front Horm Res. 2008;36:165–81. doi: 10.1159/000115364. [DOI] [PubMed] [Google Scholar]
- Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for Secretory Cell Lineage Commitment in the Mouse Intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.