Abstract
One of the prevailing theories of aging, the disposable soma theory, views aging as the result of the accumulation of damage through imperfect maintenance. Aging, then, is explained from an evolutionary perspective by asserting that this lack of maintenance exists because the required resources are better invested in reproduction. However, the amount of maintenance necessary to prevent aging, ‘maintenance requirement’ has so far been largely neglected and has certainly not been considered from an evolutionary perspective. To our knowledge we are the first to do so, and arrive at the conclusion that all maintenance requirement needs an evolutionary explanation. Increases in maintenance requirement can only be selected for if these are linked with either higher fecundity or better capabilities to cope with environmental challenges to the integrity of the organism. Several observations are suggestive of the latter kind of trade-off, the existence of which leads to the inevitable conclusion that the level of maintenance requirement is in principle unbound. Even the allocation of all available resources to maintenance could be unable to stop aging in some organisms. This has major implications for our understanding of the aging process on both the evolutionary and the mechanistic level. It means that the expected effect of measures to reallocate resources to maintenance from reproduction may be small in some species. We need to have an idea of how much maintenance is necessary in the first place. Our explorations of how natural selection is expected to act on the maintenance requirement provides the first step in understanding this.
Keywords: Aging, Natural selection, Maintenance, Disposable soma theory, Evolution, Survival
Theoretical background
Aging is the fall of fecundity and/or the rise of mortality with chronological time (Finch 1990; Baudisch 2011). This obviously being disadvantageous to evolutionary fitness, several attempts have been made to explain how evolution could allow aging to exist. The most notable theories include the mutation accumulation (Medawar 1952), antagonistic pleiotropy (Williams 1957) and disposable soma (Kirkwood 1977) theories of aging. The first two regard aging the result of genetic side effects, while the disposable soma theory regards aging the result of damage that accumulates due to imperfect maintenance of the organism. According to the disposable soma theory the reason this happens is that resources allocated to maintenance that pays off at an age at which an individual is unlikely to be alive are better allocated to reproduction. Through optimization by natural selection, maintenance effort is believed to settle below the level that is required to prevent aging (Kirkwood 1977; Kirkwood and Holliday 1979; Kirkwood and Rose 1991; Drenos and Kirkwood 2005). In this paper, ‘maintenance effort’ is defined according to the definition of Kirkwood and Rose (1991) as investments to preserve functions, distinguishing these from investments that create functions, which are captured under the term ‘growth’.
The maintenance requirement and the maintenance gap
With respect to aging most attention has been given to maintenance effort, while what we call the ‘maintenance requirement’, the level of maintenance effort required to prevent aging, has received little or no attention, especially not from an evolutionary perspective. Although overlooked, reducing the level of maintenance requirement would be an alternative strategy for the organism to prevent its aging. After all, it is the deficit of maintenance effort with respect to maintenance requirement at a point in time, we call this the ‘maintenance gap’, that causes aging. Any factor that would increase the maintenance gap would directly increase the rate of aging, be it increasing maintenance requirement or decreasing maintenance effort. All other things being equal, evolution will act to lower the maintenance requirement. It is the central question of this paper why an organism would let its maintenance requirement grow high, apparently defying this evolutionary incentive.
Evolutionary terminology
In a non-growing population the highest fitness is achieved by individuals that maximize lifetime reproductive output. This in turn is conventionally modelled as the sum of age specific fertilities multiplied by age specific survival probabilities. To increase lifetime reproductive success, fertility rate could be augmented, reproductive survival prolonged, or both. It is important here to make a clear distinction of terms. Several writers have suggested that there is a certain lifespan that the organism needs to make its reproductive contribution to the next generation. Rattan (2000) calls this ‘essential lifespan’, while Carnes (2011) has named it the ‘warranty period’. It is important to realize that this ‘essential lifespan’ has evolved, and thus is the product of evolution—we cannot assume it as a starting point in an evolutionary theory.
Where the maintenance requirement comes from and why it is important
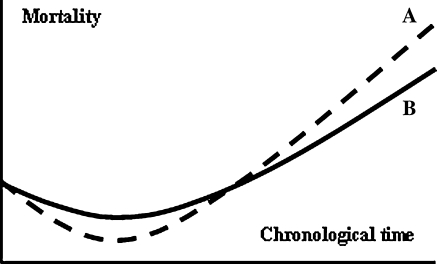
Survival of the organism is the result of the capacity to withstand challenges from extrinsic and intrinsic sources; investments in both characteristics contribute to lower all cause mortality. Death from intrinsic causes is optimized to the level of extrinsic mortality through evolved limitations on maintenance efforts (Kirkwood 1977; Kirkwood and Rose 1991). On the other hand, mortality from extrinsic causes is the outcome of the organisms capacity to respond to environmental challenges to the integrity of the organism, as well as of these challenges themselves. With incremental investments in such capacity, mortality from extrinsic causes is expected to fall. However, such capacity may be maintenance demanding, thus leading to a higher maintenance requirement and therefore to a higher rate of aging. A similar reasoning goes for reproductive capacities. We suggest that we thus have another optimization process that happens through natural selection: when growing characteristics that increase fecundity and the capacity to cope with extrinsic challenges, the maintenance requirement will increase due to the continuous investment that is necessary to maintain the soma. This higher maintenance requirement directly translates into a bigger maintenance gap. Consequently, the direct benefit of lower mortality from extrinsic causes (and higher fecundity) comes at a cost of lower intrinsic durability and aging in the long run. We show two hypothesized mortality trajectories of organisms that follow differing approaches to this trade-off (Fig. 1). Organism A grows to a state in which it is more robust to extrinsic challenges than organism B, but its state succumbs under the weight of its maintenance requirement, so that in the longer run it faces a faster acceleration of mortality rate than organism B. From this trade-off it importantly follows that nothing restricts the extent of development of the described characteristics as long as there is a net benefit for fitness (see Box 1). Maintenance requirement may grow so high that a maintenance gap would remain even if all resources were to be allocated to maintenance, especially because age-independent mortality tends to obscure disadvantageous late-life consequences, as was suggested by Medawar (1952). Thus it is conceivable that some phenotypes are selected that attain characteristics they cannot possibly maintain.
Fig. 1.
Hypothesized mortality trajectories; organism A (dashed line) gains lower midlife mortality than organism B (solid line) but pays the price of faster mortality acceleration later in life. For simplicity only mortality is considered, but a similar (inverse) graph could be drawn for fecundity
Box 1.
| Big Brains | |
|---|---|
| The rate of aging is determined by the amount of unperformed maintenance/unit of time, the ‘maintenance gap’. For the size of this gap, how much maintenance is necessary is just as important as how much maintenance is actually done. Greater size and/or maintenance-heavy tissue imply a greater maintenance requirement. An example of maintenance-heavy tissue is the (human) brain, that, in addition to the cost of its growth (even after reaching adolescence), consumes a very substantial amount of energy for its maintenance (Mink et al. 1981, Isler and Van Schaik 2006). All other things being equal the greater maintenance requirement will lead to faster aging. Nevertheless, on the whole the brain has a beneficial impact on survival (González-Lagos et al. 2010) because it allows the organism to cope better with its environment. Also, the brain may facilitate better access to resources, and energy savings through more efficient behavior and physiology (Kaplan and Robson 2002). Therefore, the brain facilitates a greater maintenance effort and interestingly affects both sides of the maintenance gap. If the brain would not have all these immediate benefits, it would have been strongly selected against. |
Positioning our contribution in the existing literature
It has been uttered before, that bigger body size goes with a bigger maintenance requirement (Munch and Mangel 2006). However, the adaptations we envision may comprise body size, but not necessarily do. Two equal masses of tissue may differ in their maintenance requirement.
Average adult mortality scales negatively with adult body size (Charnov 1993). Aging, though, is a term that relates to change and not to absolute level (Finch 1990; Baudisch 2011). Therefore, our hypothesis is in line with scaling theory. To prove or disprove the concept put forward in this paper would require a careful analysis of high quality long term individual data, correcting for reproductive effort and the effect of size on food intake. The expected finding would be that mortality rates accelerate relatively faster in individuals with lower initial mortality rates. At least suggestive is that in the wild a bigger size is associated with a longer life (Gaillard et al. 2000), whereas in laboratory and domestic environment longevity of animals typically shows a negative correlation with mass (Rollo 2002, Miller et al. 2002). After all, lifespan in a protected environment may predominantly reflect the force of mortality due to intrinsic causes (higher maintenance requirement for bigger individuals) whereas mortality in the wild may predominantly reflect death form extrinsic causes (lower mortality from extrinsic causes for bigger individuals).
Implications for the mechanistic theories of aging—IGF-1
In aging research one can distinguish proximate (mechanistic) causes of aging (Rattan 2006; Rattan 2008; Holliday and Rattan 2010), and ultimate (evolutionary) causes of aging. Possible mechanisms through which maintenance requirement may act include differences in metabolic rate and the associated production of reactive oxygen species, as well as differences in insulin/IGF-1 signalling. Insulin-IGF-1 signalling, a prime regulator of growth, is invariantly associated with lifespan regulation in mammals. The role of reduced insulin/insulin-like growth factor 1 (IGF-1) signalling in lifespan extension is well established in invertebrates (Kenyon 2010). IGF-1 and growth hormone (GH) primarily control growth and differentiation. In mice, genetic disruption of the GH/IGF-1 pathway is associated with reduced adult body size and major increases in lifespan under laboratory conditions (Bartke 2005). It is tempting to speculate that survival probabilities and fitness of these animals are low under adverse environmental conditions. Also in humans, genetic variants associated with reduced IGF-1 signalling have been associated with reduced height and enhanced survival (van Heemst et al. 2005; Suh et al. 2008); it seems that the human maintenance gap could be due to elevated maintenance requirement for a substantial part.
Discussion and conclusion
Baudisch (2005) questions: “Early in life, when individuals develop and grow, mortality falls and reproductive potential increases. Why is it that these age patterns cannot persist (…)?” Our answer is that an organism may attain a state that ultimately is not sustainable, even if all its resources were allocated to maintenance. To this moment the disposable soma theory of aging has aimed to explain why organisms do not maintain themselves, while they are considered to be able to (Drenos and Kirkwood 2005). The important novel concept that this paper aims to deliver is that just as any maintenance effort, any maintenance requirement needs an evolutionary explanation. Hence, to understand the evolutionary cause of aging, research should focus on the maintenance gap as a whole. Taking this one step further leads to the conclusion that if there is sufficient selection on traits that favor a high maintenance requirement, this maintenance requirement is unbound. The scope for mathematical models as well as research addressing the underlying mechanisms of aging is thus broadened in exciting new directions. The mechanistic cause of aging perhaps cannot be found in merely monitoring the fluxes of resources within the organism; even if all resources are found to be allocated to maintenance, the organism may still age. What contributed most to the maintenance gap in a specific organism depends on the environmental niche an organism lives in, but both factors that contribute to the maintenance gap, maintenance requirement and maintenance effort, are complementary rather than mutually exclusive and are united in the concept of the maintenance gap. Thinking in terms of the maintenance gap, then, takes all important factors into consideration when it comes to maintenance and aging, so that all questions can be grouped in two overarching questions. Where does the maintenance gap in a particular species come from? How do we close it?
Acknowledgments
The authors thank Tom Kirkwood, Annette Baudisch, Owen Jones and two anonymous reviewers for their comments on earlier manuscripts. This study was funded by the Netherlands Genomics Initiative/Netherlands Organization for scientific research (NGI/NWO;050-060-810. NCHA).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bartke A. Mini review: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Baudisch A. Hamilton’s indicators of the force of selection. Proc Nat Acad Sci USA. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudisch, A (2011) The pace and shape of ageing. Meth Ecol Evol 2. doi:10.1111/j.2041-210X.2010.00087.x
- Carnes BA. What is lifespan regulation and why does it exist? Biogerontology. 2011;12:367–374. doi: 10.1007/s10522-011-9338-3. [DOI] [PubMed] [Google Scholar]
- Charnov EL (1993) Life history invariants. Oxford University Press, Oxford
- Drenos F, Kirkwood TB. Modelling the disposable soma theory of aging. Mech Ageing Dev. 2005;126:99–103. doi: 10.1016/j.mad.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Finch CE (1990) Longevity, senescence and the genome. University Chicago Press, Chicago
- Gaillard JM, Festa-Bianchet M, Delorme D, Jorgenson J. Body mass and individual fitness in female ungulates: bigger is not always better. Proc Biol Sci. 2000;267:471–477. doi: 10.1098/rspb.2000.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Lagos C, Sol D, Reader SM. Large-brained mammals live longer. J Evol Biol. 2010;23:1064–1074. doi: 10.1111/j.1420-9101.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- Holliday R, Rattan SI. Longevity mutants do not establish any ‘‘new science’’ of ageing. Biogerontology. 2010;11:507–511. doi: 10.1007/s10522-010-9288-1. [DOI] [PubMed] [Google Scholar]
- Isler K, Van Schaik CP. Metabolic costs of brain size evolution. Biol Lett. 2006;2:557–560. doi: 10.1098/rsbl.2006.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Robson AJ. The emergence of humans: the coevolution of intelligence and longevity with intergenerational transfers. PNAS. 2002;99:10221–10226. doi: 10.1073/pnas.152502899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Evolution of aging. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Holliday R. The evolution of aging and longevity. Proc R Soc Lond B Biol Sci. 1979;205:531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An unsolved problem of biology. London: H. K. Lewis; 1952. [Google Scholar]
- Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- Munch SB, Mangel M. Evaluation of mortality trajectories in evolutionary biodemography. Proc Nat Acad Sci USA. 2006;103:16604–16607. doi: 10.1073/pnas.0601735103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS. Biogerontology: the next step. Ann NY Acad Sci. 2000;908:282–290. doi: 10.1111/j.1749-6632.2000.tb06655.x. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Minireview-increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Nat Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst D, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi: 10.2307/2406060. [DOI] [Google Scholar]