Abstract
Treatment of metastatic melanoma has long been a challenge. Over the past 8 years significant advances have been made in understanding the genetic changes that drive melanoma development and progression. These studies have shown melanoma to be a heterogeneous group of tumors, driven by a diverse array of oncogenic mutations. There is now good evidence that activating mutations in the serine/threonine kinase BRAF and the receptor tyrosine kinase KIT constitute good therapeutic targets for restricted sub-groups of melanoma. In this article, we discuss the genetics and etiology of cutaneous and non-cutaneous melanoma and review some of the latest pre-clinical and clinical data on the new targeted therapy agents that are beginning to make an impact upon the lives of melanoma patients.
1. Introduction
Despite many years of research, disseminated melanoma remains a major clinical problem. This frustrating lack of progress led some commentators to describe melanoma as being “intrinsically therapy resistant” and there are suggestions that the resistance phenotype may be a characteristic of underlying melanocyte biology 1. The recent years have seen an explosion in high throughput genomic profiling that have provided important new information about the molecular events that drive melanoma initiation and progression 2-4. Of particular note is the frequent occurrence of mutations or amplifications in oncogenes that have opened opportunities for highly selective therapeutic targeting. Based upon these studies it is now clear that melanomas are a heterogeneous group of tumors with different etiologies requiring different therapeutic strategies. The current paradigm being explored in melanoma is one of targeted therapy, an approach that matches selective small molecule inhibitors to tumors expressing specific oncogenic mutations. This strategy is exemplified by the use of imatinib in chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) and erlotinib in subsets of non-small cell lung cancer (NSCLC) that harbor activating EGFR mutations 5-7. In the current review we discuss the latest research on molecular sub-grouping of melanoma and outline promising targeted therapy strategies being developed for the treatment of the molecular subtypes of melanoma.
2. The genetic sub-groupings of cutaneous and non-cutaneous melanoma BRAF V600 mutations
The melanoma genomic revolution was kick-started by the 2002 discovery that ~50% of all melanomas harbor an activating mutation in BRAF 4 (Figure 1). Raf proteins constitute a 3 member family of Serine/Threonine kinases (ARAF, BRAF and CRAF) with closely overlapping functions 8. So far over 50 distinct mutations in BRAF have been identified 9. Of these, the BRAF V600E mutation, resulting from a valine to glutamic acid substitution, is by far the most common and accounts for over 80% of all reported BRAF mutations 4, 10. Other less common, but clinically relevant variants identified from melanoma specimens are the V600K mutation (16% of all BRAF mutations) and V600D/V600R (3% of all BRAF mutations)11. Most of the oncogenic activity of mutant BRAF is mediated through activation of the mitogen activated protein kinase (MAPK) cascade, which regulates the cell cycle entry through control of cyclin D1 expression, and the suppression of p27KIP1 12, 13 as well as through effects upon invasion and survival 14, 15. In experimental systems, the role of mutated BRAF in melanoma seems convincing with in vitro studies showing that the BRAF V600E mutation is an oncogene in immortalized mouse melanocytes 16 and that selective downregulation of the BRAF V600E mutation using RNAi leads to reversal of the melanoma phenotype 17, 18. Although mutated BRAF is undoubtedly important for melanomagenesis, introduction of BRAF V600E alone is not sufficient for the transformation of primary human or mouse melanocytes19. Instead, melanoma development seems to require both BRAF/MAPK and activity in the PI3K/AKT pathway. This was most convincingly demonstrated in a recent transgenic mouse study showing that full transformation to melanoma occurred only when the BRAF V600E mutation was activated in concert with suppression of PTEN expression20.
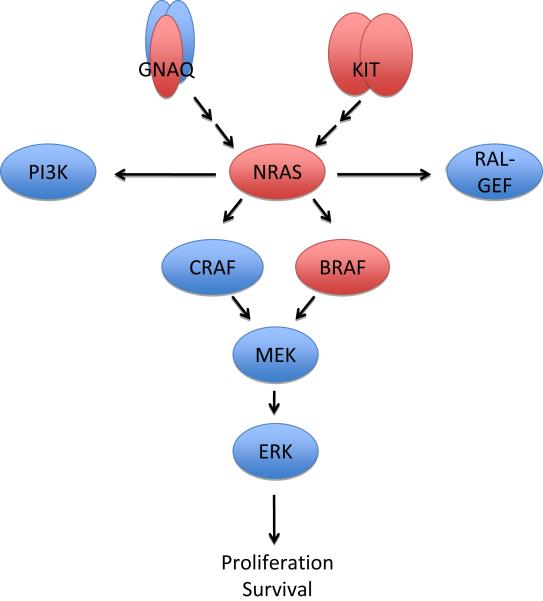
Figure 1.
Sample scheme showing some of the important molecular pathways important for melanoma progression. Genes with activating mutations in melanoma are highlighted in red.
Although BRAF mutations are not ultraviolet (UV) radiation signature mutations, they have a tendency to occur on UV-exposed skin sites and are more prevalent in individuals with a poor tanning response 21. There is also evidence that intermittent, rather than chronic sun-exposure is predictive for BRAF mutational status with BRAF mutant melanoma patients tending to be younger in age (<55 years old) with a lower cumulative UV exposure 22. BRAF mutational status is also of prognostic value and is associated with inferior survival in the metastatic setting (8.5 months in BRAF wild-type vs 5.7 months for BRAF mutant melanoma)23.
Small molecule BRAF inhibitors: Sorafenib, PLX4032/4720, GSK2118436
Since the discovery of activating BRAF mutations in melanoma, a number of BRAF inhibitors have been developed and subjected to extensive in vitro testing 24-28. The most extensively studied of these is the kinase inhibitor sorafenib (BAY43-9006, Nexxavar ®) 28. Although originally developed as a CRAF inhibitor, sorafenib was also found to inhibit BRAF with moderate potency and was initially embraced as being the proof-of-concept for BRAF inhibition in melanoma29. In animal studies, sorafenib treatment led to limited regression of BRAF V600E mutated melanoma xenografts and was associated with only minor levels of apoptosis induction 29, 30. Further pre-clinical investigations have shown sorafenib to be a relatively weak inhibitor of BRAF, with many off-target effects most notably inhibition of VEGFRs and PDGFRs 28, 31. It now seems likely that any anti-melanoma activity of sorafenib is largely independent of its putative effects upon BRAF inhibition32.
Since the evaluation of sorafenib a new generation of BRAF inhibitors has been developed. These drugs show higher potency against mutated BRAF and have fewer off-target effects; the list of those currently under pre-clinical investigation includes: SB590885, dabrafenib (GSK2118436), AZ628, XL281, GDC-0879 and vemurafenib (RG704, PLX4032/4720) 24, 25, 33-39. PLX4032 (and its analogue PLX4720) are ATP-competitive RAF inhibitors (wt BRAF IC50 100nM, mutated BRAF 31nM) that selectively inhibit growth in melanoma cell lines harboring the BRAF V600E mutation both in vitro and in vivo mouse in xenograft models 25, 38, 40. Henceforth we will refer to PLX4032 in the discussion of the preclinical studies and vemurafenib in the context of clinical studies. Responses to PLX4032 in melanoma xenograft models were BRAF V600E specific and impressive; with either partial or complete responses observed in all cases with a close relationship observed between drug exposure and response within individual xenograft models 38, 40. Interestingly, not all BRAF mutated melanoma cell lines were similarly sensitive to PLX4032 and PLX4720, with a significant proportion showing varying degrees of intrinsic resistance 33, 36, 37. Current data suggests that PLX4032/4720 induces both cell cycle arrest and apoptosis in the most sensitive cell lines and cell cycle arrest only in resistant less sensitive cell lines 33, 37. A recent genetic study, looking for patterns of mutation and genomic amplification between PLX4032 sensitive and resistant cell lines, was unable to identify any unifying differences between the two groups 37. It therefore seems that intrinsic resistance to BRAF inhibitors may be complex and multi-factorial 41. There is already evidence that sub-groups of BRAF V600E mutated melanomas exist with alterations in PTEN, cyclin D1, CDK2, CDK4, MITF and AKT3 42, 43. How the expression and mutational status of these genes impacts upon biological behavior and future therapy selection remains to be determined.
Another BRAF inhibitor currently exciting much interest in both the pre-clinical and clinical arenas is dabrafenib (GSK2118436), an ATP-competitive inhibitor of BRAF V600E/D/K, wild-type BRAF and CRAF 44. The compound has been shown to have promising activity in pre-clinical models of melanoma and is now undergoing clinical evaluation 45, 46.
Low-activity (non-V600) BRAF mutants and CRAF-dependent melanomas
A minor sub-group of melanomas have been identified with BRAF mutations in positions other than 600. Many of the non-V600 BRAF mutants tend to show impaired BRAF kinase activation in isolated kinase assays (hence the name “low-activity” BRAF mutants) and required the presence of CRAF to transactivate their MAPK signaling 10. Analysis of a large panel of melanoma cell lines and tissues revealed that ~1% of melanoma cell lines had either D594G or G469E mutation in BRAF, respectively and that 1% of melanoma specimens harbored a G469A mutation in BRAF 30. These non-V600 BRAF mutated cell lines differed in their signaling from the BRAF V600E mutants and showed high levels of phospho-ERK, low levels of phospho-MEK and resistance to MEK inhibition 30. Interestingly, these non-V600 BRAF mutants seem to form part of a broader sub-group of melanoma cell lines, including some that are BRAF wild-type and BRAF V600K mutated, that are reliant upon CRAF for their survival 47. Studies from two independent groups have now demonstrated that shRNA knockdown of CRAF in the CRAF-dependent melanoma groups leads to MEK-independent effects upon BAD phosphorylation and Bcl-2 expression, leading in turn to apoptosis and impaired tumor growth in a mouse xenograft model 30. Although sorafenib is a relatively weak BRAF inhibitor, it does show reasonable potency against CRAF. There is pre-clinical evidence demonstrating that sorafenib has good pro-apoptotic activity against melanoma cell lines with low-activity BRAF mutations, and leads to regression of these cell lines grown as mouse xenografts 30. Furthermore the development of more selective and potent inhibitors of CRAF may also offer benefit for melanomas and other malignancies expressing these low-activity/non-V600 mutations in BRAF.
NRAS, KRAS and HRAS
RAS proteins constitute a large family of low molecular weight GTP-binding proteins that localize to the plasma membrane. Three of the RAS family members, NRAS, HRAS and KRAS are often mutated in human cancers, and >20% of all tumors harbor activating mutations in one of their RAS genes 48. Mutations in NRAS, KRAS and HRAS have been identified in 20%, 2% and 1% of all melanomas, respectively 49. Mutations in NRAS are most commonly the result of a point-change leading to the substitution of leucine to glutamine at position 61 4, 50, with mutations at positions 12 and 13 being reported less frequently 2. Large-scale analysis of melanoma samples and cell lines have shown that although BRAF V600E and NRAS mutations are mutually exclusive, there is overlap between low activity BRAF mutations and NRAS mutations 2, 51. Mechanistically, mutations in NRAS lead to impairment of GTPase activity, so that the GTPase is locked into its “On” position. In its GTP-bound state RAS binds to and activates a number of effector enzymes involved in proliferation, the best characterized of these being CRAF 52. Thus, melanomas harboring activating NRAS mutations differ from melanomas with BRAF mutations in that they rely upon CRAF to induce their MAPK pathway activity 52. RAS is also known to activate the phospho inositide-3-kinase (PI3K)/AKT pathway, which contributes to tumor progression via the modulation of growth and survival of transformed cells 13. In addition to MAPK and PI3K/AKT, mutated NRAS can also activate other intracellular signaling pathways important for malignant transformation. In particular, recent studies have demonstrated the importance of Ral guanine nucleotide exchange factors (Ral-GEFs) in the anchorage-independent growth observed following the NRAS-mediated melanocytes transformation 53. A role for RAS mutations in melanoma initiation has been confirmed in animal models, where the introduction of mutated HRAS or NRAS (Q61K) leads to melanoma in transgenic mice lacking expression of the CDK inhibitor p16INK4A 54, 55.
Farnesyltransferase inhibitors, dual MEK/PI3K inhibition
NRAS is a small GTPase, and thus a difficult target for conventional drug discovery 48. Farnesyltransferase inhibitors (FTIs), a class of drugs that prevent the membrane localization (and thereby activation) of small G-proteins, were originally developed as agents to target oncogenic Ras signaling 56. Despite an extensive research effort, these compounds have shown little single-agent activity, even in colorectal carcinoma where ~40% of the tumors have activating mutations in KRAS 56. FTIs have never been evaluated in a clinical trial of melanoma patients selected for their NRAS status. Although there is limited evidence that FTIs may have some activity against melanoma cell lines in vitro, these studies preceded the era of molecular sub-grouping melanoma cell lines 57. Attention has now turned to pathways that are downstream of Ras activation that may be more tractable to pharmacological intervention. Pre-clinical evidence suggests that simultaneous blockade of the MEK and PI3K pathways leads to the regression of Ras-driven tumors in animal models 58, 59. Other experimental studies have shown that dual inhibition of BRAF and CRAF or BRAF and PI3K (using shRNA knockdown) was effective at reducing the growth and survival of NRAS-mutated human melanoma xenografts 60. Although NRAS mutated melanomas are known to rely upon CRAF for their MAPK signaling, there is little evidence that sorafenib is any more effective on the NRAS mutants than melanoma cell lines with BRAF mutations 30.
KIT
Melanomas developing on body sites with low-levels of environmental ultraviolet radiation exposure, such as on the soles of the feet or subungual sites (acral melanomas) or the mucous membranes (mucosal melanomas) are known to have a low incidence of BRAF mutations 61. Instead, these more rare histological sub-types of melanoma are often associated with genetic amplification of and/or activating mutations in the receptor tyrosine kinase KIT. A recent landmark study showed that 21% of mucosal melanomas, 11% of acral melanomas and 17% of melanomas arising on sun-damaged skin harbor activating mutations in KIT, with many of these occurring at the imatinib-sensitive juxatmembrane position 61. Sequencing of c-KIT exons 11, 13, 17 and 18 revealed the most prevalent mutations to be K642E, L576P, D816H and V559A, that interestingly are enriched at different locations from KIT mutations in GIST or hematological malignancies 62. In most cases, the acquisition of a KIT mutation was accompanied by an increase in copy number and some degree of genomic amplification. There were also instances where KIT was amplified in the absence of a mutation 61. Since the initial report of KIT aberrations in melanoma, a number of further studies have validated this finding 63-65. Pooling of the currently available data suggests the KIT mutational frequency to be 14% in acral melanoma and 18% in mucosal melanoma 66. Given that acral and mucosal melanomas each represent only 2% of all melanomas, the total percentage of melanomas with KIT mutations is likely to be quite low. Moreover in an Australian population where over 40% of melanomas are associated with chronic sun damaged skin KIT mutations were observed at a similar low frequency of 2% 67.
In experimental studies, introduction of the D814Y mutant of KIT into non-tumorigenic immortalized melanocytes did not lead to either oncogenic transformation or enhanced proliferation 68. The lack of proliferation seen in the KIT mutated melanocytes was unexpected, but is likely to be a consequence of KIT constituting only one oncogenic “hit”. In agreement with this idea, it was recently shown that the two most common c-KIT mutations found in melanoma (K642E and L576P) were only able to transform melanocytes when grown under hypoxia or following the introduction of exogenous hypoxia-inducible factor 1α(HIF-1α) 69. Mechanistically, it seemed that introduction of mutated c-KIT activated PI3K/AKT signaling but not MEK/ERK and that the combination of hypoxia and mutated c-KIT was required to fully activate both pathways. Interestingly, these data again support the transformation model seen in BRAF mutated melanoma where dual MAPK/AKT signaling is required for tumor initiation and progression 69.
Imatinib, sunitinib and dasatanib
The relative scarcity of melanoma cell lines harboring activating KIT mutations has made pre-clinical studies a challenge. It is becoming clear that the presence of a KIT mutation, rather than genomic amplification is predictive of response to small molecule KIT inhibitors 66. It further seems likely that the nature of the KIT mutation dictates which KIT inhibitor should be used 66. To date, only two pre-clinical studies have been published on melanoma cell lines derived from either acral or mucosal melanomas 65, 70. The first of these characterized 3 primary mucosal melanoma cell lines, of which one was noted to have an exon-11 V559D mutation in c-KIT 65. Treatment of this cell line with imatinib led to cell cycle arrest and apoptosis induction and was associated with inhibition of JAK/STAT, PI3K/AKT and MAPK signaling and the inhibition of Bcl-2, survivin and Mcl-1 expression 65. A second study reported the identification of a mucosal melanoma cell line with a D820Y exon-17 mutation in c-KIT (the mutation often associated with imatinib resistance in GIST) with sensitivity to sunitinib (only at high concentrations) 70. One other recent publication reported the identification of a non-acral/non-mucosal melanoma cell lines harboring an L576P KIT mutation 71. In this instance, the cell line was found to be resistant to imatinib, nilotinib and sorafenib but sensitive to dasatanib 71. There is also limited evidence suggesting that the presence of constitutive KIT activity (as shown by phospho-KIT) may be predictive of KIT inhibitor responsiveness 72.
Another subtype of melanoma that seems to be associated with activation of KIT signaling are those arising in the pigmented cells of the eye. Uveal melanoma is the most common primary eye tumor in adults; these derive from the melanocytes of the choroid, ciliary body and iris. In common with other forms of non-cutaneous melanoma, uveal melanomas generally lack activating mutation in BRAF and NRAS 73 and instead maintain expression of c-KIT in up to 87% of cases. Although unlike acral and mucosal melanomas, uveal melanomas typically lack activating c-KIT mutations 74. Cell culture experiments have demonstrated that uveal melanoma cell lines harbor phospho-KIT expression and undergo imatinib-mediated cell cycle arrest 75. Clinical results of KIT inhibitors in uveal melanoma have not been reported.
GNAQ and GNA11
Activation of MAPK signaling seems to be a requisite for melanoma development. In uveal melanomas, most of which lack BRAF and RAS mutations, there is emerging evidence that MAPK activity is driven through activating Q209 mutations in the heterotrimeric G-protein alpha subunit GNAQ 76 or the equivalent residue in the closely related G-protein alpha subunit GNA11. Mutations at Q209 of GNAQ are analogous to those seen in NRAS (at Q61), and result in impaired GTPase activity leading to constitutive signaling. Large scale molecular profiling has identified GNAQ Q209 mutations in 46-49% of uveal melanoma samples and 27% of uveal melanoma cell lines 76, 77. In vitro studies showed that although introduction of GNAQ-Q209L alone was unable to transform primary human melanocytes, it did lead to anchorage-independent growth when transfected into immortalized melanocytes (mutated p53/CDK4 and hTERT) 76. Although no small molecule inhibitors of GNAQ currently exist, the potential therapeutic relevance of this G-protein was demonstrated by that fact that siRNA knockdown of GNAQ led to increased cell death in uveal melanoma cell lines that harbored the mutation 76.
4. Matching treatments to mutational profiles: clinical data
The preclinical data described above has led to a number of hypothesis-driven clinical trials to target the oncogenic mutations found in melanoma. The initial attempts to target oncogenic mutations in melanoma were to treat all melanomas regardless of genotype with targeted agents. This has now been replaced by clinical studies where patients are selected based on mutational profile that is leading to some striking results.
MEK inhibitors
The findings of frequent mutations in BRAF and NRAS in melanoma led to the evaluation of MEK inhibitors in melanoma including AZD6244, CI-1040 and PD0325901. These early studies did not select patients based on genotype and it was not clear if these agents were able to reliably inhibit MEK and phosphorylation of ERK in melanoma cells at the maximum tolerated doses78-80. Results were disappointing with ~10% objective response rates and retrospective analyses of genotypes in a subset of treated patients failing to predict clinical benefit. Dosing with MEK inhibitors has been limited by diarrhea and visual disturbance including retinal vein thrombosis in a small subset of patients. Recently the MEK inhibitor GSK1120212 with more reliable and sustained inhibition of MEK and pERK at clinically achievable doses has been evaluated in melanoma patients harboring BRAF V600E mutations with preliminary data indicating response rates in excess of 20% 81, 82. Definitive randomized studies with this agent are planned that should determine the duration and rate of clinical response to inhibition of MEK in patients with advanced disease.
BRAF inhibitors
Sorafenib was the first RAF inhibitor to enter clinical development in patients with melanoma. In these initial studies patients were not selected on the basis of genotype. Due to its poor selectivity for the active conformation of BRAF induced by the V600E mutation, coupled with significant “off-target” activity, results were disappointing with very low response rates 83. Randomized studies of sorafenib in combination with chemotherapy have given variable results with low response rates but provided no evidence that clinical effects were mediated through inhibition of BRAF 31, 84.
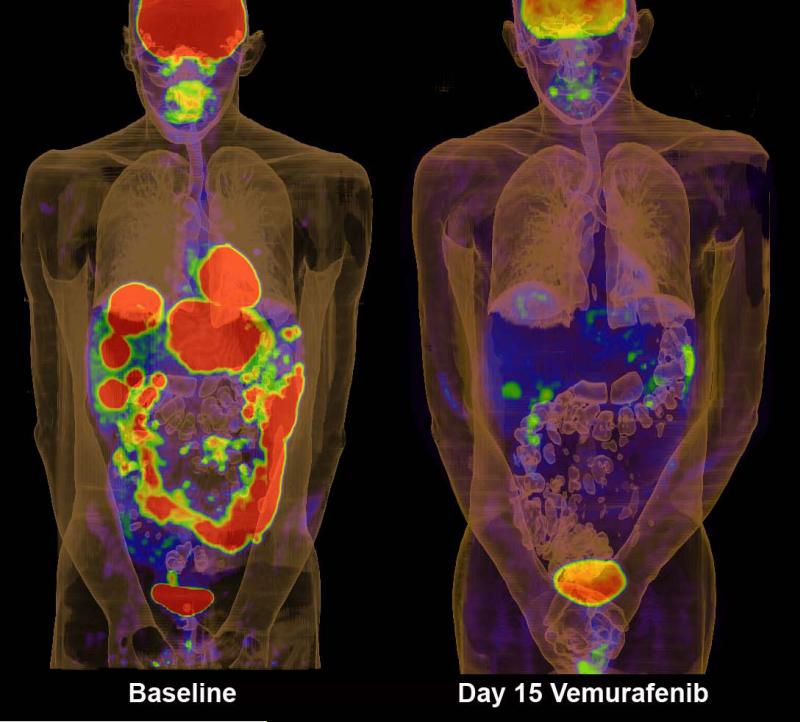
In contrast, the development of BRAF-inhibitors that are selective for the active conformation of the kinase have given very encouraging results. The BRAF-inhibitor vemurafenib (PLX4032) when delivered at the maximum-tolerated dose, induces strong inhibition of downstream signaling as determined by inhibition of pERK, reduction in Ki67 and inhibition of glucose uptake into melanoma metastases as measured by FDG-PET 40, 85 (Figure 2). In a recent phase III trial of individuals with previously untreated BRAF V600E mutant melanoma (n=675) vemurafenib treatment (960mg BID twice daily) led to responses in 48% of patients 86. The response rate of the dacarbazine control group was 5%. At 6 months, the overall survival was 84% and 64% for the vemurafenib and dacarbazine treated groups, respectively86. Similar impressive effects on signaling and response have been observed with another selective BRAF inhibitor GSK2118436 45 that has also entered phase III clinical trials.
Figure 2.
FDG-PET scans of patient on the phase I trial of vemurafenib. Panels show scans of tumor burden at baseline and after 15 days of treatment at 960mg bid.
Interestingly all BRAF inhibitors including sorafenib, vemurafenib, GSK2118436 and XL281 that have been evaluated clinically have induced proliferative squamous lesions in the skin that closely resemble squamous cell carcinomas of the keratoacanthoma type 39, 45, 85-87. These lesions are frequently rapidly growing and can be managed with surgery or other local measures. Although the mechanism by which inhibition of RAF kinases induces these lesions remains to be fully elucidated there is now clinical evidence that the paradoxical activation of MAPK signaling, arising as a result of BRAF inhibition, may play a role in SCC development (see below) 46.
KIT inhibitors
Inhibition of KIT by tyrosine kinase inhibitors has been reported to induce clinical responses in melanomas harboring KIT mutations. Case reports and small series have reported objective responses to the KIT-inhibitors imatinib and sorafenib 88-92. Definitive randomized studies are underway to determine if KIT-inhibition improves progression-free survival in melanoma (clinicaltrials.gov number NCT01028222). As 5 KIT inhibitors are currently approved for GIST or CML (imatinib, dasatnib, nilotinib, sorafenib and sunitinib) that have varying inhibitory profiles against a range of KIT mutations, it may be possible to match an individual KIT mutation to a particular drug. Indeed clinical response has been reported to sorafenib in a case of metastatic anal melanoma with the imatinib resistant mutation D820Y91.
5. Resistance and the development of combination therapies
Although the presence of an activating BRAF V600E mutation generally predicts for sensitivity to BRAF inhibition, not all patients with BRAF V600E mutant melanoma respond to vemurafenib and there is evidence that some patients may be intrinsically resistant 85. Recent preclinical studies have demonstrated that amplification of cyclin D1 (in up to 17% of BRAF V600E mutant melanomas) or loss of the tumor suppressor RB together with loss of the tumor suppressor phosphatase and tensin homolog (PTEN) may both contribute to intrinsic BRAF inhibitor resistance 93-95. In the case of PTEN loss, BRAF inhibition was found to paradoxically activate AKT which prevented cell death by suppressing the levels of the pro-apoptotic protein BIM 94.
Almost all BRAF mutant melanoma patients who respond to vemurafenib ultimately fail therapy and become resistant. These observations mirror the pattern of response seen to targeted therapy in CML, GIST 96, 97 and most recently medulloblastoma 98, 99, where an initial period of tumor regression is later followed by relapse. Even before the development of BRAF specific inhibitors it was already known that both growth factors and cytokines could rescue melanoma cells from apoptosis following the siRNA-induced knockdown of BRAF100, 101, 82. A number of studies have now begun to address the mechanisms of acquired BRAF inhibitor resistance in both melanoma cell lines and specimens from BRAF mutant melanoma patients failing vemurafenib therapy. So far a large number of potential acquired resistance mechanisms have been reported; these include upregulated receptor tyrosine kinase signaling (both PDGFRβ and IGF1R), the emergence of apparently de novo mutations in NRAS, acquisition of novel mutations in MEK1 and the increased expression of MAP3K8 (otherwise known as COT) 102-106. Although the resistance mechanisms reported so far are diverse, nearly all involve the reactivation of a common set of signaling pathways already known to be important for melanoma progression, such as MEK/ERK and PI3K/AKT/mTOR106. There is already preclinical data showing that dual BRAF + MEK inhibition may prevent or delay the onset of resistance to PLX4720 and may overcome resistance mediated by MEK1 mutations, COT overexpression and the acquisition of de novo NRAS mutations 104, 107, 108. In contrast, resistance mediated through IGFR1 signaling can be overcome by dual MEK + PI3K inhibition and resistance mediated through PDGFR signaling can be ameliorated through the targeting of the mTOR/PI3K/AKT pathway 103. As a number of these mechanisms reactivate MEK phase I/II trials of the BRAF inhibitor GSK2118436 in combination with the MEK inhibitor GSK1120212 (NCT01072175) and vemurafenib combined with the MEK inhibitor GDC-0973 (NCT01271803) are underway. There are already indications that these combinations may be effective. In a recent presentation at ASCO, the phase I/II trial of the GSK112012 + GSK2118436 combination was associated with objective response rates (complete response + partial response) of 77% at a dose level of 150mg GSK118436/1mg GSK112012 and 74% at the dose level of 150mg GSK118436/2mg GSK1120212 46. Even more significantly, the combination of the BRAF + MEK inhibitor was associated with significantly reduced levels of squamous cell carcinoma (<1%, n=109) 46. Other clinical studies combining BRAF inhibitors with inhibitors of the mTOR/PI3K/AKT pathway are due to commence in the near future.
The approach being taken to manage therapeutic escape in melanoma differs from the current model of treating acquired targeted therapy drug resistance seen in other cancers, where resistance is often associated with secondary mutations in drug target proteins. The most well known examples of this phenomenon are imatinib resistance in CML and GIST that arises as the result of de novo “gatekeeper” mutations in BCR-ABL and c-KIT, respectively 96, 109, 110. Although preclinical studies identified Threonine-529 to be the BRAF gatekeeper site, there is currently little evidence that chronic treatment of melanoma patients with vemurafenib leads to acquisition of secondary mutations in BRAF at the gatekeeper site or at any others 32, 85, 102.
6. Future perspectives
The importance of patient selection
A future can be envisaged where the molecular profiling of patient tumors will become an integral part of therapy selection for medical oncologists. The importance of matching the right targeted therapy to the correct melanoma genotype is illustrated by recent pre-clinical studies showing that inhibitors of BRAF paradoxically activate MAPK signaling in tumors that lack activating BRAF mutations. Reports from at least 6 independent groups have shown that BRAF inhibition activates MAPK in cell lines with NRAS and KRAS mutations as well as those cell lines where the MAPK pathway is activated through other oncogenes such as HER2 34, 111-115. Mechanistic studies showed that although vemurafenib and other BRAF inhibitors were able to profoundly inhibit the activity of BRAF V600E containing complexes in melanoma cells they instead promoted the activity of CRAF-CRAF dimers in cells with RAS mutations, leading in turn to MEK activation 34, 115. There is also evidence that PLX4032 increases the invasive potential of NRAS-mutated melanoma cells through the through the activation of ERK and FAK signaling 113. Additional studies demonstrated that BRAF inhibitors may even contribute to the progression of NRAS mutated melanomas in part by suppressing apoptosis through the modulation of Mcl-1 expression 114. Following these observations a new generation of BRAF inhibitors were recently unveiled that apparently prevent the paradoxical activation of MAPK signaling. Although data is currently lacking on these new drugs, it is hoped that their improved selectivity profile may prevent the onset of SCC and delay the time to resistance.
These studies are extremely important in the approaching the development of new cancer therapies as they indicate that simple empiric evaluation of novel cancer therapeutics in patients could be associated with adverse outcomes. Instead they affirm the approach of rationally developing therapies in cancer patients based on strong preclinical data and individual patient molecular profiling. It is indeed time to get personal in treatment of melanoma.
Acknowledgments
The authors thank Mr Jason Callahan and Prof Rod Hicks for the FDG-PET image.
Grant support (to KS): The Harry Lloyd Trust, The Bankhead-Coley Research Program of the State of Florida (09BN-14), An Institutional Research Grant from the American Cancer Society #93-032-13 and the NIH/National Cancer Institute grants U54 CA143970-01 and R01 CA161107-01. Grant support (to GM): GM is a recipient of the Sir Edward Dunlop Clinical Research Fellowship of the Cancer Council of Victoria. Grants from the Victorian Cancer Agency, the Cancer Council of Victoria and the National Health and Medical Research Council Australia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003 May 19;22(20):3138–51. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 2.Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, Marmion JM, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 2008 Feb 1;68(3):664–73. doi: 10.1158/0008-5472.CAN-07-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005 Nov 17;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Duensing S, Duensing A. Targeted therapies of gastrointestinal stromal tumors (GIST)--the next frontiers. Biochem Pharmacol. 2010 Sep 1;80(5):575–83. doi: 10.1016/j.bcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001 Apr 5;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Zhang G, Haura EB. Targeting epidermal growth factor receptor: central signaling kinase in lung cancer. Biochem Pharmacol. 2010 Sep 1;80(5):613–23. doi: 10.1016/j.bcp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004 Nov;5(11):875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 9.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004 Oct;6(4):313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004 Mar 19;116(6):855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, Bacchiocchi A, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, Aplin AE. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005 May 12;24(21):3459–71. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- 13.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002 Feb;2(2):133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 14.Smalley KSM. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? International Journal of Cancer. 2003 May 1;104(5):527–32. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 15.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008 May 22;27(23):3301–12. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 16.Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004 Apr 1;64(7):2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Research. 2003 Sep 1;63(17):5198–202. [PubMed] [Google Scholar]
- 18.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004 Aug 19;23(37):6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 19.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005 Aug 4;436(7051):720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 20.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009 Mar 12; doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006 Jul 28;313(5786):521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 22.Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS medicine. 2008 Jun 3;5(6):e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011 Apr 1;29(10):1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 24.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006 Dec 1;66(23):11100–5. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 25.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008 Feb 19; doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haass NK, Sproesser K, Nguyen TK, Contractor R, Medina CA, Nathanson KL, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008 Jan 1;14(1):230–9. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 27.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006 Jan 19;439(7074):358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005 Mar 15;65(6):2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 30.Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009 Jan 8;28(1):85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009 Jun 10;27(17):2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 32.Whittaker S, Kirk R, Hayward R, Zambon A, Viros A, Cantarino N, et al. Gatekeeper Mutations Mediate Resistance to BRAF-Targeted Therapies. Science translational medicine. Jun 9;2(35):35ra41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 33.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. Jun 8;102(12):1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. Mar 18;464(7287):427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008 Jun 15;68(12):4853–61. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sondergaard JN, Nazarian R, Wang Q, Guo D, Hsueh T, Mok S, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med. 2010;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tap WD, Gong KW, Dering J, Tseng Y, Ginther C, Pauletti G, et al. Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma. Neoplasia. 2010 Aug;12(8):637–49. doi: 10.1593/neo.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. Jul 1;70(13):5518–27. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GK, Robertson S, Shen A, Wang E, Pace L, Dials H, et al. A phase I study of XL281, a selective oral RAF kinase in patients with advanced solid tumors. Journal of Clinical Oncology. 2009;27(15s):3513. [Google Scholar]
- 40.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010 Sep 7;467:596–99. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smalley KSM, Sondak VK. Melanoma - an unlikely poster child for targeted therapy. N Engl J Med. 2010;363:876–78. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 42.Smalley KS, Nathanson KL, Flaherty KT. Genetic subgrouping of melanoma reveals new opportunities for targeted therapy. Cancer Res. 2009 Apr 15;69(8):3241–4. doi: 10.1158/0008-5472.CAN-08-4305. [DOI] [PubMed] [Google Scholar]
- 43.Nathanson KL. Using genetics and genomics strategies to personalize therapy for cancer: focus on melanoma. Biochem Pharmacol. 2010 Sep 1;80(5):755–61. doi: 10.1016/j.bcp.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva J, Cipolla A, Kong J, Neri MK, Nathanson KL, Lee J, et al. A kinase switch underlies acquired resistance to BRAF inhibitors. Pigment Cell Melanoma Res. 2009;22(6):136. [Google Scholar]
- 45.Kefford R, Arkenau H, Brown MP, Millward M, Infante JR, Long GV, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. Journal of Clinical Oncology. 2010;28(15s):8503. [Google Scholar]
- 46.Infante JR, Falchook GS, Lawrence DA, Weber J, Kefford R, Bendell J, et al. Phase I/II Study of the oral MEK1/2 inhibitor GSK1120212 dosed in combination with the oral BRAF inhibitor GSK2118436. J Clin Oncol. 2011;29(18S) CRA8503. [Google Scholar]
- 47.Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009 Feb 1;15(3):1076–85. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 49.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010 Jul 1;70(13):5549–57. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Research. 2002 Dec 1;62(23):6997–7000. [PubMed] [Google Scholar]
- 51.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010 Jan 22;140(2):209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, et al. In Melanoma, RAS Mutations Are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006 Oct 1;66(19):9483–91. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 53.Mishra PJ, Ha L, Rieker J, Sviderskaya EV, Bennett DC, Oberst MD, et al. Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation. Oncogene. Apr 22;29(16):2449–56. doi: 10.1038/onc.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997 Nov 1;11(21):2822–34. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005 May 15;65(10):4005–11. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 56.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007 Jul;6(7):541–55. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 57.Smalley KSM, Eisen TG. Farnesyl transferase inhibitor SCH66336 is cytostatic, pro-apoptotic and enhances chemosensitivity to cisplatin in melanoma cells. International Journal of Cancer. 2003 Jun 10;105(2):165–75. doi: 10.1002/ijc.11064. [DOI] [PubMed] [Google Scholar]
- 58.Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009 Jul 15;15(14):4649–64. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 59.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008 Dec;14(12):1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaiswal BS, Janakiraman V, Kljavin NM, Eastham-Anderson J, Cupp JE, Liang Y, et al. Combined targeting of BRAF and CRAF or BRAF and PI3K effector pathways is required for efficacy in NRAS mutant tumors. PLoS One. 2009;4(5):e5717. doi: 10.1371/journal.pone.0005717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006 Sep 10;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 62.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002 Mar 15;20(6):1692–703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 63.Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, Galbincea J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009 Nov;22(11):1446–56. doi: 10.1038/modpathol.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonescu CR, Busam KJ, Francone TD, Wong GC, Guo T, Agaram NP, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007 Jul 15;121(2):257–64. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 65.Jiang X, Zhou J, Yuen NK, Corless CL, Heinrich MC, Fletcher JA, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin Cancer Res. 2008 Dec 1;14(23):7726–32. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 66.Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010 Sep 1;80(5):568–74. doi: 10.1016/j.bcp.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Handolias D, Salemi R, Murray W, Tan A, Liu W, Viros A, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res. 2010 Apr;23(2):210–5. doi: 10.1111/j.1755-148X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- 68.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006 May;126(5):1102–10. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 69.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1alpha to transform melanocytes. Oncogene. 2010 Jan 14;29(2):227–36. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 70.Ashida A, Takata M, Murata H, Kido K, Saida T. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009 Feb 15;124(4):862–8. doi: 10.1002/ijc.24048. [DOI] [PubMed] [Google Scholar]
- 71.Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009 Aug;8(8):2079–85. doi: 10.1158/1535-7163.MCT-09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smalley KS, Contractor R, Nguyen TK, Xiao M, Edwards R, Muthusamy V, et al. Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression. Cancer Res. 2008 Jul 15;68(14):5743–52. doi: 10.1158/0008-5472.CAN-08-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rimoldi D, Salvi S, Lienard D, Lejeune FJ, Speiser D, Zografos L, et al. Lack of BRAF mutations in uveal melanoma. Cancer Res. 2003 Sep 15;63(18):5712–5. [PubMed] [Google Scholar]
- 74.Pache M, Glatz K, Bosch D, Dirnhofer S, Mirlacher M, Simon R, et al. Sequence analysis and high-throughput immunohistochemical profiling of KIT (CD 117) expression in uveal melanoma using tissue microarrays. Virchows Arch. 2003 Dec;443(6):741–4. doi: 10.1007/s00428-003-0883-2. [DOI] [PubMed] [Google Scholar]
- 75.All-Ericsson C, Girnita L, Muller-Brunotte A, Brodin B, Seregard S, Ostman A, et al. c-Kit-dependent growth of uveal melanoma cells: a potential therapeutic target? Invest Ophthalmol Vis Sci. 2004 Jul;45(7):2075–82. doi: 10.1167/iovs.03-1196. [DOI] [PubMed] [Google Scholar]
- 76.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009 Jan 29;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5230–4. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005 Aug 10;23(23):5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 79.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004 Nov 15;22(22):4456–62. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 80.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008 May 1;26(13):2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Infante JR, Fecher LA, Nallapareddy S, Flaherty KT, Cox DC, De Marini DJ, et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSL1120212. Journal of Clinical Oncology. 2010;28(15s):2503. [Google Scholar]
- 82.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011 Mar 1;17(5):989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 83.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006 Sep 4;95(5):581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008 May 1;26(13):2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 85.Flaherty KT, Puzanov I, Kim KB, Ribas A, MacArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011 Jun 5; doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnault JP, Mateus C, Wechsler J, Spatz A, Tomasic G, Sibaud V, et al. Paradoxical cutaneous squamous cell proliferations in patients treated with sorafenib. Journal of Clinical Oncology. 2009;27(15s):9564. [Google Scholar]
- 88.Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008 Apr 20;26(12):2046–51. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 89.Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nature clinical practice. 2008 Dec;5(12):737–40. doi: 10.1038/ncponc1251. [DOI] [PubMed] [Google Scholar]
- 90.Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008 May 29; doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 91.Handolias D, Hamilton AL, Salemi R, Tan A, Moodie K, Kerr L, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer. 2010 Apr 13;102(8):1219–23. doi: 10.1038/sj.bjc.6605635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satzger I, Kuttler U, Volker B, Schenck F, Kapp A, Gutzmer R. Anal mucosal melanoma with KIT-activating mutation and response to imatinib therapy--case report and review of the literature. Dermatology. 2010;220(1):77–81. doi: 10.1159/000265558. [DOI] [PubMed] [Google Scholar]
- 93.Smalley KS, Lioni M, Palma MD, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008 Sep;7(9):2876–83. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011 Apr 1;71(7):2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB, et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene. 2011 Jul 4; doi: 10.1038/onc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007 Nov 29;26(54):7560–8. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 97.Sawyers C. Targeted cancer therapy. Nature. 2004 Nov 18;432(7015):294–7. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 98.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009 Sep 17;361(12):1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened Mutation Confers Resistance to a Hedgehog Pathway Inhibitor in Medulloblastoma. Science. 2009 Sep 2;326:572–74. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christensen C, Guldberg P. Growth factors rescue cutaneous melanoma cells from apoptosis induced by knockdown of mutated (V 600 E) B-RAF. Oncogene. 2005 Sep 15;24(41):6292–302. doi: 10.1038/sj.onc.1208758. [DOI] [PubMed] [Google Scholar]
- 101.Gray-Schopfer VC, Karasarides M, Hayward R, Marais R. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007 Jan 1;67(1):122–9. doi: 10.1158/0008-5472.CAN-06-1880. [DOI] [PubMed] [Google Scholar]
- 102.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010 Nov 24;468:973–77. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010 Dec 14;18(6):683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 Nov 24; doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011 Mar 7; doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011 May 25; doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010 Jun 8;102(12):1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20411–6. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009 Nov 6;16(5):401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005 Jun 30;435(7046):1267–70. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 111.Carnahan J, Beltran PJ, Babij C, Le Q, Rose MJ, Vonderfecht S, et al. Selective and potent Raf inhibitors paradoxically stimulate normal cell proliferation and tumor growth. Mol Cancer Ther. 2010 Aug;9(8):2399–410. doi: 10.1158/1535-7163.MCT-10-0181. [DOI] [PubMed] [Google Scholar]
- 112.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. Jan 22;140(2):209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010 Apr;23(2):190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the ongenic B-RAF inhibitor, PLX4720, in mutant N-Ras melanoma cell lines. Oncogene. 2010;30:366–71. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010 Mar 18;464(7287):431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]