Highlights
* We examined neural activity in children with a history of preschool-onset depression. * We examined change in brain activity during mood induction and emotion regulation. * A history of preschool depression related to decreased prefrontal cortex activity. * Severity of current depressed mood was associated with increased limbic activity.
Keywords: Preschool depression, Imaging, Emotion regulation, Prefrontal, Amygdala
Abstract
While major depressive disorder has been shown to be a significant mental health issue for school-age children, recent research indicates that depression can be observed in children as early as the preschool period. Yet, little work has been done to explore the neurobiological factors associated with this early form of depression. Given research suggesting a relation between adult depression and anomalies in emotion-related neural circuitry, the goal of the current study was to elucidate changes in functional activation during negative mood induction and emotion regulation in school-age children with a history of preschool-onset depression. The results suggest that a history of depression during the preschool period is associated with decreased activity in prefrontal cortex during mood induction and regulation. Moreover, the severity of current depressed mood was associated with increased activity in limbic regions, such as the amygdala, particularly in children with a history of depression. Similar to results observed in adult depression, the current findings indicate disruptions in emotion-related neural circuitry associated with preschool-onset depression.
1. Introduction
A large body of research has supported the validity of major depressive disorder (MDD) during childhood such that MDD is widely recognized as a significant mental health issue for school-aged (6–12 years old) children (Birmaher et al., 1996, Kaufman and Charney, 2001). Recent work has extended these findings to suggest that MDD can also occur in preschool-aged children (3–5 years old). Preschool-onset major depressive disorder (PO-MDD), an early occurring form of depression, has been shown to exhibit a chronic and recurrent course similar to MDD in school-aged children or in adults (Luby, 2009). In particular, PO-MDD is more strongly predictive of the occurrence of later childhood depression than of other disorders, suggesting that PO-MDD exhibits homotypic continuity with later MDD rather than acting as a nonspecific precursor to general psychopathology (Luby et al., 2009b). In addition, children with PO-MDD show functional impairments in multiple contexts (Luby et al., 2009a) and show increased cortisol responses to separation and frustration stressors (Luby et al., 2003). Although a substantial body of data has been published to date on the validity of preschool MDD including its discriminant validity, longitudinal stability, and biological correlates, some skepticism about the clinical significance of preschool depression has remained. Part of this skepticism comes from a reluctance to label young children with psychiatric diagnoses as well as legitimate concerns about the rapidly changing nature of the mental status of young children and potential bias on the part of parental informants who may themselves be depressed. Neuroimaging data provides a critical area of investigation to more rigorously test the validity of preschool depression. Data such as that provided by this investigation and by related studies may address questions of bias or measurement that can arise using other forms of data as well as helping to characterize the neurobiological alterations associated with PO-MDD.
Research examining the behavioral and neural correlates of adult and adolescent MDD has frequently demonstrated aberrations in neural systems supporting emotion processing and regulation. For example, a number of studies have provided evidence for a heightened amygdala response to negative emotional stimuli in adult and adolescent depression (Beesdo et al., 2009, Fales et al., 2008, Hamilton and Gotlib, 2008, Peluso et al., 2009, Sheline et al., 2001, Victor et al., 2010, Yang et al., 2010). Additionally, multiple studies have suggested that altered functional activity within dorsal and ventral prefrontal cortices (PFC) is associated with the disrupted affective processing and emotional control characteristically exhibited by depressed individuals (Beauregard et al., 2006, Brody et al., 2001, Davidson et al., 2003, Keedwell et al., 2005, Mayberg, 2003, Phillips et al., 2003, Pizzagalli et al., 2001; see Gotlib and Hamilton, 2008, for a review). This has been most evident in studies of depressed adults demonstrating increased recruitment and activation of ventral PFC control regions to downregulate negative emotions (Johnstone et al., 2007, Sheline et al., 2009) rather than more dorsal regions typically seen in healthy subjects (Ochsner et al., 2004a, Ochsner et al., 2004b). Some work has also been done to characterize anomalous neural activity associated with childhood and adolescent depression. For example, abnormal amygdala activation to fearful emotional faces has been noted in a small sample of depressed children (Thomas et al., 2001). Heightened amygdala activity to emotional faces was also shown to predict later recognition of these faces in depressed as compared to healthy adolescents (Roberson-Nay et al., 2006). Altered amygdala activity was also observed in children and adolescents at risk for depression (offspring of parents with MDD) as compared to low risk individuals while passively viewing emotional faces (Monk et al., 2008).
Despite a growing body of research indicating that emotion-related neural systems are disrupted in depression, little is known about the early developmental course of these anomalies. This is a compelling question given the rapid pace at which emotion regulation skills develop during childhood and findings that early dysfunction in this domain predicts clinically relevant problems later in childhood. For example, low levels of emotion regulation during infancy and toddlerhood have been found to predict later behavioral problems (Eisenberg et al., 2003, Stifter et al., 1999). In examining neurobiology, models of development across childhood and adolescence have suggested that differential rates of maturation between prefrontal and limbic regions may account for children's sensitivity to emotional and appetitive cues (see Casey et al., 2011). The relative maturity of limbic regions relative to prefrontal regions and the overall immaturity of both systems described in these models would help to account for results indicating amygdala hyperactivity to emotional faces in children and adolescents as compared to adults (e.g. Guyer et al., 2008). These periods of early development may also be particularly vulnerable to disruption by depression-related influences, like early life stress, and this may depend on the region-specific rates of growth and pruning (Andersen and Teicher, 2008). This line of evidence would suggest that emotion regulation, rooted in interactions between limbic and prefrontal circuitry, may be particularly vulnerable to disturbances (e.g. experiences of depression) in early childhood.
Given the evidence for alterations in the neural systems associated with emotion regulation in adult and adolescent depression, the rapid early development of emotion regulation (Cole et al., 1994) and previous work showing alterations in emotion development in preschoolers at risk for MDD (e.g. Dawson et al., 2003), we examined potential anomalies in brain activity associated with emotion processing and emotion regulation in children with the earliest known manifestation of depression, PO-MDD. Previous work in this sample and in related samples has noted disruptions in affective neural circuitry beginning during the preschool period. In particular, the severity of PO-MDD has been correlated with amygdala reactivity during emotional face viewing (Barch et al., submitted, Gaffrey et al., 2011b). PO-MDD has also been associated with decreased functional connectivity between the amygdala and prefrontal cortex (Luking et al., 2011). These results provide support for the hypothesis that emotion processing and regulation are disrupted in children with PO-MDD. In order to more directly examine this hypothesis, we adopted a sad mood elaboration paradigm developed by Cooney et al. (2007) to examine the neural systems involved in emotion regulation. This study noted activation in orbital frontal cortex, parahippocampal gyrus, and ventral striatal areas associated with the induction of and elaboration on a sad mood in adult females. Furman et al. (2010) recently used this paradigm to demonstrate altered amygdala responsivity in children with the short allele of the serotonin transporter gene, such that children with one or two short alleles displayed a lower latency to peak of amygdala activity suggesting that they may be primed to maintain and elaborate on negative mood. This genetic variation in the serotonin transporter gene has been associated with heightened amygdala responsivity to emotionally evocative information (Hariri et al., 2002, Munafo and Hariri, 2008) and, in interaction with life events, with increased risk for depression (Caspi et al., 2003, Karg et al., 2011; but see also Risch et al., 2009). We used functional magnetic resonance imaging (fMRI) to characterize brain activity during this paradigm in school-aged children with a previous diagnosis of PO-MDD and in healthy children with no history of psychiatric disorder. In the paradigm used in the current study, children elaborated on a sad mood induced by a film clip with the goals of examining induction of and elaboration on negative affect. While other studies on emotion regulation in adults often explicitly require participants to decrease their negative emotional responses to individual word or picture stimuli, the current approach is more consistent with studies of implicit regulation; moreover, using complex film stimuli may represent a more ecologically valid approach to the induction of emotions. Based on previous findings, we predicted that children with a history of PO-MDD would exhibit altered activity in dorsal prefrontal regions and increased limbic activity during the elaboration of a sad mood.
2. Materials and methods
2.1. Participants
Participants in this study were a subsample of children involved in the Preschool Depression Study (PDS), a prospective longitudinal investigation of preschoolers and their families conducted at the Early Emotional Development Program (EEDP) at Washington University in St. Louis. Three- to six-year-old children and their caregivers were recruited using a screening checklist at daycares, preschools, and primary care sites in the St. Louis metropolitan area and preschoolers with symptoms of depression were oversampled (see Luby et al., 2009b, for additional details on recruitment for the PDS). The current study reports on 55 children (26 male, for demographic information see Table 1) from the PDS at school age who participated in the neuroimaging phase of this project. A history of head trauma, neurological disease, developmental delay, and the use of psychoactive medications were exclusionary criteria for the current analyses. Participants were all between the ages of 7 and 11 years at the time of scan (mean age = 9.3). Results of the diagnostic assessment tools described in the following section allowed the participants to be divided into two groups. One group had been diagnosed with preschool-onset depression between the ages of 3–5 (PO-MDD; N = 24) while the control group did not exhibit a diagnosis of PO-MDD (CONT; N = 31). At the time of scan, 12.73% of the participants exhibited a diagnosis of MDD (23.6% of the entire PDS sample met a diagnosis of MDD at the time of scan). In addition, 9.09% of participants were diagnosed with attention deficit-hyperactive disorder (ADHD), 3.64% were diagnosed with generalized anxiety disorder, and 9.09% were diagnosed with social anxiety disorder. Parental written consent was obtained prior to study participation and the Institutional Review Board at Washington University approved all experimental procedures. It is important to note that only children without a history of medication use were included in this study.
Table 1.
Demographic and clinical characteristics of healthy control and MDD groups.
| Control group |
MDD group |
|||
|---|---|---|---|---|
|
N = 31 |
N = 24 |
|||
| Mean | SD | Mean | SD | |
| Age at scan (years) | 9.70 | 0.87 | 9.80 | 1.04 |
| Sex (% male) | 45% | 38% | ||
| Ethnicity:* % Caucasian | 50.00% | 74.19% | ||
| % African American | 33.33% | 22.58% | ||
| % Other ethnicity | 16.67% | 3.23% | ||
| Family incomea | 2.97 | 1.24 | 2.79 | 1.25 |
| Maternal education levelb | 2.77 | 1.06 | 2.50 | 1.03 |
| Initial MDD severity** | 1.58 | 1.41 | 7.28 | 3.30 |
| Average MDD severity** | 1.27 | 0.85 | 3.55 | 1.37 |
| Average externalizing symptomology** | 2.42 | 2.06 | 8.75 | 7.19 |
| Average internalizing symptomology** | 2.08 | 0.99 | 4.69 | 2.49 |
| CDI-parent | 44.22 | 7.73 | 48.24 | 9.08 |
| CDI-child | 42.19 | 5.33 | 44.92 | 9.07 |
Family income per year was obtained at the first diagnostic wave using the following categories: (1) ($0–20k), (2) ($20,001–40k), (3) ($40,001–60k), and (4) ($60,001+).
Maternal education level was obtained using the following categories: (1) some grade school, (2) completed grade school, (3) some high school, (4) completed high school, (5) some college or a 2-year degree, (6) completed a 4 year college degree, (7) some school beyond college and (8) completed a professional or graduate degree.
p < 0.05.
p < 0.01.
2.2. Diagnostic assessment
Trained staff from the EEDP at Washington University conducted up to four in-person interview sessions with participants and parents/guardians over the course of 4–6 years. Nearly all of the children (90%) were assessed four times while the remaining children were assessed three times. We do not think that this small variation influenced our results or biased the use of measures in the current analyses. The children were between the ages of 3–5 years at the time of their first interview and between the ages of 7–11 years at the time of scanning. Diagnoses were derived using the Preschool-Age Psychiatric Assessment (PAPA), a reliable and age appropriate semi-structured diagnostic interview widely used in research on preschool psychopathology (Egger et al., 2003). Interviews were audiotaped and reviewed for reliability as previously reported (Luby et al., 2009b). The PAPA is a parent measure with developmentally appropriate questions covering the DSM-IV criteria for a range of disorders, including major depression, oppositional defiant disorder, ADHD, and anxiety disorders. The two-week duration of symptoms requirement for major depression was set aside (no minimum duration was necessary for diagnosis) for the purposes of this study based on data indicating that it may not be developmentally appropriate for this age group (Gaffrey et al., 2011a, see also Luby et al., 2009b for information about duration of PO-MDD in larger sample). Once study subjects reached the age of 8, the Childhood and Adolescent Psychiatric Assessment (CAPA) was used. The CAPA probes for symptoms of a variety of psychiatric disorders as above but, unlike the PAPA, also makes use of report from the child or adolescent in addition to the primary caregiver (Angold and Costello, 2000). Importantly, the PAPA/CAPA also allow for quantification of symptom severity. Children were classified as having a history of MDD if at any time point the child met all DSM-IV symptom criteria for MDD on the PAPA (see Luby et al., 2002). In addition, for each in-person diagnostic time point, depression sum scores (the total number of MDD symptoms endorsed on the PAPA/CAPA) were calculated and used to assess symptom severity (Luby et al., 2004). We also computed sum severity scores for non-depression internalizing disorders and externalizing disorders at each time point. We used these scores to examine whether results were specific to depression or were related more generally to childhood internalizing or externalizing psychopathology.
To assess the severity of current MDD symptoms, child and parent versions of the Children's Depression Inventory (CDI-C and CDI-P, respectively) were completed at the time of scan (Kovacs, 1985). Total scores for the CDI-C and CDI-P were converted to t-scores.
2.3. Procedure
Once consent for participation was obtained, children participated in a training sequence designed to increase their comfort and understanding of the scanning procedure in order to minimize movement during the actual scan session. As part of their training, children watched a video, which familiarized them with the scanner facility and staff by depicting another child participating in the actual scanner procedure. Following this, children were introduced to the scanner environment using a mock scanner (i.e., MRI simulator). Children participated in this experience at their own pace and each training session was altered to meet the needs of the individual participant. Generally, this experience included lying down on the scanner table, having headphones placed on their head and entering the bore of the simulator. Children practiced lying still while listening to recorded MRI sounds and receiving visual feedback of their head movement. Movement feedback was provided in real time through the use of motion tracking software, MoTrak. Specifically, children viewed a crosshair, which moved to represent their head motion and were instruct to try to keep the crosshair within a bull's eye target. After approximately 10 min of practice, children were removed from the simulator and any remaining questions regarding the practice or actual scan were addressed.
The protocol for the sad mood elaboration task was adapted from Cooney et al. (2007) and Furman et al. (2010). Participants first focused on a fixation cross for a one-minute baseline scan. Participants then observed a clip from the film “My Girl” (Gazer et al., 1991) which depicts a young girl at a funeral for her best friend. The film clip lasted 5 min and 19 s including brief task instructions before the start of the clip. The time during the film clip was used to collect structural scans that were used in the scan co-registration process. Before the next scanning period, participants were given structured auditory prompts to encourage active elaboration on the induced sad mood (e.g. ‘Imagine yourself being in this situation’ and ‘Have you ever been in a similar situation?’) and were asked to think about the clip and the induced sad mood during the next scanning period which lasted for one minute following the prompts. (The exact procedures are provided in the Supplemental Materials.) Participants rated their mood on a five-point scale (1 = very sad, 5 = very happy) before watching the film clip and after the sad mood elaboration period. Higher scores, therefore, reflect more positive mood.
2.4. fMRI scanning
Imaging data were collected using a 3T TIM TRIO Siemens scanner. T1 structural images were acquired using a sagittal MP-RAGE 3D sequence (TR = 2400 ms, TE = 3.16 ms, flip = 8°; voxel size = 1 mm × 1 mm × 1 mm). Functional data was collected with a 12-channel head coil using an asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (T2*) (TR = 2500 ms, TE = 27 ms, FOV = 256 mm, flip = 90°, voxel size = 4 mm × 4 mm × 4 mm, slices = 36).
2.5. fMRI preprocessing
Scan data were preprocessed using the following steps: (1) compensation for slice-dependent time shifts; (2) removal of first 5 images of each run to allow BOLD signal to reach steady state; (3) elimination of odd/even slice intensity differences due to interpolated acquisition; (4) realignment of data acquired in each subject within and across runs to compensate for rigid body motion (Ojemann et al., 1997); (5) intensity normalization to a whole brain mode value of 1000; (6) registration of the 3D structural volume (T1) to the atlas representative template in the Talairach coordinate system (Talairach and Tournoux, 1988) using a 12-parameter affine transform and re-sampling to 1 mm cubic representation (Ojemann et al., 1997, Buckner et al., 2004); and (7) co-registration of the 3D fMRI volume to the T2, and the T2 to the participants structural image; (8) transformation of the fMRI to atlas space using a single affine 12-parameter transform and (9) spatial smoothing using a 6 mm full-width half-maximum Gaussian filter.
2.6. fMRI analysis
Analysis of fMRI data was performed using in-house software (FIDL analysis package, http://www.nil.wustl.edu/Bfidl). Estimates of functional activation during sad mood elaboration were obtained using block-design analyses. This involved using a general linear model (GLM) incorporating regressors for linear trend and baseline shifts. A hemodynamic response shape was assumed (Boynton function) and used to derive magnitude estimates relative to fixation baseline. Signal to noise ratios (SNR) for elaboration and fixation images were examined for both groups. SNR did not significantly differ across groups for either the elaboration (t(42) = 0.32, p = 0.75) or for the fixation (t(42) = 0.06, p = 0.96) images. The average movement during the fixation period was a RMS (per frame) of 0.13 (median = 0.09, range = 0.04–0.60) and the average movement during the elaboration period was 0.17 (median = 0.12, range = 0.05–0.77). These values did not differ across conditions (t(55) = 1.79, p > 0.05) and an analysis of variance (ANOVA) indicated that there was no interaction with group (F(52) = 0.635, p > 0.50).
To examine the main effect of sad mood induction and elaboration, we performed a one-sample t-test to isolate regions whose activity during the elaboration vs. baseline was significantly different than zero across groups. The resulting whole-brain maps were thresholded for significance to obtain a whole-brain false positive rate of 0.05 based on Monte Carlo simulations implemented with in-house software. A threshold of 17 contiguous voxels with a z value of >3.0 was used for these corrections. This analysis was also performed using a priori regions of interest (ROIs) including amygdala/hippocampus, the striatum, dorsolateral prefrontal cortex, dorsal anterior cingulate, pregenual anterior cingulate, and subgenual cingulate. A threshold of 10 contiguous voxels with a z value of >2.6 was used for these results to obtain a false positive rate of 0.05 across the whole ROI mask based on Monte Carlo simulations implemented in AlphaSim (Ward, 2000).
To examine group differences in brain activity during sad mood elaboration, we grouped participants based on clinical diagnosis and conducted whole-brain, voxel-wise group t-tests. As above, the resulting whole-brain maps were thresholded for significance to obtain a whole-brain false positive rate of 0.05 based on Monte Carlo simulations. A threshold of 17 contiguous voxels with a z value of >3.0 was used for these corrections. Areas showing a significant group difference in activity during the sad mood elaboration vs. baseline were converted to ROIs for further analyses to assess potential interactions with other clinical variables. In particular, we examined whether any diagnostic group differences in functional brain activity during sad mood elaboration were related to or accounted for by: (1) severity of depression at the time of the scan; (2) cumulative history of depression severity across all assessment time points; or (3) cumulative history of internalizing and externalizing psychopathology. All correlations and partial correlations that were computed related the clinical variable of interest to the activity (averaged across voxels) in the selected ROI.
In addition, to explore whether individual differences in the severity of depression predicted differences in brain activity, we computed whole-brain correlations with scores on the CDI and PAPA/CAPA. Specifically, we examined whether any of the following variables predicted brain activity during sad mood elaboration: (1) depression severity at the initial pre-school assessment time point; (2) cumulative history of depression severity across all 4 assessment points; or (3) depression severity at the time of the scan (CDI scores from parents and children). Similar to the previous analyses, these whole-brain correlation maps were thresholded for significance to obtain a whole-brain false positive rate of 0.05 based on Monte Carlo simulations. A threshold of 17 contiguous voxels with a z value of >3.0 was used for these corrections.
3. Results
3.1. Demographic and clinical characteristics
Table 1 presents demographic and clinical characteristics of the two participant groups. The PO-MDD and control groups did not differ significantly in the age or sex of participants, or in family income and level of maternal education. However, the two groups did differ in ethnicity, with a significantly higher percentage of the control group identifying as white. As shown in Table 1, there were no significant group differences in scores on the CDI-C or CDI-P at the time of scanning. In contrast, there was a highly significant group difference in depression severity scores measured at the initial timepoint and averaged across the all of the assessment timepoints. Significant differences were also found between groups in their levels of internalizing and externalizing symptomatology. As expected, for all of these variables children in the PO-MDD group had higher scores than did control children. Across groups, initial depression severity assessed at baseline positively correlated with average depression severity across the timepoints, as expected (r(53) = 0.70, p < 0.001), but did not correlate with scores on the CDI-C (r(53) = −0.01, p = 0.96) or the CDI-P (r(53) = 0.15, p = 0.28) acquired at the time of scan. Average depression severity correlated positively with scores on the CDI-P (r(53) = 0.35, p = 0.01) but not on the CDI-C (r(53) = 0.19, p = 0.16). Scores on the CDI-C and CDI-P were significantly correlated(r(53) = 0.44, p < 0.001). As a reminder, the average depression severity scores were based on the PAPA, which is a parent report instrument. Thus, it is not surprising that average depression severity correlated more strongly with parent report CDI at the time of scan than with child-report CDI.
As shown in Fig. 1, mood ratings confirmed the effectiveness of the mood induction and elaboration procedures. A two-way (group repeated over time) ANOVA indicated that participants endorsed significantly more negative/less positive mood after the elaboration period than they did before viewing the film (main effect of time, F(1,92) = 49.71, p < 0.001). Neither the main effect of group (F(1,92) = 0.26, p = 0.61) nor, the interaction of group and time (F(1,92) = 0.09, p = 0.76) was significant.
Fig. 1.
Mood ratings for the pre-induction and post-elaboration periods for healthy control and MDD groups.
3.2. Main effect of elaboration across group
Of the a priori ROIs examined, significantly increased in activity during the sad mood elaboration as compared to baseline fixation was found in the caudate, putamen, middle frontal gyrus, and cingulate cortex. In the whole-brain contrast, a significant increase in activity during the sad mood elaboration as compared to the baseline fixation was found in a variety of regions including the caudate, putamen, superior frontal gyrus, cingulate cortex, occipital cortex and cerebellum (see Fig. 2, and for details on the coordinates of specific regions see Table S1 in Supplemental Materials). No activity was found to be significantly less in the elaboration period than the baseline fixation period.
Fig. 2.
Regions found to be significantly more active during sad mood elaboration as compared to baseline fixation across group. Red regions represent results of the whole-brain analyses. Blue regions represent results of the a priori ROI analysis. Purple areas are those which overlap between the whole-brain and ROI analyses.
3.3. Whole brain group comparison
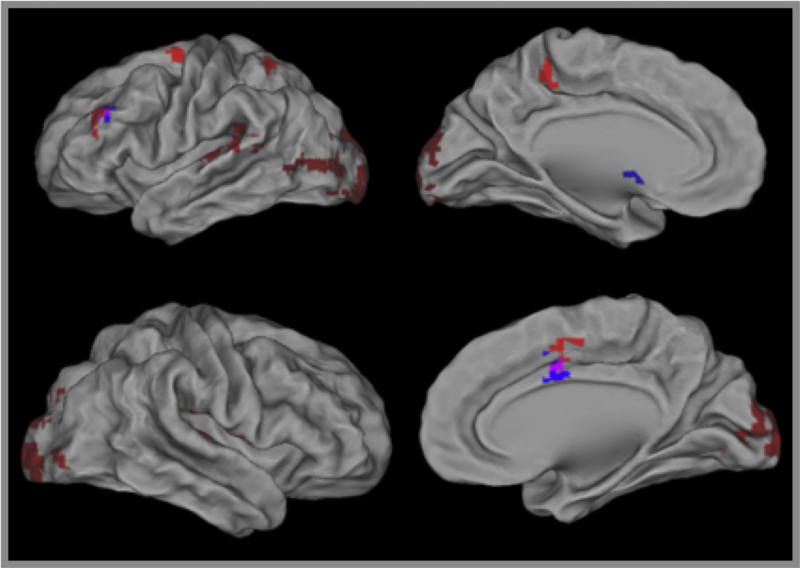
A significant decrease in activity during the sad mood elaboration was found for the PO-MDD group compared to controls in the left dorsolateral PFC (BA 46) and the right superior frontal gyrus (BA 6; see Fig. 3 and Table 2). This difference remained significant in both clusters after controlling for the severity of depression scores at Time 1, the cumulative severity of depression across all assessment points, CDI-P scores, CDI-C scores, and severity of internalizing and externalizing psychopathology. Thus, group differences were not attributable to current depression, comorbid psychopathology, or initial depression severity.
Fig. 3.
Regions of interest found in categorical and dimensional analysis of the relation between depression and brain activity during sad mood elaboration. Blue regions are those that showed diagnostic group differences in the categorical analyses. Red regions are those that showed significant correlations with current depression scores (CDI).
Table 2.
Categorical and dimensional analysis of the relation between depression and brain activity during negative mood induction and elaboration.
| BA | Voxels | Side | X | Y | Z | r | Partial correlation with original predictor variable after controlling for: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDD vs. control | T1 MDD severity | Average MDD severity | CDI-P | CDI-C | Internalizing/externalizing | ||||||||
| MDD vs. control children | |||||||||||||
| Dorsolateral prefrontal cortex | 46 | 74 | L | −44 | 39 | 18 | −0.55** | N/A | −0.48** | −0.38** | −0.57** | −0.55** | −0.44** |
| Superior frontal gyrus | 6 | 82 | R | 22 | 15 | 60 | −0.63** | N/A | −0.49** | −0.44** | −0.61** | −0.62** | −0.52** |
| History of depression | |||||||||||||
| Initial MDD severity | |||||||||||||
| Visual cortex | 18 | 17 | L | −32 | −96 | 3 | −0.48** | −0.39** | N/A | −0.46** | −0.43** | −0.37** | −0.56** |
| Superior frontal gyrus | 6 | 19 | R | 22 | 9 | 63 | −0.50** | −0.29** | N/A | −0.29** | −0.37** | −0.37** | −0.49** |
| Average MDD severity | |||||||||||||
| Dorsolateral prefrontal cortex | 46 | 19 | L | −40 | 45 | 15 | −0.47** | −0.23 | −0.43** | N/A | −0.43** | −0.47** | −0.27 |
| Superior frontal gyrus | 6 | 19 | R | 26 | 6 | 60 | −0.52** | −0.17 | −0.37** | N/A | −0.54** | −0.51** | −0.31* |
| Current depression | |||||||||||||
| CDI-parent | |||||||||||||
| Middle temporal gyrus | 21 | 19 | R | 62 | −51 | −3 | −0.56** | −0.55** | −0.56** | −0.57** | N/A | −0.55** | −0.56** |
| Medial frontal gyrus | 8/9 | 48 | L | −2 | 45 | 33 | −0.52** | −0.52** | −0.51** | −0.48** | N/A | −0.45** | −0.47** |
| Inferior frontal gyrus | 9/44 | 31 | R | 32 | 18 | 27 | −0.55** | −0.55** | −0.55** | −0.50** | N/A | −0.57** | −0.50** |
| Cingulate | 24/30 | 119 | L | −14 | 21 | 30 | −0.55** | −0.56** | −0.54** | −0.49** | N/A | −0.49** | −0.45** |
| CDI-child | |||||||||||||
| Amygdala/hippocampus | 21 | 10 | L | −21 | −18 | −14 | 0.48** | 0.51** | 0.49** | 0.47** | 0.49** | N/A | 0.47** |
R values for MDD vs. control groups represent point-biserial correlation coefficients comparing group status as a dichotomous variable with brain activity.
p < 0.05.
p < 0.001.
3.4. Individual differences in history of depression severity
To assess the effect of depression severity, we computed whole-brain, voxel-wise correlations of activity during sad mood elaboration with MDD severity as assessed by the PAPA at the initial pre-school age assessment (T1 MDD) and with MDD severity averaged across the PAPA/CAPA interviews between the ages of 3 and 11 years (average MDD). These analyses included both the control and the PO-MDD children. As shown in Table 2 (scatterplots of data provided in Supplemental Materials), we found significant negative correlations between brain activity and T1 MDD severity in left visual (BA 18) and right superior frontal (BA 6) cortex. These correlations remained significant after controlling for current depression severity with either CDI-P or CDI-C scores, for cumulative history of MDD severity, or for a history of internalizing and externalizing symptoms (see Table 2). Furthermore, these correlations remained significant after controlling for group status (MDD vs. Control), suggesting that the initial severity of T1 MDD predicted current brain activity beyond differences due to diagnostic status.
We also found significant negative correlations between brain activity and average MDD severity in left dorsolateral PFC and right superior frontal gyrus (see Table 2) similar to those found in the categorical group comparisons described above, though not overlapping with these ROIs. The correlation in the right superior frontal cluster remained significant when controlling for either depression at the time of scan or for a history of internalizing or externalizing disorders (Table 2). The correlation found in the BA 46 region remained significant when controlling for current depression, but not when variance due to internalizing and externalizing symptoms was partialled out. Furthermore, neither of these correlations remained significant after controlling for group status, suggesting that they reflect underlying diagnostic group differences or correlated internalizing and externalizing symptomology in the case of the BA 46 region rather than relations with individual differences in depression severity.
3.5. Individual differences in current depression severity
To assess the effects of depression severity at the time of scan, we correlated functional brain activity during sad mood elaboration with scores on the parent and child versions of the CDI. We found significant negative correlations between brain activity and CDI-P scores at the time of scan in regions of the middle temporal gyrus, medial frontal cortex, inferior frontal gyrus, and the anterior cingulate cortex (see Table 2, Fig. 3, and scatterplots in Supplemental Materials). All of these correlations remained significant after controlling for diagnostic group status, T1 MDD severity, average MDD severity and internalizing and externalizing psychopathology. In addition, we found a significant positive correlation between brain activity and depression severity on the CDI-C in the amygdala/hippocampal complex (see Table 2 and Fig. 3). This correlation also remained significant after controlling for diagnostic group status, T1 MDD severity, average MDD severity and internalizing and externalizing psychopathology.
4. Discussion
A growing body of research has documented dysfunction in the processing and regulation of emotion in adult and adolescent depression, particularly in limbic regions, such as the amygdala, and in the prefrontal cortex (Brody et al., 2001, Davidson et al., 2003, Johnstone et al., 2007, Keedwell et al., 2005, Phillips et al., 2003, Sheline et al., 2009). Given this, the present study sought to investigate whether such alterations could also be identified at even earlier points in development, specifically in children who experienced an episode of depression during the preschool period. The sad mood elaboration paradigm induced a negative mood in children and called for them to actively engage with this mood by elaborating on their sad affect. As in previous studies using this task (Cooney et al., 2007, Furman et al., 2010), we found increased activity during the elaboration period as compared to the baseline period in limbic regions, like the parahippocampal gyrus, as well as prefrontal cortex regions. As discussed in more detail below, several altered patterns of brain activity were observed when comparing children with symptoms of MDD to healthy children during this induction and elaboration task.
Comparing children with a history of PO-MDD to children with no history of psychopathology, we identified two regions of PFC that showed differential activity during sad mood elaboration. Both regions were significantly less active for those with a history of PO-MDD than for healthy children. Two similar but smaller regions were found to correlate negatively with a cumulative history of depression. Importantly, previous work has shown increased activity during a negative mood induction in healthy individuals in a BA 46 region similar to that isolated by the group comparison in the present study (Goldin et al., 2008). Other research has highlighted a similar BA 6 region as a positive mediator in the relation between ventrolateral PFC and success in regulating negative emotions (Wager et al., 2008). The diagnostic group effects in both the BA 6 and BA 46 regions remained significant when controlling for current depression or cumulative history of internalizing and externalizing psychopathology. Further, the relation between activity in BA 6 and cumulative history of depression remained significant when controlling history of internalizing and externalizing disorders, although the correlation with BA 46 did not. These results suggest that the findings of reduced activity in BA 6 during sad mood elaboration are specific to the cumulative history MDD. These findings are consistent with other imaging findings from the same study population suggesting an association between an episode of MDD experienced prior to the age of 6 and alterations in brain structure and function even after controlling for past and current co-morbidity and current depression. As described in the introduction, depression severity in PO-MDD has been related to amygdala reactivity while viewing faces expressing negative emotion (Barch et al., submitted, Gaffrey et al., 2011b). Children with PO-MDD also displayed decreased functional connectivity between the amygdala and prefrontal cortex as well as other limbic regions (Luking et al., 2011). Additionally, these children displayed disrupted default mode network connectivity (Gaffrey et al., submitted) and decreased connectivity between the subgenual cingulate and prefrontal regions (Gaffrey et al., 2010). Taken together with these prior results, the current findings indicate disruptions in affective neural circuits related to depression during the preschool period.
In addition to the effects of PO-MDD, depressive severity at the time of scan was negatively correlated with activity during sad mood elaboration in a more medial PFC region and a more inferior PFC region, as well as in middle temporal gyrus and the anterior cingulate. Interestingly, these associations remained significant even when controlling for diagnostic group status and history of depression, suggesting that they were not secondary to illness history and were present in both healthy controls and children with a history of PO-MDD. Examining emotion regulation in healthy participants, Ochsner et al. (2004b), identified increased activity in similar medial frontal and cingulate regions during upregulation of negative emotions. Furthermore, greater depression severity as reported by the child at the time of the scan was associated with increased amygdala activity during sad mood elaboration. Again, this correlation remained significant even after controlling for diagnostic group status and history of internalizing and externalizing psychopathology.
Taken together, these results point to a dysfunctional hypoactivity in neural regions involved in upregulating or elaborating on negative emotions in children with current depressive symptoms and in children with a history of PO-MDD. These results might reflect one of two possibilities. First, it is possible that children with greater depression severity at the time of the scan engaged more readily in negative mood elaboration and, therefore, did not need to recruit these regions as much as less depressed children. The positive correlation between activity in the amygdala/hippocampus and scores on the CDI-C may also be consistent with this hypothesis, potentially indicating a greater negative affective response to the sad mood induction/elaboration. It is important to note, however, that there were no significant correlations between depression severity at the time of scan assessed by the parent or child CDI and changes in mood ratings. Alternatively, these associations may reflect dysfunction of mood-regulation regions that are associated with depression. Such findings would be consistent with the literature on adult MDD documenting altered activity in prefrontal regions during the regulation of emotion. For example, several studies have reported general hypoactivity in PFC associated with depression (Baxter et al., 1989, Bench et al., 1993, Drevets, 1999, Siegle et al., 2007). Research examining emotion regulation in depression has documented alterations in PFC activity and in top-down prefrontal-limbic coupling (Johnstone et al., 2007, Sheline et al., 2009, Erk et al., 2010). Consequently, it is possible that our results reflect similar dysfunction in top-down control of emotion in a sample of children with PO-MDD. The negative correlation between historical depression severity and activity in PFC could thus indicate that increasing MDD symptomology is related to decreasing ability to effectively exert cognitive or prefrontal control over one's emotions. This is the first work documenting changes in neural activity in school-age children with a history of PO-MDD during emotion regulation allowing us to observe anomalous activity patterns similar to what is observed in adolescent and adult MDD. This and other work described above in this young sample with a history of PO-MDD may suggest similarities in the mechanism underlying emotional dysfunction in depression.
We should note several limitations of the current study. First, the nature of this task made it difficult to parse the effects of the film induction from the effects of active elaboration. Thus, it is difficult to differentiate enhanced limbic responsivity to the induction film clip from disrupted mood regulation circuitry during the active elaboration. Although the current results could represent an effect of either portion of the task, it is likely that they indicate an alteration in emotion circuitry elicited by both the negative mood induction and the active elaboration. Further investigation of brain activity following sad mood induction separate from elaboration would help to differentiate anomalies in limbic reactivity in children with PO-MDD from potential dysfunction in top-down emotional control during active elaboration. Second, the children were not given any explicit instructions as to what type of strategy to use during the elaboration phase. Previous research comparing the use of reappraisal and distraction to down-regulate negative affect has demonstrated differential activation in various regions of the PFC (Goldin et al., 2008) and in limbic regions (McRae et al., 2010). In a comparison of self-focused and situation-focused strategies for upregulating negative emotions, Ochsner et al. (2004a) found differences in activation mainly in the middle temporal gyrus. While all participants in the current study were given the same elaboration prompts, which hopefully minimized the different strategies being used, a more directed examination of elaboration strategies is necessary to explore the possible effects of strategy on emotion regulation in pre-school onset MDD. Third, by necessity, the emotion regulation scan always came after the baseline scan raising the possibility of order or time on task effects. Though it is possible that these effects would account for some activation in the main effect of elaboration across group, we do not expect the categorical or dimensional analyses to be preferentially biased by order or time on task. Hopefully, this can be further controlled in future studies. Fourth, we collected only one relatively short run of data for fixation and for baseline. As such, it is possible that further relationships to depression would be found if more data were collected, given that this would likely further increase power to detect effects.
In summary, the current results add to mounting evidence for dysfunction in neural systems supporting emotion processing and regulation in depression by documenting alterations in both prefrontal and limbic brain activity in 7–11 year-old children during elaboration of sad mood. Notably, a history of MDD occurring during the pre-school period correlated with hypoactivity in PFC regions previously associated with emotional control, while child-reported depressive symptomology at the time of scan correlated with limbic overactivity. These results clearly suggest a link between an episode of preschool depression and altered brain activity associated with sad mood elaboration in later childhood. However, we cannot determine with the current data the causal nature of the relation between alterations in brain activity relating to sad mood elaboration and PO-MDD. Specifically, such alterations may precede the onset of depression and, potentially, play a role in the development of depression or serve as a marker of risk for the development of depression. Alternatively, these anomalies may represent the lasting effects of early experiences of mood dysregulation. Regardless of which etiology is present, our findings underscore the need for further investigations of earlier developmental processes that are associated with alterations in affective neurocircuitry in younger depressed or at-risk populations. Further prospective research investigating the causal role that neurobiological changes play in PO-MDD is also indicated and may provide key insights into understanding the developmental psychopathology of MDD.
Conflict of interest statement
The authors have no financial interest(s) or conflicts to disclose.
Acknowledgements
Grant numbers MH64769 (JL) and MH090786 (JL, DB, KB) from the National Institute of Mental Health funded the current study. Dr. Belden's work on this manuscript was supported by a grant from the National Institute of Mental Health (1K01MH090515-01). The NIMH had no further role in the design and conduct of the study, collection, management, analysis, and interpretation of data, or preparation, review, or approval of the manuscript. Author DP had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. We would like to thank all participants and their families that provided time and effort to making this study possible.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.11.008.
Appendix A. Supplementary data
References
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Angold A.E., Costello J. The Child and Adolescent Psychiatric Assessment (CAPA) J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Baxter L.R., Jr., Schwartz J.M., Phelps M.E., Mazziotta J.C., Guze B.H., Selin C.E., Gerner R.H., Sumida R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Barch, D.M., Gaffrey, M.S., Botteron, K.N., Belden, A.C., Luby, J.L. Functional brain activation to emotionally valenced faces in school aged children with a history of preschool onset depression, submitted for publication. [DOI] [PMC free article] [PubMed]
- Beauregard M., Paquette V., Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau J.Y., Guyer A.E., McClure-Tone E.B., Monk C.S., Nelson E.E., Fromm S.J., Goldwin M.A., Wittchen H.U., Leibenluft E., Ernst M., Pine D.S. Common and distinct amygdala-function perturbations in depressed vs. anxious adolescents. Arch. Gen. Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench C.J., Friston K.J., Brown R.G., Frackowiak R.S., Dolan R.J. Regional cerebral blood flow in depression measured by positron emission tomography the relationship with clinical dimensions. Psychol. Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Ryan N.D., Williamson D.E., Brent D.A., Kaufman J., Dahl R.E., Perel J., Nelson B. Childhood and adolescent depression: a review of the past 10 years. J. Am. Acad. Child Adolesc. Psychiatry. 1996;36:1325–1326. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Brody A.L., Barsom M.W., Bota R.G., Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin. Clin. Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Somerville L.H. Braking and accelerating of the adolescent brain. J. Res. Adolesc. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cole P., Michael M.K., O’Donnell Teti L. The development of emotion regulation and dysregulation: a clinical perspective. Monogr. Soc. Res. Child Dev. 1994;59:73–100. [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Atlas L.Y., Eugene F., Gotlib I.H. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–1774. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Dawson G., Ashman S.B., Panagiotides H., Hessl D., Self J., Yamada E., Embry L. Preschool outcomes of children of depressed mothers: role of maternal behavior, contextual risk, and children's brain activity. Child Dev. 2003;74:1158–1175. doi: 10.1111/1467-8624.00599. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Prefrontal cortical amygdalar metabolism in major depression. Ann. N.Y. Acad. Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Egger H.L., Ascher B., Angold A. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Duke University Medical Center; Durham, NC: 2003. The Preschool Age Psychiatric Assessment: Version 1.4. [Google Scholar]
- Eisenberg N., Valiente C., Morris A.S., Fabes R.A., Cumberland A., Reiser M., Gershoff E.T., Shepard S.A., Losoya S. Longitudinal relations among parental emotional expressivity, children's regulation and quality of socio-emotional functioning. Dev. Psychol. 2003;39:3–19. doi: 10.1037//0012-1649.39.1.3. [DOI] [PubMed] [Google Scholar]
- Erk S., Mikschl A., Stier S., Ciaramidaro A., Gapp V., Weber B.B., Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. J. Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C., Barch D.M., Rundle M.M., Mintun M.A., Snyder A.Z., Cohen J.D., Mathews J., Sheline Y.I. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol. Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D., Hamilton P., Joormann J., Gotlib I.H. Altered timing of amygdala activation during sad mood elaboration as a function of 5-HTTLPR. Biol. Psychiatry. 2010 doi: 10.1093/scan/nsq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Belden A.C., Luby J.L. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. J. Affect. Disorder. 2011;133:537–545. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Luby J.L., Repovs G., Belden A.C., Botteron K., Luking K., Barch D.M. Subgenual cingulate connectivity in children with a history of preschool-onset depression. Neuroreport. 2010;21:1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Luby J.L., Belden A.C., Hirshberg J.S., Volsch J., Barch D.M. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. J. Affect. Disord. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey, M.S., Luby, J.L., Botteron, K., Repovs, G., Barch, D.M. Default mode network connectivity in children with a history of preschool onset depression, submitted for publication. [DOI] [PMC free article] [PubMed]
- Gazer B., Zieff H. Columbia Pictures; United States: 1991. My Girl [Motion picture] [Google Scholar]
- Goldin P.R., McRae K., Wamel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Hamilton J.P. Neuroimaging and depression: current status and unresolved issues. Curr. Dir. Psychol. Sci. 2008;17:159–163. [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R., Adler A.D., Fromm S.J., Leibenluft E., Pine D.S., Ernst M. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Gotlib I.H. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol. Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K., Burmeister M., Shedden K., Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev. Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Andrew C., Williams S.C., Brammer M.J., Phillips M.L. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol. Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children's depression, inventory (CDI) Psychopharmacol. Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- Luby J.L. Early childhood depression. Am. J. Psychiatry. 2009;166:974–979. doi: 10.1176/appi.ajp.2009.08111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A.C., Pautsch J., Si X., Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J. Affect. Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Heffelfinger A., Mrakotsky C., Brown K., Hessler M., Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch. Gen. Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Mrakotsky C., Heffelfinger A., Brown K., Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am. J. Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Si X., Belden A.C., Tandom M., Spitznagel E. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J. Am. Acad. Child Adolesc. Psychiatry. 2002;42:928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Si X., Belden A.C., Tandom M., Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch. Gen. Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Repovs G., Belden A.C., Gaffrey M.S., Botteron K.N., Luby J.L., Barch D.M. Functional connectivity of the amygdala in early-childhood-onset depression. JAAC. 2011;50:1027–1104. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H.S. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D.E., Gross J.J., Ochsner K.N. The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C., Klein R., Telzer E., Schroth E., Mannuzza S., Moulton J.L., III, Guardino M., Masten C.L., McClure-Tone E.B., Fromm S., Blair R.J., Pine D.S., Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165:90. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Munafo S.M.B., Hariri A.R. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol. Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D., Hanelin J., Ramachandran T., Mackey S. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D.E., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ojemann J.G., Akbudak E., Snyder A.Z., McKinstry R.C., Raichle M.E., Conturo T.E. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Peluso M.A., Glahn D.C., Matsuo K., Monkul E.S., Najt P., Zamarripa F., Li J., Lancaster J.L., Fox P.T., Gao J.H., Soares J.C. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res.: Neuroimaging. 2009;173:158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D., Pascual-Marqui R.D., Nitschke J.B., Oakes T.R., Larson C.L., Abercrombie H.C., Schaefer S.M., Koger J.V., Benca R.M., Davidson R.J. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am. J. Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Risch N., Herrell R., Lehner T., Liang K.Y., Eaves L., Hoh J., Griem A., Kovacs M., Ott J., Merikangas K.R. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R., McClure E.B., Monk C.S., Nelson E.E., Guyer A.E., Fromm S.J., Charney D.S., Leibenluft E., Blair J., Ernst M., Pine D.S. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol. Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Donnelly J.M., Ollinger J.M., Snyder A.Z., Mintun M.A. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry. 2001;9:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G., Thompson W., Carter C., Steinhauer S., Thase M. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol. Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Stifter C.A., Spinrad T., Braungart-Rieker J. Toward a developmental model of child compliance: the role of emotion regulation in infancy. Child Dev. 1999;70:21–32. doi: 10.1111/1467-8624.00003. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; Stuttgart: 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Thomas K.M., Drevets W.C., Dahl R.E., Ryan N.D., Birmaher B., Eccard C.H., Axelson D., Whalen P.J., Casey B.J. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Victor T., Furey M.L., Fromm S.J., Ohman A., Drevets W.C. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch. Gen. Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, B.D., 2000. AlphaSim. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Yang T.T., Simmons A.N., Matthews S.C., Tapert S.F., Frank G.K., Max J.E., Bischoff-Grethe A., Lansong A.E., Brown G., Strigo I.A., Wu J., Paulus M.P. Adolescents with major depression demonstrate increased amygdala activation. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.