Abstract
Context
Although drug-eluting stents reduce restenosis rates relative to bare-metal stents, concerns have been raised that drug-eluting stents may also be associated with an increased risk of stent thrombosis. Our study focused on the effect of stent type on population-based interventional outcomes.
Objective
To compare outcomes of Medicare beneficiaries who underwent non-emergent coronary stenting before and after the availability of drug-eluting stents.
Design, Setting, and Patients
Observational study of 38 917 Medicare patients who underwent nonemergent coronary stenting from October 2002 through March 2003 when only bare-metal stents were available (bare-metal stent era cohort) and 28 086 similar patients who underwent coronary stenting from September through December 2003, when 61.5% of patients received a drug-eluting stent and 38.5% received a bare-metal stent (drug-eluting stent era cohort). Follow-up data were available through December 31, 2005.
Main Outcome Measures
Coronary revascularization (percutaneous coronary intervention, coronary artery bypass surgery), ST-elevation myocardial infarction, survival through 2 years of follow-up.
Results
Relative to the bare-metal stent era, patients treated in the drug-eluting stent era had lower 2-year risks for repeat percutaneous coronary interventions (17.1% vs 20.0%, P<.001) and coronary artery bypass surgery (2.7% vs 4.2%, P<.01). The difference in need for repeat revascularization procedures between these 2 eras remained significant after risk adjustment (hazard ratio, 0.82; 95% confidence interval, 0.79-0.85). There was no difference in unadjusted mortality risks at 2 years (8.4% vs 8.4%, P=.98), but a small decrease in ST-elevation myocardial infarction existed (2.4% vs 2.0%, P<.001). The adjusted hazard of death or ST-elevation myocardial infarction at 2 years was similar (hazard ratio, 0.96; 95% confidence interval, 0.92-1.01).
Conclusion
The widespread adoption of drug-eluting stents into routine practice was associated with a decline in the need for repeat revascularization procedures and had similar 2-year risks for death or ST-elevation myocardial infarction to bare-metal stents.
There has been a growing concern about the possibility of an increased risk of stent thrombosis (ST) associated with the use of drug-eluting stents compared with bare-metal stents. Although a relatively rare event, stent thrombosis is associated with a high risk of myocardial infarction (MI) and death1-9 and any suggestion of possible excess risk has justifiably been the focus of intense investigation. Although the initial US Food and Drug Administration (FDA) public health warning raised the specter of an increased risk of early (within 30 days), subacute thrombosis,10 trial-level meta-analyses raised concerns about an increased risk of late thrombosis (>30 days).11,12 Despite several recent studies, including patient-level meta-analyses8,13-15 and large registry reports,16-21 there remains uncertainty about the trade-offs between the safety and effectiveness of drug-eluting stents.22-26
Published studies have focused on comparing samples of patients receiving drug-eluting stents with those receiving bare-metal stents. Another important perspective on the safety and effectiveness of drug-eluting stents could be obtained by assessing how the rapid adoption of drug-eluting stent use affected the health of the entire stented population. That is, given the choices physicians and patients are making about who receives a drug-eluting stent and who receives a bare-metal stent, what are the outcomes compared with the era when bare-metal stents were the only option.
To address this question, we performed a population-based study of all Medicare-eligible patients aged 65 years or older who received a nonemergent coronary stent. Rates of subsequent revascularization, MI, and survival were compared for cohorts of patients before and after the availability of drug-eluting stents. We hypothesized that a decreased rate of restenosis and subsequent revascularization, with their attendant risks, could more than compensate for a small increased risk of stent thrombosis from drug-eluting stents.9,27,28
METHODS
Data
We used a 100% national sample of all Part A hospital claims submitted to the Centers for Medicare & Medicaid Services for the 2002-2005 period to identify patients 65 years or older enrolled in traditional, fee-for-service Medicare programs who had received a coronary stent. The Part A data contain a record for each Medicare hospitalization that includes unique identifiers for the hospital and patient, the dates of admission and discharge, an admitting diagnosis and priority, procedures performed, and additional diagnoses representing comorbid conditions, among other information. Procedures and diagnoses are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)convention.29 We used the Medicare Denominator File to identify which patients were enrolled in the fee-for-service program and their vital status throughout the period of follow-up. The study was approved by the Institutional Review Board of the Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
The Cohort
Patients undergoing a percutaneous coronary intervention (PCI) with stent placement were identified by the presence of a hospital claim for a bare-metal stent (ICD-9-CM code 36.06), drug-eluting stent (ICD-9-CM code 36.07), or both. For the analysis, patients coded as having placement of both types of stents during their first PCI hospitalization were classified as having a drug-eluting stent. Using the unique patient identifier, all claims for each patient were then linked and put in chronological order to create patient histories. We used exclusion criteria based on the Stent Anticoagulation Restenosis Trial Study (STARS).30 Patients were excluded if (1) the admission was emergent as noted on the index claim; (2) a diagnosis code for MI (ICD-9-CM codes 410-410.6, 410.8-410.9, 5th digit 0 or 1) was present on the index claim; (3) they were admitted within 7 days of discharge from a prior hospitalization; (4) they were within 1 year of coronary artery bypass (CABG) surgery (ICD-9-CM codes 36.1-36.19) or a prior PCI (ICD-9-CM codes 36.0-36.09); and (5) they had evidence of bypass graft disease on their index claim (ICD-9-CM codes 414.02-414.05, 996.72) to eliminate patients who might have had an intervention on a bypass graft rather than on a native coronary artery. Patients were eligible for study if they were enrolled in Medicare for at least a month before undergoing a PCI with stenting to allow for censoring based on entry criteria.
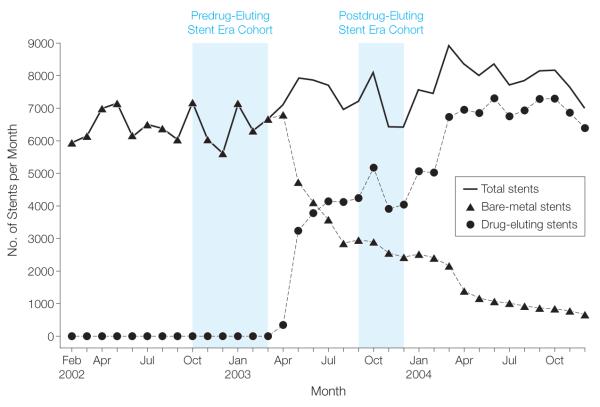
Because of concerns about the selective use of the CYPHER, a sirolimus-elutingstent (Cordis Corp, Miami Lakes, Florida) shortly after it had received FDA approval on April 24, 2003, we created adrug-eluting stent era cohort involving patients undergoing stent placement from September 1 through December 31, 2003, by which time drug-eluting stent use had stabilized (Figure 1). This predated FDA approval in March 2004 of the second-to-market TAXUS, a paclitaxel-eluting stent (Boston Scientific, Natick, Massachusetts). For the comparison to the drug-eluting stent era experience, we created a historical cohort of patients undergoing bare-metal stent placement between October 1, 2002, and March 31, 2003, the time frame immediately before the introduction of the first drug-eluting stent. By comparison with the drug-eluting stent era, we extended the period of enrollment for the bare-metal stent era by 2 months to improve the power of our analysis. All stent procedures from April 2003 were excluded to avoid assignment error during the introduction of a new procedure code.
Figure 1.
Numbers of Medicare Enrollees Undergoing Coronary Stenting, February 2002 through December 2004
Comorbid Conditions
The Part A claim includes age, sex, and race, which is classified according to the Social Security Administration enrollment; and up to 10 medical diagnoses. Using information from the index admission, we identified the following comorbid conditions as defined by Romano et al31: history of MI, congestive heart failure, peripheral vascular disease, pulmonary disease, diabetes without complications, diabetes with complications, mild liver disease, moderate or severe liver disease, dementia, renal disease, nonmetastatic cancer, and metastatic solid tumor.
Outcomes
Our measure of effectiveness was the rate of repeat coronary revascularization, defined as any PCI, whether or not the patient received a stent (ICD-9-CM codes 36.0-36.09), or crossed over to CABG (ICD-9-CM codes 36.1-36.19). Only the first subsequent revascularization was counted for each patient. Outcomes of interest were death and ST-elevation MI (STEMI), which would include, but not be limited to, the sequelae of stent thrombosis. Death was obtained from the Medicare Denominator File. STEMI was based on the presence of specific codes on a Part A claim (ICD-9-CM codes 410-410.6, 410.8-410.9, 5th digit 0 or 1). To avoid confusing a technical complication of the procedure with an adverse outcome secondary to subacute thrombosis, only patients who survived for at least a day following their procedure were included in the analysis and the outcomes of CABG and repeat PCI were assessed starting on the day after the procedure. Because we could not distinguish STEMI as the indication for the procedure vs STEMI as a procedural outcome, patients with a STEMI coded on their index admission were excluded from the analysis. Event-free survival is defined as survival absent a STEMI. Follow-up data through December 31, 2005, were available, ensuring complete ascertainment of event rates to 2 years for all patients.
Analysis
Differences in baseline patient characteristics between the the 2 cohorts were assessed using a χ2 test for categorical variables and a t test for continuous variables. Crude risks of outcomes were compared using a χ2 test. Crude rates of outcomes were plotted using Nelson-Aelen cumulative hazard estimators.32 The hazard distributions were compared using the log-rank test.33
We used propensity scoring to control for any differences between the population of patients undergoing coronary stenting during the bare-metal stent era, whose only option for stenting was a bare-metal stent and those receiving stents during the drug-eluting stent era, when patients could receive either a bare-metal stent or drug-eluting stent at the discretion of the interventionalist.34 The propensity score, accounting for differences in age, sex, race (including interactions of age and sex), and comorbid conditions (Table 1), was calculated using logistic regression with cohort membership as the dependent variable. Fit of the propensity score model was assessed using the C statistic, a measure of the area under the receiver operating characteristic curve. The C statistic from the propensity score weighting logistic regression model was 0.51, indicating that the 2 samples were similar in terms of attributes. (A randomized trial would yield a C statistic of 0.50.)
Table 1.
Patient Characteristics for Bare-Metal Stent vs Drug-Eluting Stent Era
| No. (%) |
P Value |
||
|---|---|---|---|
| Bare-Metal Stent Era (n = 38 917) |
Drug-Eluting Stent Era (n = 28 086) |
||
| Drug-eluting stent | 0 (0.0) | 17 273 (61.5) | <.001 |
| Age, mean (SD), y | 74.7 (5.89) | 74.6 (5.84) | .01 |
| Women | 15 917 (40.9) | 11 656 (41.5) | .07 |
| African American | 4320 (11.1) | 3174 (11.3) | .51 |
| History of MI | 4047 (10.4) | 2949 (10.5) | .82 |
| Congestive heart failure | 3347 (8.6) | 2331 (8.3) | .29 |
| Vascular disease | 4553 (11.7) | 3342 (11.9) | .48 |
| Pulmonary disease | 5059 (13.0) | 3764 (13.4) | .20 |
| Dementia | 117 (0.3) | 112 (0.4) | .02 |
| Diabetes | 9807 (25.2) | 7330 (26.1) | .009 |
| Liver disease | 39 (0.1) | 28 (0.1) | .97 |
| Renal disease | 156 (0.4) | 84 (0.3) | .001 |
| Cancer | 1635 (4.2) | 1123 (4.0) | .18 |
| Multivessel PCI | 6995 (18.0) | 5046 (18.0) | .83 |
Abbreviation: MI, myocardial infarction; PCI, percutaneous coronary intervention.
We estimated adjusted survival weighting by the propensity score and expressed differences in survival between the 2 cohorts as a hazard ratio (HR) with 95% confidence intervals (95% CIs). We repeated the propensity score adjustment using both nearest neighbor matching (with random matching for ties) and stratification matching, using the “pscore” command in STATA 10.0.35 We also compared outcomes between the 2 era cohorts using direct adjustment for risk adjusters, as suggested by Hirano and Imbens.34 Because all results were similar, we only report adjusted HRs based on the propensity score–weighted data.
To determine whether there was an increased risk of death or STEMI for patients who stopped taking their thienopyridine after stent placement, we performed a landmark analysis.18 This analysis examined the adverse event rates for patients who had not died or had a STEMI during the first 3 months following stent placement, the recommended duration of dual antiplatelet therapy in 2003 for patients receiving a sirolimus-eluting stent.
Data were managed using SAS software, version 9.1.3 (SAS Institute Inc, Cary, North Carolina) while statistical testing, including survival analysis and the generation of output, was performed using Stata statistical software, release 10.0 (Stata Corp; College Station, Texas). This study was approved by the Committee for the Protection of Human Subjects of Dartmouth College and with their approval, consent and authorization were waived per 45 Code of Federal Regulations 46.116(d) and 45 Code of Federal Regulations 164.512(1) (2)(ii), respectively.
RESULTS
Prior to April 2003, only bare-metal stents were placed (Figure 1), but within 6 months of FDA approval of the sirolimus-eluting stent, more than 60% of the stents placed in the Medicare population were drug-eluting stents. By June 2004, about 3 months after the paclitaxel-eluting stent had received FDA approval, close to 90% of patients were receiving a drug-eluting stent. From October 2002 to March 2003, 38 917 patients received at least 1 coronary stent, all of which were bare metal stents, and these patients represent the bare-metal stent era cohort. From September 2003 to December 2003, 28 086 patients received at least 1 coronary stent. Of those, 17 275 (61.5%) received a drug-eluting stent and 10 811 (38.5%) received a bare-metal stent, and these patients represent the drug-eluting stent era cohort.
Patient Characteristics
On average, patients who received stents were in their mid 70s, 41% were women, 11% were African American, 10% had a history of MI, 8% had congestive heart failure, 12% had vascular disease, 13% had pulmonary disease, 25% had diabetes, and 4% had cancer (Table 1). There were few observable differences in patient characteristics between the 2 cohort eras with the exception that slightly more patients in the drug-eluting stent era had diabetes. In each era cohort, 18.0% of patients had undergone a multivessel PCI.
Revascularization
During the 2 years of observation, 22.8% of patients in the bare-metal stent era cohort underwent a repeat revascularization (20.0%, PCI; 4.2%, CABG, not mutually exclusive) with an average time to revascularization of 228.7 days. In the drug-eluting stent era cohort, 19.0% of patients underwent a repeat revascularization (17.1% PCI; 2.7% CABG, not mutually exclusive) at an average of 214.8 days.
The unadjusted risks of repeat PCI were significantly lower (P<.001) in the drug-eluting stent than in the bare-metal stent era cohorts: 9.7% vs 10.8% at 6 months; 13.1% vs 15.2% at 12 months, and 17.1% vs 20.0% at 24 months (Table 2). Risks for CABG following stent placement were significantly (P<.001) higher in the bare-metal stent era than in the drug-eluting stent era cohorts: 0.7% vs 0.5% at 3 months, 1.9% vs 1.0% at 6 months, 2.9% vs 1.7% at 12 months, and 4.2% vs 2.7% at 24 months.
Table 2.
Risks and Hazard Ratios of Repeat Percutaneous Coronary Intervention and Coronary Artery Bypass Graft Surgery for Drug-Eluting Stent vs Bare-Metal Stent Eras
| Outcome | No. (%) |
P Value |
Hazard Ratios (95% Confidence Interval) of Drug-Eluting Stent vs Bare-Metal Stent |
||
|---|---|---|---|---|---|
| Bare-Metal Stent Era (n = 38 917) |
Drug-Eluting Stent Era (n = 28 086) |
Crude | Adjusteda | ||
| PCI, mo 3 |
2569 (6.5) | 1939 (6.8) | .05 | 1.06 (1.00-1.13) | 1.06 (1.00-1.13) |
| 6 | 4243 (10.8) | 2753 (9.7) | <.001 | 0.90 (0.86-0.94) | 0.90 (0.86-0.94) |
| 12 | 5917 (15.2) | 3681 (13.1) | <.001 | 0.86 (0.83-0.90) | 0.86 (0.83-0.90) |
| 24 | 7785 (20.0) | 4804 (17.1) | <.001 | 0.85 (0.82-0.88) | 0.85 (0.82-0.88) |
| CABG surgery, mo 3 |
272 (0.7) | 140 (0.5) | <.001 | 0.66 (0.53-0.81) | 0.66 (0.53-0.81) |
| 6 | 740 (1.9) | 281 (1.0) | <.001 | 0.54 (0.47-0.62) | 0.54 (0.47-0.62) |
| 12 | 1129 (2.9) | 478 (1.7) | <.001 | 0.57 (0.51-0.64) | 0.57 (0.51-0.63) |
| 24 | 1635 (4.2) | 759 (2.7) | <.001 | 0.64 (0.58-0.69) | 0.63 (0.58-0.69) |
| PCI or CABG surgery, mo 3 |
2803 (7.0) | 2051 (7.2) | .40 | 1.03 (0.97-1.09) | 1.03 (0.97-1.09) |
| 6 | 4827 (12.3) | 2978 (10.5) | <.001 | 0.85 (0.82-0.90) | 0.85 (0.82-0.89) |
| 12 | 6734 (17.3) | 4046 (14.4) | <.001 | 0.82 (0.79-0.86) | 0.82 (0.79-0.86) |
| 24 | 8875 (22.8) | 5338 (19.0) | <.001 | 0.82 (0.79-0.85) | 0.82 (0.79-0.85) |
Abbreviations: CABG, coronary artrery bypass graft, PCI, percutaneous coronary intervention.
Adjusted for age, sex, race, history of myocardial infarction, congestive heart failure, vascular disease, pulmonary disease, dementia, diabetes, liver disease, kidney disease, cancer.
There were small differences in the indications for repeat revascularization between the 2 eras (P=.001) with fewer repeat revascularizations associated with STEMI (3.4% drug-eluting vs 3.9% bare-metal stent era) and with non-STEMI (4.3% drug-eluting vs 5.6% bare-metal stent era) during the drug-eluting stent era and more revascularizations associated with chronic stable angina or other symptoms (92.3% drug-eluting stent vs 90.6% bare-metal stent era).
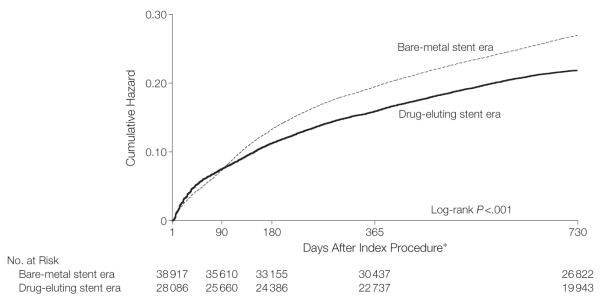
Figure 2 shows unadjusted estimates of the cumulative hazard of any repeat revascularization through 2 years of follow-up by which time the absolute benefit associated with the drug-eluting stent era was a 5.1% lower risk of repeat revascularization (Table 2, HR, 0.82; 95% CI, 0.79-0.85). After adjusting for differences in case mix, there remained a significantly lower cumulative hazard of revascularization at 2 years in the drug-eluting stent era than in the bare-metal stent era (Table 2, HR, 0.82; 95% CI, 0.79-0.85).
Figure 2.
Unadjusted Cumulative Hazard of Repeat Revascularization for Patients Undergoing Coronary Stenting in the Bare-Metal Stent vs Drug-Eluting Stent Eras
*Analysis criteria required that the patient had to be event free for at least 1 day from receiving the index stent; day 0 was the day of the index procedure.
STEMI and Death
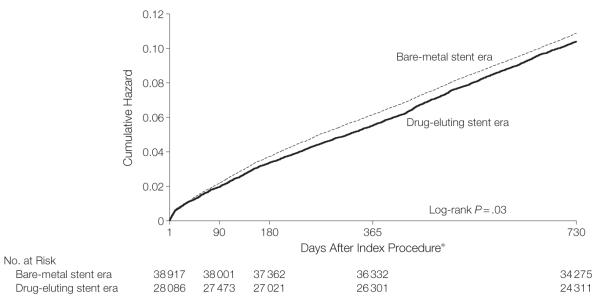
There were no significant differences in crude mortality (Table 3) in the drug-eluting stent than in the bare-metal stent cohort eras: 1.4% vs 1.4% at 3 months, 2.5% vs 2.5% at 6 months, 4.3% vs 4.5% at 12 months, and 8.4% vs 8.4% at 24 months of follow-up. Crude risks of subsequent STEMIs were significantly (P=.02) lower in the drug-eluting stent vs the bare-metal stent cohort eras: 0.7% vs 0.9% at 3 months, 1.0% vs 1.3% at 6 months, 1.4% vs 1.8% at 12 months, and 2.0% vs 2.4% at 24 months of follow-up, although the absolute magnitude of this benefit at 2 years was small (0.4%). The lower risk of STEMI was responsible for the combined end point of death or STEMI being lower in the drug-eluting stent era as well. Figure 3 shows unadjusted estimates of the cumulative hazard of death or STEMI through 2 years of follow-up, by which time there were slightly fewer adverse events in the drug-eluting stent era cohort. Although the unadjusted hazard curves were statistically different (P=.03), the adjusted hazard of death or STEMI at 2 years (Table 3; HR, 0.96; 95% CI, 0.92-1.01) indicated there was no difference in these outcomes between the 2 eras.
Table 3.
Crude Risks and Hazard Ratios of Death and STEMI for Bare-Metal Stent vs Drug-Eluting Stent Eras
| Outcome | No. (%) |
P Value |
Hazard Ratios (95% Confidence Interval) of Drug-Eluting Stent vs Bare-Metal Stent |
||
|---|---|---|---|---|---|
| Bare-Metal Stent Era (n = 38 917) |
Drug-Eluting Stent Era (n = 28 086) |
Crude | Adjusteda | ||
| Death, mo 3 |
545 (1.4) | 393 (1.4) | .57 | 0.96 (0.84-1.09) | 0.97 (0.85-1.11) |
| 6 | 973 (2.5) | 702 (2.5) | .98 | 1.00 (0.90-1.10) | 1.01 (0.92-1.11) |
| 12 | 1751 (4.5) | 1208 (4.3) | .22 | 0.96 (0.89-1.03) | 0.97 (0.91-1.05) |
| 24 | 3269 (8.4) | 2359 (8.4) | .98 | 1.01 (0.96-1.06) | 1.02 (0.97-1.07) |
| STEMI, mo 3 |
350 (0.9) | 197 (0.7) | .02 | 0.81 (0.68-0.97) | 0.81 (0.68-0.97) |
| 6 | 506 (1.3) | 281 (1.0) | <.001 | 0.75 (0.65-0.86) | 0.75 (0.65-0.86) |
| 12 | 701 (1.8) | 393 (1.4) | <.001 | 0.77 (0.68-0.87) | 0.77 (0.68-0.87) |
| 24 | 934 (2.4) | 562 (2.0) | <.001 | 0.82 (0.74-0.92) | 0.82 (0.74-0.92) |
| Death or STEMI, mo 3 |
856 (2.2) | 534 (1.9) | .06 | 0.90 (0.81-1.00) | 0.91 (0.82-1.01) |
| 6 | 1401 (3.6) | 927 (3.3) | .02 | 0.90 (0.83-0.98) | 0.91 (0.84-0.99) |
| 12 | 2296 (5.9) | 1489 (5.3) | .001 | 0.90 (0.84-0.96) | 0.91 (0.85-0.97) |
| 24 | 3931 (10.1) | 2696 (9.6) | .04 | 0.95 (0.91-1.00) | 0.96 (0.92-1.01) |
Abbreviation: STEMI, ST-elevated myocardial infarction.
Adjusted for age, sex, race, history myocardial infarction, congestive heart failure, vascular disease, pulmonary disease, dementia, diabetes, liver disease, kidney disease, cancer.
Figure 3.
Unadjusted Cumulative Hazard of Death or ST-Elevation Myocardial Infarction for Patients Undergoing Coronary Stenting in the Bare-Metal Stent vs Drug-Eluting Stent Eras
*Analysis criteria required that the patient had to be event free for at least 1 day from receiving the index stent; day 0 was the day of the index procedure.
Subgroup Analysis
Results of subgroup analyses were similar to those from the analysis of the overall cohort. The adjusted hazard of repeat revascularization through 2 years of follow-up was significantly lower in the drug-eluting stent era cohort than in the bare-metal stent era cohort for all patient subgroups including women (HR, 0.80; 95% CI, 0.76-0.85) and men (HR, 0.83; 95% CI, 0.79-0.86), those 75 years or older (HR, 0.81; 95% CI, 0.77-0.85) and those younger than 75 years (HR, 0.83; 95% CI, 0.79-0.87), African Americans (HR, 0.88; 95% CI, 0.75-1.04) and non-African Americans (HR, 0.81; 95% CI, 0.79-0.84), and those with diabetes (HR, 0.80; 95% CI, 0.76-0.86) and those without diabetes (HR, 0.82; 95% CI, 0.79-0.86).
The adjusted hazard of death or STEMI through 2 years of follow-up was nonsignificantly lower in the drug-eluting stent era cohort than in the bare-metal stent era cohort for women (HR, 0.98: 95% CI, 0.90-1.06) and men (HR, 0.95; 95% CI, 0.89-1.01), those aged 75 years or older (HR, 0.98; 95% CI, 0.92-1.04) and those younger than 75 years (HR, 0.93; 95% CI, 0.86-1.01), African Americans (HR, 0.93; 95% CI, 0.75-1.15) and non-African Americans (HR, 0.96; 95% CI, 0.92-1.01), and those with diabetes (HR, 0.93; 95% CI, 0.85-1.02) and those without diabetes (HR, 0.98; 95% CI, 0.92-1.03).
Landmark Analysis
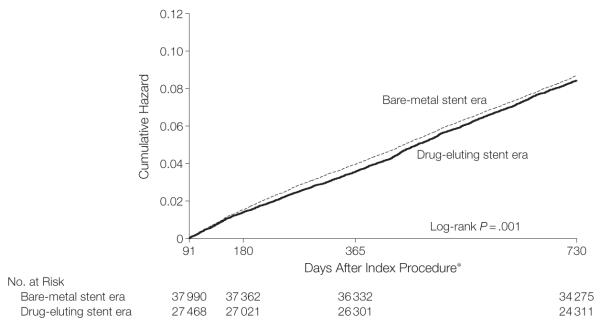
At 3 months after PCI, some proportion of patients receiving a drug-eluting stent would have stopped their dual antiplatelet therapy and would have been exposed to any increased risk associated with a drug-eluting stent that might have been mitigated by aggressive antiplatelet therapy. The landmark analysis (Figure 4) of unadjusted cumulative hazard showed a small decrease in the risk of death or STEMI in the drug-eluting stent era cohort compared with the bare-metal stent era cohort with a log-rank test (P=.001) indicating the shape of the cumulative hazard curves were different. However, the adjusted cumulative hazard by 2 years of follow-up showed that outcomes were comparable (HR, 0.97; 95% CI, 0.92-1.03).
Figure 4.
A Landmark Analysis of the Undjusted Cumulative Hazard of Death or ST-Elevation Myocardial Infarction for Patients Undergoing Coronary Stenting in the Bare-Metal stent vs Drug-Eluting Stent Eras Among Patients Who Were Event-Free for 3 Months Following Their Initial Stent Procedure
*Analysis criteria required that the patient had to be event free for at least 90 days from receiving the index stent.
COMMENT
Determining the magnitude of any increased risk of stent thrombosis associated with the use of drug-eluting stents has proven difficult. The randomized trials were designed to compare the effectiveness of drug-eluting vs bare-metal stents in preventing the rate of restenosis. Attempts to use isolated trial data to detect a significant difference in the lower rate of stent thrombosis have been routinely underpowered, even when data have been aggregated into meta-analyses.15 In addition, the trial experience represents only on-label stent use. A substantial percentage of stents are placed in circumstances for which they have not been FDA-approved,36,37 which means that trial results have limited generalizability.
Observational studies have been better powered to detect a difference in stent thrombosis and better reflect on-label and off-label stent use but are limited by potential biases resulting from unobserved differences in case-mix, severity of illness, and coronary anatomy between drug-eluting and bare-metal stent cohorts. All studies have been challenged by difficulties in attributing the clinical events of death and MI to stent thrombosis when cardiac catheterization data were not available, and up until recently,38 there has been no consistent approach to this issue.
We have assessed the impact of drug-eluting stents on the outcomes of percutaneous coronary revascularization by comparing outcomes for the population of patients undergoing coronary stenting in the drug-eluting stent era with outcomes in the bare-metal stent era. Although such an analysis will not answer the question of what is the true rate of stent thrombosis with drug-eluting stent vs the rate with bare-metal stent, it does address the important question of whether, on-average, the population of stented patients is being helped or hurt by the widespread use of this technology.
Based on the experience of 67 003 Medicare enrollees, we found that following the introduction of drug-eluting stent repeat revascularization rates significantly decreased. The relative risk of repeat revascularization decreased by 16.7% in the drug-eluting stent era compared with the bare-metal stent era. This occurred without any discernable increase in the incidence of death and STEMI, at least some of which would be attributable to stent thrombosis. This was true for all patient subgroups and both shortly after the procedure, when subacute stent thrombosis might occur, and between 4 and 24 months after the procedure, when late stent thrombosis might occur. Contemporaneous with the introduction of drug-eluting stent, there has been a small decrease in the rate of STEMI.
The magnitude of the decrease in repeat revascularization at 2 years that we found in the drug-eluting stent era compared with the bare-metal stent era (3.8%) is smaller than has been reported for clinically driven revascularization rates in the randomized trials (11.8%-20.6%).14,39,40 In part, this is because the trials compared cohorts of patients who received drug-eluting stents only with patients who received bare-metal stents only while our drug-eluting stent era cohort contained a mix of patients, 61.5% who had received a drug-eluting stent and 38.5% who had received a bare-metal stent. It is also a consequence of trials having strict angiographic criteria consistent with the on-label use of stents while in everyday clinical practice the decision about when and where to stent is not under such stringent control. This likely explains why our results are more comparable with those from several observational studies. The Emilia Romagna Italian registry19 of 10 629 patients (28.8% drug-eluting stent) reported an adjusted 2-year incidence of target vessel revascularization of 9.1% for patients receiving drug-eluting stents vs 12.9% for those receiving bare-metal stents (absolute difference, 3.8%; adjusted HR, 0.68; 95% CI, 0.57-0.80). The Swedish registry17 of 19 771 patients (30.5% drug-eluting stent) reported a crude 3-year rate of any repeat revascularization of 15.2% for a drug-eluting stent cohort vs 16.5% for a bare-metal stent cohort (absolute difference, 1.3%; adjusted HR, 0.84; 95% CI, 0.77-0.92). The Ontario registry21 of 3751 matched pairs of patients receiving drug-eluting and bare-metal stents reported 2-year target vessel revascularization rates of 7.4% and 10.7%, respectively (absolute difference, 3.3%; P<.001).
Most recently, the New York State PCI registry reported a target vessel revascularization at 2 years of 11.2% in adrug-eluting stent era cohort vs 17.9% in the bare-metal stent era (adjusted HR, 0.60; 95% CI, 0.56-0.64).41
It is possible that if the incremental hospital reimbursement for the use of drug-eluting vs bare-metal stents did not meet the incremental cost of using drug-eluting stents, particularly for procedures requiring multiple stents, there may have been some incentive in the drug-eluting stent era to stage procedures, which would have resulted in our underestimating the true early benefit of drug-eluting stents on rates of repeat revascularization.
We found no difference in survival between the drug-eluting stent and bare-metal stent era cohorts. A small but statistically significant decrease in the unadjusted rate of STEMI in the drug-eluting stent era cohort began to emerge at 3 months and increased slightly through 2 years of follow-up. This coincided with the lower rate of repeat PCI in the drug-eluting stent era cohort, some of which was presumably target-lesion related and due to restenosis. Because PCI in a stable patient population has not been shown to decrease the risk of STEMI, it is possible that our findings could be attributable to temporal improvements in the overall quality of cardiovascular care, even though the maximum difference in enrollment dates between the bare-metal and drug-eluting stent era cohorts was only 15 months. We recognize that this difference in event rates for STEMI is small, that its statistical significance is driven by our large sample size, and that the differences in death or STEMI were not significant after risk adjustment (Table 3).
The patient-level meta-analyses of randomized trials comparing drug-eluting with bare-metal stents13-15 have not shown a significant difference in the rates of death or STEMI out to 4 years of follow-up, even though an insignificant trend toward a higher event rate existed among patients who received drug-eluting stents in the pooled analysis of the sirolimus stent group.15 The large Italian registry19 reported results similar to ours with 2-year rates of death or STEMI of 12.3% for bare-metal vs 10.9% for drug-eluting stent use (adjusted HR, 0.87; 95% CI, 0.73-1.04). The Ontario study21 reported 2-year rates of death or MI of 10.5% for bare-metal stent vs 9.3% for drug-eluting stent use, a significant difference (P=.02) driven by a lower mortality rate among patients receiving drug-eluting stents (4.3% vs 6.1%) with no difference in MI rates.
The New York State PCI registry41 reported 2-year rates of death or MI of 9.9% in the drug-eluting stent era vs 10.8% in the bare-metal stent era (adjusted HR, 0.90; 95% CI, 0.83-0.97), with no significant difference in survival (adjusted HR, 0.94; 95% CI, 0.84-1.04) but a significant difference in non-fatal MI (adjusted HR, 0.86; 95% CI, 0.76-0.97).
Although the Swedish registry,17 reported no significant difference in the overall incidence of the combined end point between the drug-eluting and bare-metal stent cohorts when the entire 3 years of follow-up were assessed, a significant increased risk of drug-eluting stents did emerge at 6 months when a “landmark analysis” was performed and patients who died or had a STEMI from enrollment to 5 months were removed from the analysis. This was done to investigate any effect that might emerge when dual antiplatelet therapy was stopped, as had been suggested by others.4,18,42 From 6 months to 3 years, drug-eluting stent use was associated with a significantly increased risk of death or STEMI (adjusted HR, 1.20; 95% CI, 1.05-1.37). We performed a landmark analysis of our data, conditioned on the absence of death or STEMI during the first 3 months of observation and assuming that after 3 months patients would no longer be taking their thienopyridine, as was the recommendation for the sirolimus-eluting stent at that time. Unlike the Swedish registry, our landmark analysis (Figure 4) showed no increased risk of death or STEMI in the drug-eluting stent era cohort. Left censoring at 6 months (data not shown) did not change our results. To what extent differences in patient selection, procedural practices, or postprocedure medical management (including the use of antiplatelet agents) explain why the results of our landmark analysis differed from that of the Swedish registry is unknown.
Our study has several limitations. We could not assess the true rate of stent thrombosis associated with the use of drug-eluting vs bare-metal stents because the administrative data does not contain the details of coronary anatomy and procedural process necessary to control for any selection bias in the use of 2 types of stents.17,19 We did not have data on medication use and could not directly assess the influence of dual-antiplatelet therapy on our findings. We did perform a landmark analysis and could detect no increase in adverse event rates in the drug-eluting stent era after 3 months of observation when there was no recommendation for the ongoing use of a thienopyridine agent.
Our results are limited to the experience with the sirolimus-eluting stent, not the paclitaxel-eluting stent. We excluded patients who may have presented with a STEMI. There remains uncertainty as to whether compared with bare-metal stents, drug-eluting stents are associated with improved outcomes in this setting.43-45 Our results are also limited to patients who were at least 65 years (who undergo approximately half of all PCIs) undergoing a first revascularization. Differences between our study cohort and other patient populations with those comorbidities, coronary, lesion, and procedural characteristics predictive of adverse events following a percutaneous intervention, or differences in the use of antiplatelet agents or resistance to them could alter our findings.
Similarly, our data do not permit us to assess to what extent temporal changes in patient characteristics (eg, coronary anatomy and function), procedural characteristics (eg, completeness of revascularization), the use of antiplatelet agents, or the overall quality of cardiovascular care contribute to our findings. For example, we know that in a relatively short period, the availability of drug-eluting stents was associated with an increased number of patients receiving a coronary stent (Figure 1). Assuming no large change in the population at risk, this suggests that physicians’ thresholds for stent placement may have changed. They may have been more willing to stent lesions in vessels already at somewhat low risk for restenosis or other adverse events, or they may have been more willing to attempt multivessel stenting in patients who previously would have undergone CABG, a patient population at high risk for restenosis and other adverse events. Our data do not allow us to disentangle the extent to which changing practice patterns such as these contributed to the results of this study. We do know there was no evidence of secular changes in the observable characteristics of patients over time (Table 1) and there was no evidence of a positive trend in survival during the pre–drug-eluting stent era prior to 2003 (data not shown).
In summary, we have found that for the Medicare population undergoing nonemergent coronary stenting, the availability of drug-eluting stents has decreased the incidence of repeat revascularization and resulted in no increase in the risk of death or STEMI over 2 years of follow-up. Although other data may suggest some incremental risk of stent thrombosis with the use of drug-eluting stents, we can detect no adverse consequence to the health of the population. We speculate that whatever the increased risk of stent thrombosis associated with drug-eluting stent use is, it is more than offset by a decrease in the risk of developing restenosis and the attendant risk of a procedure to treat that restenosis.
Acknowledgments
Funding/Support: This study was supported by grant P01 AG019783 from the National Institute on Aging and the Robert Wood Johnson Foundation.
Role of the Sponsors: The National Institute on Aging, the Robert Wood Johnson Foundation, and the Centers for Medicare & Medicaid Services had no role in the design and conduct of the study; the analysis and interpretation of data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Malenka has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Study concept and design: Malenka, Kaplan, Lucas, Skinner
Acquisition of data: Malenka, Lucas, Sharp, Skinner
Analysis and interpretation of data: Malenka, Kaplan, Lucas, Sharp, Skinner
Drafting of the manuscript: Malenka, Kaplan, Lucas, Skinner
Critical revision of the manuscript: Malenka, Kaplan, Lucas, Sharp, Skinner
Statistical analysis: Malenka, Lucas, Sharp, Skinner
Obtained funding: Lucas, Skinner
Administrative, technical, or materal support: Malenka, Lucas, Sharp, Skinner
Study supervision: Malenka, Lucas, Skinner
Financial Disclosures: Dr Malenka reports receiving research funding from Guidant Endovascular Systems. Dr Kaplan reports receiving research funding from Abbott Vascular and is a Founder and Director of Tryton Medical Inc, a venture-backed “start up” company focused on the development of a stent technology to treat bifurcation lesions.
Additional Contributions: We thank Lori Haffner, MS (Center for Outcomes Research and Evaluation, Maine Medical Center, Portland), for analytic assistance and editorial assistance in preparation of the manuscript. Ms Haffner received no compensation for her assistance apart from her employment at the institution where the study was conducted.
REFERENCES
- 1.Karrillon GJ, Morice MC, Benveniste E, et al. Intracoronary stent implantation without ultrasound guidance and with replacement of conventional anticoagulation by antiplatelet therapy: 30-day clinical outcome of the French Multicenter Registry. Circulation. 1996;94(7):1519–1527. doi: 10.1161/01.cir.94.7.1519. [DOI] [PubMed] [Google Scholar]
- 2.Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997;29(1):6–12. doi: 10.1016/s0735-1097(96)00452-4. [DOI] [PubMed] [Google Scholar]
- 3.Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–1971. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 4.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 5.Ong ATL, Hoye A, Aoki J, et al. Thirty-day incidence and six-month clinical outcome of thrombotic occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45(6):947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 6.Kuchulakanti PK, Chu WW, Torguson R, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis of sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113(8):1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 7.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. BASKET LATE Investigators Late clinical events after clopidogrel discontinuation may limit the benefit ofdrug-eluting stents. J Am Coll Cardiol. 2006;48(12):2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Mauri L, Hsieh WC, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Ellis SG, Columbo A, et al. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007;115(22):2842–2847. doi: 10.1161/CIRCULATIONAHA.106.687186. [DOI] [PubMed] [Google Scholar]
- 10.Information for physicians on sub-acute thromboses (SAT) and hypersensitivity reactions with use of the Cordis CYPHER coronary stent [Accessed April 18, 2008];FDA Public Health Web Notification. 2003 October 29; http://www.fda.gov/cdrh/safety/cypher.html.
- 11.Camenzind E, Steg P Gabriel, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115(11):1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 12.Nordmann AJ, Briel M, Bucher HC. Long-term mortality in randomized controlled trials comparing drug-eluting versus bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27(23):2784–2814. doi: 10.1093/eurheartj/ehl282. [DOI] [PubMed] [Google Scholar]
- 13.Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030–1039. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 15.Spaulding C, Daemen J, Boersma E, et al. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):989–997. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]
- 16.Williams DO, Abbott JD, Kip KE, et al. Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug-eluting stents: report of the DEScover registry. Circulation. 2006;114(20):2154–2162. doi: 10.1161/CIRCULATIONAHA.106.667915. [DOI] [PubMed] [Google Scholar]
- 17.Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356(10):1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 18.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term outcomes after drug-eluting stent implantation. JAMA. 2007;297(2):159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 19.Marzocchi A, Saia F, Piovaccari G, et al. Long-term safety and efficacy of drug-eluting stents: two-year results of the REAL (REgistro AngioLastiche dell’Emilia Romagna) Multicenter Registry. Circulation. 2007;115(25):3181–3188. doi: 10.1161/CIRCULATIONAHA.106.667592. [DOI] [PubMed] [Google Scholar]
- 20.Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369(9562):667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 21.Tu JV, Bowen J, Chiu M, et al. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007;357(14):1393–1402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]
- 22.Shuchman M. Trading restenosis for thrombosis? new questions about drug-eluting stents. N Engl J Med. 2006;355(19):1949–1952. doi: 10.1056/NEJMp068234. [DOI] [PubMed] [Google Scholar]
- 23.Harrington RA, Ohman EM. The enigma of drug-eluting stents: hope, hype, humility, and advancing patient care. JAMA. 2007;297(18):2028–2030. doi: 10.1001/jama.297.18.2028. [DOI] [PubMed] [Google Scholar]
- 24.Krucoff MW, Boam A, Schultz DG. Drug-eluting stents “deliver heartburn”: how do we spell relief going forward? Circulation. 2007;115(23):2990–2994. doi: 10.1161/CIRCULATIONAHA.107.707778. [DOI] [PubMed] [Google Scholar]
- 25.Kaul S, Shah PK, Diamong GA. As time goes by: current status and future directions in the controversy over stenting. J Am Coll Cardiol. 2007;50(2):128–137. doi: 10.1016/j.jacc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8 2006. Circulation. 2007;115(17):2352–2357. doi: 10.1161/CIRCULATIONAHA.107.688416. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, Daemen J. Late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation. 2007;115(11):1433–1439. doi: 10.1161/CIRCULATIONAHA.106.666826. [DOI] [PubMed] [Google Scholar]
- 28.Chen MS, John JM, Chest DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent rest enosis is not a benign clinical entity. Am Heart J. 2006;151(6):1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.International Classification of Diseases, 9th Revision, Clinical Modification. Practice Management Information Corporation; Los Angeles: 1999. [Google Scholar]
- 30.Cutlip DE, Leon MB, Ho KK, et al. Acute and nine-month clinical outcomes after “suboptimal” coronary stenting: results from the STent Antithrombotic Regimen Study (STARS) registry. J Am Coll Cardiol. 1999;34(3):698–706. doi: 10.1016/s0735-1097(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 31.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 32.Nelson WA. Theory and application of hazard plotting for censored failure data. Technometrics. 1972;14:945–966. [Google Scholar]
- 33.Bland JM, Altman DG. The logrank test. BMJ. 2004;328(7447):1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: an application to data on right heart catheterization. Health Serv Outcomes Res Methodol. 2001;2:259–278. [Google Scholar]
- 35.Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. STATA Journal. 2002;2:358–377. [Google Scholar]
- 36.Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label use of drug-eluting stents. JAMA. 2007;297(18):1992–2000. doi: 10.1001/jama.297.18.1992. [DOI] [PubMed] [Google Scholar]
- 37.Win HK, Caldera AE, Maresh K. EVENT Registry Investigatiors. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007;297(18):2001–2009. doi: 10.1001/jama.297.18.2001. [DOI] [PubMed] [Google Scholar]
- 38.Cutlip DE, Windecker S, Mehran R, et al. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 39.Weisz G, Leon MB, Holmes DR, Jr, et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the sirolimus-eluting stent in de novo native coronary lesions (SIRIUS) Trial. J Am Coll Cardiol. 2006;47(7):1350–1355. doi: 10.1016/j.jacc.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 40.Fajadet J, Morice MC, Bode C, et al. Maintenance of long-term clinical benefit with sirolimus-eluting coronary stents: three-year results of the RAVEL Trial. Circulation. 2005;111(8):1040–1044. doi: 10.1161/01.CIR.0000156334.24955.B2. [DOI] [PubMed] [Google Scholar]
- 41.Hannan EL, Racz M, Holmes DR, et al. Comparison of coronary artery stenting outcomes in the eras before and after the introduction of drug-eluting stents. Circulation. 2008;117(16):2071–2078. doi: 10.1161/CIRCULATIONAHA.107.725531. [DOI] [PubMed] [Google Scholar]
- 42.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER Registry. Circulation. 2006;113(24):2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 43.Spaulding C, Henry P, Teiger E, et al. TYPHOON Investigators. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093–1104. doi: 10.1056/NEJMoa062006. [DOI] [PubMed] [Google Scholar]
- 44.Laarman GJ, Suttorp MJ, Dirksen MT, et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355(11):1105–1113. doi: 10.1056/NEJMoa062598. [DOI] [PubMed] [Google Scholar]
- 45.Daemen J, Tanimoto S, Garcia-Garcia HM, et al. Comparison of three-year clinical outcome of sirolimus- and paclitaxel-eluting stents versus bare metal stents in patients with ST-segment elevation myocardial infarction (from the RESEARCH and T-SEARCH Registries) Am J Cardiol. 2007;99(8):1027–1032. doi: 10.1016/j.amjcard.2006.11.070. [DOI] [PubMed] [Google Scholar]