Abstract
Chemical compounds have emerged as powerful tools for modulating embryonic stem cell (ESC) functions and deriving induced pluripotent stem cells (iPSCs), but documentation of compound-induced efficient directed differentiation in human ESC (hESCs) and human iPSC (hiPSCs) is limited. By screening a collection of chemical compounds, we identified compound C (also denoted as dorsomorphin), a protein kinase inhibitor, as a potent regulator of hESC and hiPSC fate decisions. Compound C suppresses mesoderm, endoderm and trophoectoderm differentiation and induces rapid and high-efficiency neural conversion in both hESCs and hiPSCs (88.7% and 70.4%, respectively). Interestingly, compound C is ineffective in inducing neural conversion in mouse ESCs (mESCs). Largescale kinase assay revealed that compound C targets at least seven TGF-β superfamily receptors, including both type I and type II receptors, and thereby blocks both the Activin and BMP signaling pathways in hESCs. Dual inhibition of Activin and BMP signaling accounts for the effects of compound C on hESC differentiation and neural conversion. We also identified muscle segment homeobox gene 2 (MSX2) as a downstream target gene of compound C and a key signaling intermediate of the BMP pathway in hESCs. Our findings provide a single-step cost-effective method for efficient derivation of neural progenitor cells in adherent culture from human pluripotent stem cells. Therefore, it will be uniquely suitable for the production of neural progenitor cells in large scale and should facilitate the use of stem cells in drug screening and regenerative medicine and study of early human neural development.
Keywords: human embryonic stem cells, neural conversion, compound C, TGF-β, superfamily receptors, induce pluripotent stem cells
INTRODUCTION
Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) reprogrammed from somatic cells can differentiate into multiple cell types, allowing them to be utilized for biomedical research, drug discovery and cell-based therapies [1, 2]. A deeper understanding of the signaling mechanisms and pathways that maintain pluripotency and induce direct differentiation of hESCs and iPSCs will shed light on early human development and facilitate the applications of these powerful cellular systems.
Several developmentally important signals have been extensively studied in hESCs. For example, basic FGF (bFGF) supports long-term undifferentiated growth of hESCs [3]. The TGF-β superfamily, comprising TGF-β, Activin, Nodal and bone morphogenesis proteins (BMPs), has diverse roles in hESCs [4, 5]. TGF-β/Activin/Nodal was shown to co-operate with FGF signaling to maintain pluripotency of hESCs by controlling NANOG expression. Activation of BMP signaling in hESCs induces mesoderm and trophoectoderm activities depending on the duration of activation [6–8], while activation of Activin/Nodal pathway can trigger endoderm differentiation [9]. Conversely, inhibition of Activin/Nodal and BMP signaling, alone or in combination, promotes neuroectoderm specification [10–12].
Chemical compounds can be used to control cellular processes and have been applied successfully to the modulation of fate decisions of stem cells including hESCs and hiPSCs. Chemical compounds can be synthesized at a large scale and great purity, offer temporal and tunable control of molecular and cellular functions, and has a low cost. Earlier studies showed that the ROCK inhibitor Y-27632 promotes survival of dissociated hESCs, while the small-molecule valproic acid (VPA) improves the efficiency of hiPSC derivation [13, 14]. Recently, cell-permeable small molecules that direct differentiation of hESCs into the endoderm or early pancreatic lineage were indentified [15, 16]. Despite these encouraging advances, there is limited documentation about using chemical compounds to efficiently induce differentiation to other lineages such as neuroectoderm in hESCs and hiPSCs.
Here, we report the identification and use of a single chemical compound, compound C/dorsomorphin, for suppressing endoderm, mesoderm and trophoectoderm differentiation of human pluripotent stem cells and for rapid and highly efficient neural conversion from hESCs and hiPSCs in defined adherent cultures. We also show that compound C targets at least seven type I and type II TGF-β superfamily receptors and blocks both the Activin and the BMP signaling pathway, which account for compound C's ability to induce highefficiency neural conversion.
MATERIALS AND METHODS
Cell Lines and Cell Culture
H1 and H9 hESCs (WiCell Research Institute) were routinely maintained under feeder conditions as described [1]. The culture medium consists of DMEM/F12 with 20% knockout serum replacement (KSR), 1 mM glutamine, 1% non-essential amino acid, 0.1 mM β-mercaptoethanol and 4 ng/ml bFGF. KSR is a defined supplement. For feeder-free cultures, cells were cultured on plates coated with Matrigel (BD Biosciences) in the presence of conditioned medium (CM) from mouse embryonic fibroblasts (MEFs), which replaces the MEF feeders. Fresh medium was added every day. For neural conversion of hESCs, mTeSR medium (StemCell Technologies) and DMEM/F12 medium with 20% knockout serum replacement (KSR), 1 mM glutamine, 1% non-essential amino acid and 0.1 mM β-mercaptoethanol were used. mTeSR is a defined feeder-independent medium. The hiPSC line RND5 (passage 8–10) was from ArunA Biomedical, Inc (Athens, GA) and was maintained under the same conditions as H1 and H9 hESCs. Derivation and validation of the hiPSCs are described: http://www.openbiosystems.com/GeneTargeting/viPS%20Lentiviral%20Vector%20Kit/. NTera-2 (American Type Culture Collection, ATCC) cells were cultured according to ATCC recommendations. The medium consists of 10% fetal bovine serum (FBS, ATCC). For low-density single-cell culture, cells were dissociated into single cells with 0.025% trypsin-0.02% EDTA and plated at the density of 10,000 cells/cm2. W4 mouse ESC (mESC) line was from Taconic and was cultured as described [17].
Western Blotting
For the analysis of proteins except phospho-Smad2/3, cells (5×106) were lysed directly with 200 µl laemmli sample buffer (Biorad). For detection of phospho-Smad2/3, nuclear fraction of hESCs was isolated with a NEPER® Nuclear and Cytoplasmic Extraction Kit (Pierce). Samples (25 µg) were analyzed by western blot. Dilutions for various antibodies were described in Table S4. The blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce), and signals were quantified with Image J.
EB Formation Assay
H1 or H9 hESCs (5×106/well) cultured on MEFs were dissociated as clusters with dispase (1 mg/mL), plated onto Ultra-Low Attachment 6-well plates (Corning) and grown in embryoid body (EB) medium (DMEM/F12, 20% FBS, 1 mM glutamine, 1 mM 1% non-essential amino acid and 0.1 mM β-mercaptoethanol). To change medium, EBs and medium were gently transferred into a 15-ml tube and left at RT for 5 min to allow the EBs to sink to the bottom. The medium was gently removed and replaced by fresh medium. EBs were collected at different time points for further analysis.
Induction of Neural Differentiation
For neural differentiation, H1 or H9 hESCs were cultured on Matrigel with mTeSR medium to eliminate variations introduced by the MEF feeders. hESCs were gently dissociated into single-cell suspension with 1 mg/mL accutase for 5–7 min at 37°C. The dissociated cells were plated on Matrigel at high density (106/cm2) and cultured to confluency in mTeSR medium before induction of differentiation. For compound C-induced neural conversion, mTeSR medium was replaced with DMEM/F12 containing 20% KSR, 1 mM glutamine, 1% non-essential amino acid and 0.1 mM β-mercaptoethanol. This condition was used for all treatment groups. Medium was replaced every day. For early neural patterning, SHH (100 ng/mL) was incubated with compound C-induced neural progenitor cells for 5 d. For late neural terminal differentiation/maturation, SHH-treated cells were gently dissociated into small clusters with dispase and plated on laminin-coated plates. Cells were cultured in neural differentiation medium, which consists of neural basal medium, 1x N2 supplement, purmorphamine (1 μM), ascorbic acid (200 μM), BDNF (20 ng/mL), GDNF (20 ng/mL), SHH (100 ng/mL) and FGF8 (100 ng/mL), for 2 weeks before collected for analysis.
Luciferase Assay
The ID-120-luc reporter construct for BMP signaling activity, kindly provided by Dr. Renhe Xu (University of Connecticut), was described [18]. To measure the reporter activity, H1 hESCs were plated on Matrigel-coated 24-well plates before transfection. The ID-120-luc plasmid was co-transfected into cells with the Rellina plasmid (Promega), and 24 h after transfection, medium was replaced with hESC growth medium without bFGF. BMP-4 (10 ng/mL) in combination with different concentrations of compound C was administered to cells. The cells were lysed 24 h after the addition of BMP-4 and compound C by using lysis buffer provided with a dual-luciferase assay kit (Promega). The luciferase activity was measured with LUMIstar plate reader (BMG Labtech). The luciferase-based Activin reporter plasmid (pARE-GL3, [19]) was kindly provided by Dr. Yisrael Sidis (Partners Healthcare). To measure the reporter activity, H1 hESCs was co-transfected with 0.25 μg pARE-GL3, 0.25 μg FAST-1 (forkhead activin signal transducer-1), and Rellina plasmid. Activin A (100 ng/mL) and various concentrations of SB421542 or compound C were added 24 h after transfection. The luciferase activity was measure as described above.
Additional experimental procedures and associated references are available in the Supporting Information.
RESULTS
Compound C Modulates Differentiation Activities in hESCs
We recently screened a collection of chemical compounds, identified and reported mTOR as a key positive regulator of pluripotency [17]. In the current study, we screened for compounds that prevent hESC differentiation. We cultured two hESC lines with distinct genotypes [WA01 (H1), XY; WA09 (H9), XX] [1] in the absence of bFGF and conditioned medium (CM) produced by mouse embryonic fibroblasts (MEFs), both required to support hESC self-renewal and pluripotency [1, 20]. Human ESCs grown without bFGF and CM continued to proliferate at a slower rate, but began to differentiate from the center of the colonies and gradually lost the expression of pluripotency markers OCT-4 and SOX2 as described [17] (Fig. 1A). We applied the collection of chemical compounds and inhibitors (Table S1) to hESCs cultured in the absence of bFGF and CM. We found that compound C (1 µM), a kinase inhibitor, supported the growth of hESCs as compact colonies, reminiscent of control cells cultured with bFGF and CM (Fig. S1A). In addition to preventing the differentiation morphology, compound C greatly attenuated the reduction of OCT-4, SOX2 and NANOG proteins (Fig. 1A, Fig. S1B). The supportive effects of compound C on OCT-4, NANOG and SOX2 expression were further confirmed at the transcription level: the mRNA levels of POU5F1 (the gene encoding OCT-4), SOX2 and NANOG in compound C-treated hESCs were significantly higher than in cells without the treatment (Fig. 1B). Interestingly, the rescuing effect of compound C on NANOG expression was weaker than POU5F1 and SOX2. Furthermore, compound C markedly prevented the differentiation morphology and down-regulation of pluripotency markers in a pluripotent human embryonal carcinoma cell line, NTera-2 cells (Supporting Information) (Fig. S1C and S1D), suggesting a conserved effect in human pluripotent stem cells.
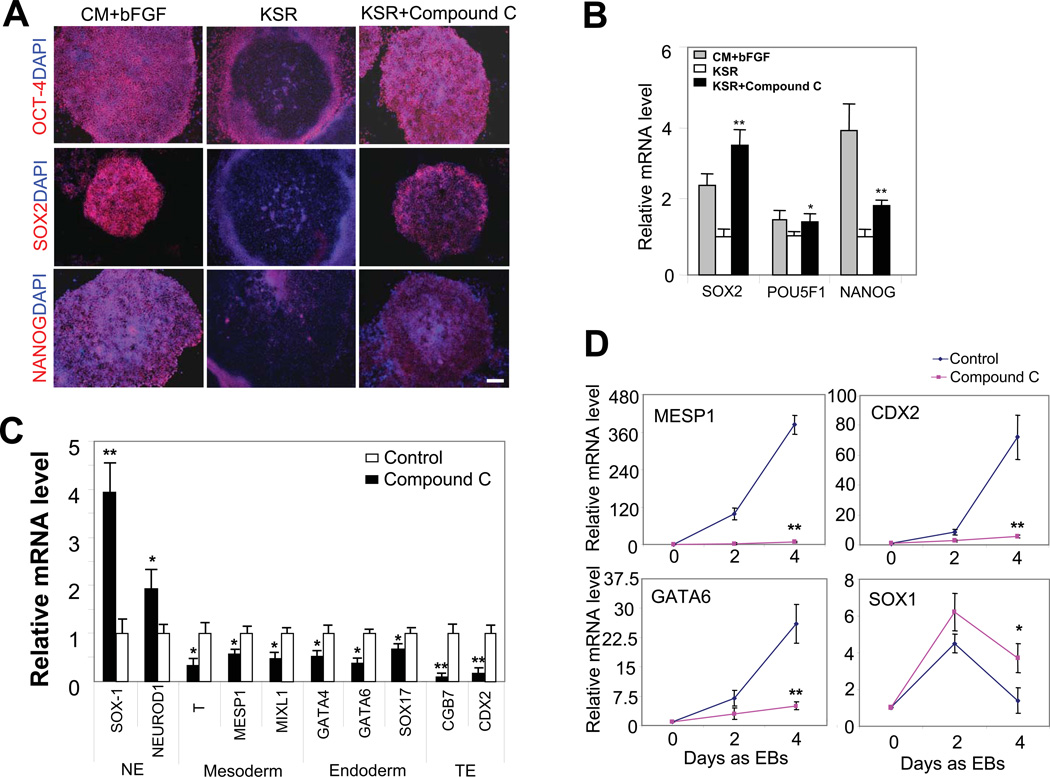
Figure 1. Compound C suppresses mesoderm, endoderm and trophoectoderm differentiation in hESCs.
(A) Immunofluorescence studies of OCT-4, SOX2 and NANOG proteins (red) in H1 cell colonies cultured in complete growth medium (CM + bFGF), medium with KSR only, and medium with KSR and 1 µM compound C for 6 d. All three pluripotency markers were expressed in 92.5%±4.7% of the colonies (i.e., with CM and bFGF). Removal of CM and bFGF reduced the OCT-4-, SOX2- and NANOG-positive colonies to 8.1%±3.2%, and addition of compound C increased it to 88.4%±6.5%. Nuclei were stained with DAPI (blue). H9 cells showed similar response to compound C treatment. Dose-dependent experiments with compound C (100 nM, 200 nM, 500 nM, 1 µM and 2 µM) showed that 1 µM was necessary to give rise to the strong anti-differentiation effect. Scale bar, 200 μm. (B) mRNA levels of SOX2, POU5F1 and NANOG in H1 hESCs cultured in aforementioned three conditions for 6 d, assessed by real-time PCR. All values were normalized to the level (=1) of mRNA in the cells treated with KSR alone. *, p<0.05; **, p<0.01. Cells treated with compound C were compared to control cells (i.e., with KSR only) treated with vehicle. ACTB (β-actin gene) was used as an internal control. (C) mRNA levels of differentiation markers in H1 hESCs cultured in medium with KSR only and medium with KSR and 1 µM compound C for 6 d, assessed by real-time PCR. All values were normalized to the level (=1) of mRNA in the cells treated with KSR alone. *, p<0.01; **, p<0.001. Cells treated with compound C were compared to control cells treated with vehicle. NE and TE denote neuroectoderm and trophoectoderm, respectively. (D) mRNA levels of control EBs or compound C-treated EBs assessed by real-time PCR. Four separate experiments were conducted, and quantification of three replicates of a typical experiment 30 is shown. Each bar represents the mean ± SEM (error bars). All values were normalized to the mRNA level (=1) of in the control cells on day 0. *, p<0.05; **, p<0.01. EBs treated with compound C were compared to control EBs on day 4.
Compound C profoundly influenced the differentiation activities in hESCs. It significantly reduced the expression of markers for mesoderm (MESP1, T and MIXL1), endoderm (GATA4, GATA6) and trophoectoderm (CDX2 and CGB7) in H1 cells cultivated in the absence of bFGF and CM (Fig. 1C), but caused the expression of neural ectoderm markers SOX1 and NEUROD1 to moderately increase (Fig. 1C). The effect of compound C on hESC differentiation was further verified with the embryoid body (EB) formation assay. During EB formation, expression of differentiation markers was consistently up-regulated in untreated control cells (Fig. 1D and Fig. S1E) [17]. In this assay, compound C treatment markedly prevented the up-regulation of markers for endoderm, mesoderm and trophoectoderm, but enhanced the expression of neuroectoderm markers after EB induction (Fig. 1D and Fig. S1E). Compound C treatment had no effects on the size or the number of EBs (Fig. S1F).
High-Efficiency Induction of Neural Conversion by Compound C
Because compound C potently suppressed endoderm, mesoderm and trophoectoderm but enhanced neural ectoderm activities in hESCs, we asked whether it could be applied to the induction of neural conversion. To test this, we developed differentiation conditions for hESCs using adherent cell culture, which is more consistent and easier to manipulate than the EB condition. Cells dissociated by accutase (1 mg/mL) were plated on Matrigel and continuously cultured to confluency in mTeSR medium, which could eliminate variations introduced by the MEF feeders. Differentiation was initialized by replacing mTeSR with DMEM/F12 medium containing knock-out serum replacement (KSR) (but lacking bFGF and CM) and compound C (1 μM). Another small-molecule inhibitor, SB431542, which inhibits type I TGF-β receptors and reportedly promotes neural conversion in hESCs [12], was used with or without compound C. We used PAX6, a neural progenitor marker, to monitor early neural conversion. Human ESCs, when plated as single cells in the absence of bFGF and CM, began to exhibit differentiation morphology by d 3, and the differentiation was more apparent by d 5~d 6 with the majority of cells spread out and losing the expression of OCT-4 (Fig. 2A and Fig. S2A). Interestingly, cells treated with compound C grew as a compact uniform monolayer, in contrast to untreated cells, which often showed areas with disassociated cells and cell clumps (Fig. S2A). Immunofluorescence studies revealed that PAX6 protein was nearly uniformly expressed in compound C-treated cells (Fig. 2A, middle panel), whereas much fewer untreated cells exhibited anti-PAX6 fluorescence (Fig. 2A, upper panel). Other early neural progenitor markers such as SOX1 and SOX2 were also highly expressed in compound C-treated cells (Fig. 2A, middle panel). Western blot analysis confirmed increased expression of PAX6 and SOX2 proteins (Fig. 2B). Furthermore, western blot analysis also showed that expression of Nestin protein, a neural stem cell marker, was elevated in compound C-treated cells (Fig. 2B). In contrast, addition of SB431542 caused only a slight increase of PAX6 protein and failed to induce further increase of PAX6, Nestin and SOX2 proteins in compound C-treated cells (Fig. 2A and 2B). The expression of pluripotency marker OCT-4 was almost undetectable 7 d after differentiation induction (data not shown). In keeping with the increased level of protein, a gradual increase of PAX6 mRNA was also observed during the differentiation process, which lasted for at least 10 d (Fig. S2B).
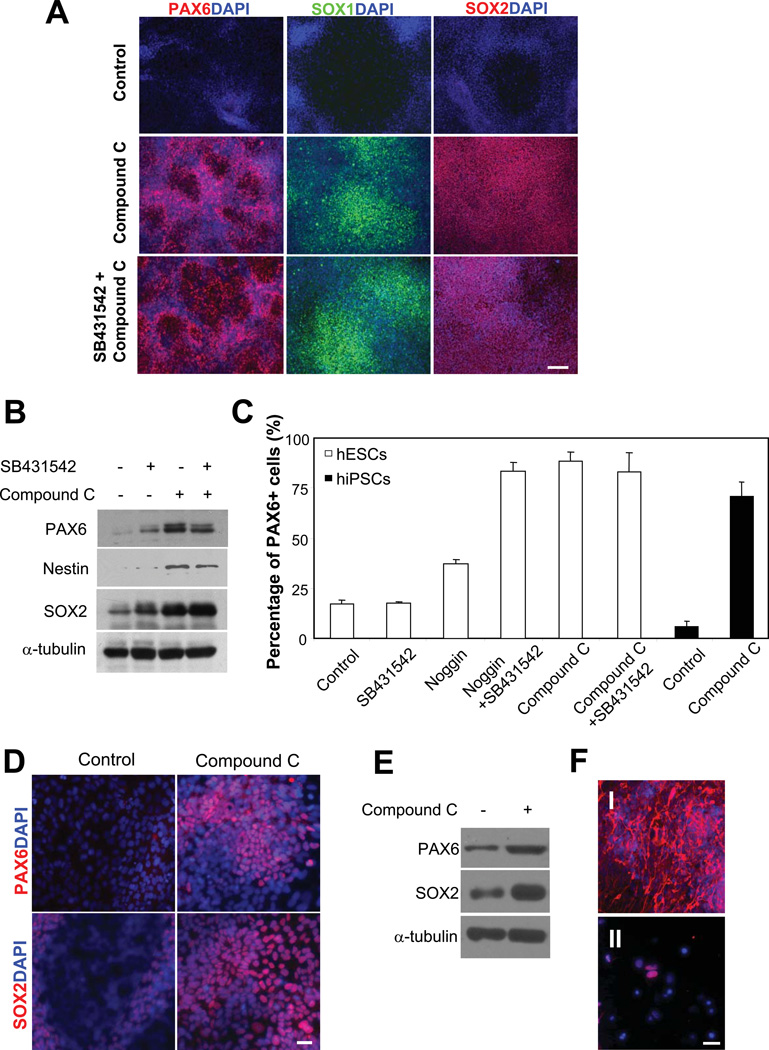
Figure 2. Compound C induces high-efficiency neural conversion.
(A) Immunofluorescence studies of PAX6 (left panel, red) and SOX1, (middle panel, green) and SOX2 (right panel, red) in differentiating H1 hESCs. Cell nuclei were stained with DAPI (blue). In contrast to control cells, which exhibited minimal anti-PAX6 fluorescence, H1 cells treated with compound C alone (1 μM) or with compound C (1 µM) and SB431542 (50 nM) combined expressed high level of PAX6 after 7-d differentiation. SOX1 and SOX2 were also highly expressed in compound C or compound C and SB431542 treated cells. Bar, 200 µm. (B) Western blot analysis of PAX6, Nestin and SOX2 in H1 cells with three treatments. Cells were induced to differentiate for 7 d. A typical experiment from five separate experiments is shown. α-tubulin was a loading control. (C) The percentage of PAX6+ cells assessed by flow cytometry. H1 hESCs treated with compound C alone or with compound C and SB431542 contained 88.7±2.5% and 80.3±10.3% (mean ± SEM) PAX6+ cells, respectively. In contrast, control cells and cells treated with SB431542 showed 16.9±1.9% and 17.7±0.4% PAX6 + cells. Treatment with Noggin (500 ng/mL) alone or combined with SB431542 gave rise to 36.9±1.6% and 84.7±3.9% PAX6+ cells, respectively. Similarly, compound C treatment of IMR-90-derived hiPSCs induced a high population of PAX6+ cells (70.3%±5.5%), in contrast to untreated cells (4.8%±2.1%). (D) Immunofluorescence studies of PAX6 (top panel, red) and SOX2 (bottom panel, red) in differentiating hiPSCs. Cell nuclei were stained with DAPI (blue). Cells were treated or not treated with 1 µM compound C for 7 d. scale bar, 50 μm. (E) Western blot analysis of PAX6 and SOX2 in hiPSCs treated or not treated with 1 µM compound C. Cells were induced to differentiate for 7 d. α-tubulin was a loading control. (F) Immunofluorescence studies of TUJ1 and NURR1 in terminally differentiated H1 hESCs. Anti-TUJ1 (I) and NURR1 (II) fluorescence (red) was observed in H1-derived differentiated cells after 2-w neural maturation. Nuclei were stained with DAPI (blue). Scale bar, 50 μm.
To determine the fraction of PAX6+ cells, we analyzed the differentiated cells by flow cytometry 7 d to 10 d after compound C treatment. Remarkably, the treatment yielded a high percentage of PAX6+ cells (88.7±2.5%, Fig. 2C and Fig. S2C). There was no significant difference between d 7 to d 10 cells (data not shown). Interestingly, the dual treatment of compound C and SB431542 failed to increase the percentage of PAX6+ cells (80.3%±10.3%) (Fig. 2C and Fig. S2C). Furthermore, while Noggin treatment alone moderately increased the percentage of PAX6+ cells (36.9±1.6%), combined treatment of SB431542 and Noggin significantly increased the percentage (84.7±3.9%) (Fig. 2C and Fig. S2C), consistent with a previous report [10]. The percentage of PAX6+ cells acquired with compound C was slightly higher than with combined treatment of Noggin and SB431542 (Fig. 2C). Thus, the use of compound C, mTeSR medium and KSR-containing DMEM/F12 medium enabled us to develop a single-step cost-effective procedure for high-efficiency neural conversion in hESCs in defined adherent cultures.
Compound C also induced high-efficiency neural conversion in hiPSCs. We used hiPSCs derived from human fetal lung fibroblasts (IMR-90) for neural induction by following the same procedure developed for hESCs. Immunofluorescence and western blot analyses revealed a drastic increase of PAX6 and SOX2 proteins in cells treated with compound C (1 µM, 7 d) (Fig. 2D and 2E). Similar to hESCs, compound C treatment of hiPSCs induced a high population of PAX6+ cells (70.4%±5.5%) (Fig. 2C). These results demonstrate that compound C alone induces high-efficiency neural conversion in hESCs and hiPSCs and could be used as a powerful tool in large-scale generation of neural progenitors for therapeutic applications.
The terminal differentiation potential of PAX6+ cells derived with compound C was confirmed. Immunofluorescence of Neuron specific class III β-tubulin (TUJ1), a pan-neuronal marker, was nearly uniformly expressed in cells after 2-week neural maturation (Fig. 2F, I). In contrast, TUJ1 immunofluorescence was not detected in undifferentiated hESCs or compound C-induced PAX6+ cells (data not shown). In addition, a fraction of the cells also expressed Nur-related factor 1 (NURR1), a dopaminegic neuron marker (Fig. 2F, II). Thus, the PAX6+ neural progenitors derived by the use of compound C have the potential to undergo terminal differentiation and to produce neuronal subtypes.
It was reported that compound C/dorsomorphin can promote cardiomyogenesis in mouse ESCs (mESCs) [21], prompting us to examine whether it could exert the same effect in hESCs. However, we found that compound C failed to induce cardiomyogenesis in hESCs (Supplementary Text; Fig. S3). In addition, treatment of mESCs with compound C failed to induce early neural conversion (Supplementary Text; Fig. S3). The differential effects of compound C in mESCs and hESCs might be due to their differences in species, origin of development or other undefined characteristics. Some known signaling pathways mediating self-renewal and differentiation of mESCs are not conserved in hESCs [22, 23].
Compound C Targets Several Families of Protein Kinases
Compound C is widely used as a ATP-competitive inhibitor of AMP-activated kinase (AMPK), a multimeric protein complex that regulates cellular and organismal metabolism [24, 25], but recently its specificity has been questioned [26, 27]. In keeping with this, compound C has been shown to also target the type I BMP receptors (ALK2, ALK3 and ALK6) and likely other protein kinases [27, 28]. To gain a broader, more comprehensive view of the potential targets of compound C, we assessed its effects on a panel of kinases (402) in a large-scale in-vitro kinase assay, as described [29]. This assay identified far more targets of compound C than previously recognized (Fig. 3A and Table S2). Notably, compound C robustly inhibited many other members of TGF-β superfamily receptors in addition to ALK2, ALK3 and ALK6 and of the SNF/AMPK family kinases in addition to AMPKα1 and α2 (Fig. 3A). Furthermore, compound C potently inhibited PDGF receptors (PDGFRα and PDGFRβ), VEGFR receptors [VEGFR1 (FLT1) and VEGFR2 (KDR)], FLT3 and KIT (Fig. 3A).
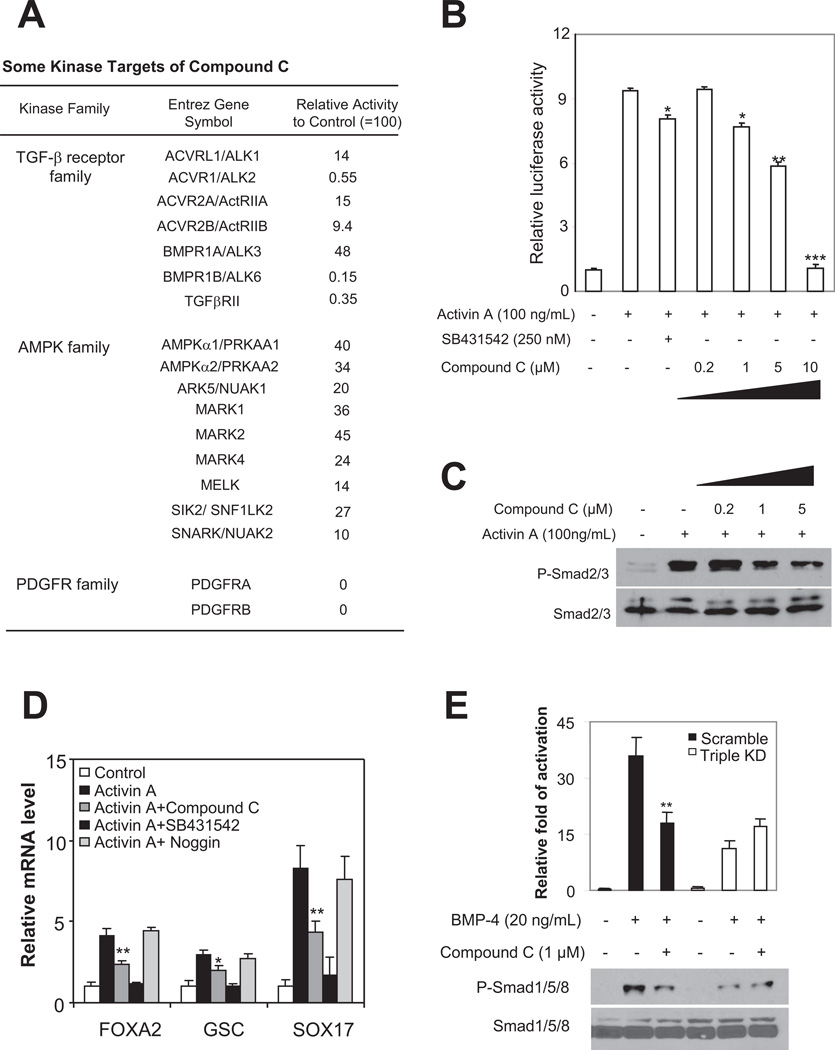
Figure 3. Compound C targets the TGF-β superfamily receptors and blocks Activin and BMP signaling.
(A) Some of the kinases inhibited by 1 μM compound C and the degree of inhibition, as revealed by the in vitro kinase assay. The complete list of kinases examined is shown in Supplementary Table S2. (B) Relative luciferase activity of the pARE-GL3 reporter in H1 cells with or without Activin A (100 ng/mL, 24 h), treated with different concentrations of SB421542 or compound C. Results from four separate experiments are shown as mean±SEM. *, p<0.05 ; **, P<0.01; ***, p<0.001. Cells with various treatments were compared to control (with Activin stimulation). Hint amount of Rellina plasmid was co-transfected as an internal control. (C) Western blot analysis of phosphorylated Smad2/3 and total Smad2/3 in the nuclear fraction of H1 cells treated with various concentrations of compound C and stimulated with Activin-A (100 ng/mL) for 24 h. A typical experiment from four separate experiments is shown. Total Smad2/3 was a loading control. Compound C (1 µM) reduced the nuclear p-Smad2/3 levels by approximately 25%. (D) mRNA levels of endoderm markers SOX17, FOXA2 and GSC in H1 cells with various treatments, assessed by real-time PCR. Cells were stimulated with Activin A (100 ng/mL) for 5 d. Four separate experiments were conducted, and quantification of three replicates of a typical experiment is shown. Each bar represents the mean ± SEM (error bars). All values were normalized to the mRNA level (=1) of in the control cells. Activin and compound C treatments vs Activin alone were compared (*, p<0.05; **, p<0.01). (E) Western blot analysis of Smad1/5/8 phosphorylation under various conditions. Similar to 1 µM compound C treatment, depletions of ALK2, ALK3 and ALK6 altogether (denoted as Triple KD) attenuated the phosphorylation of Smad1/5/8 induced by BMP-4 (20 ng/mL, 30 min). Further addition of 1 µM compound C to cells lacking the three receptors exerted no detectable effects. A typical blot is shown in the bottom panel, and quantification of blots from four separate experiments is shown in the top panel. α-tubulin was a loading control. Y-axis represents relative intensities (measured with Image J) with values normalized to the signal (=100%) without BMP-4 or compound C treatment. Each bar represents mean ± SEM (error bars). Student t tests compared data between cells treated with compound C vs control cells after BMP-4 addition (**, p<0.01).
To determine if AMPK inhibition was responsible for the effects of compound C on hESCs, we abolished the function of AMPK by depleting the α1 and α2 subunits. Surprisingly, depletion of either α1 or α2 subunit failed to support the undifferentiated growth and pluripotency of hESCs and instead markedly decreased the levels of SOX2 protein (Fig. S4). Furthermore, AICAR, an AMPK activator, failed to rescue the phenotypic changes caused by compound C in hESCs. Based on these results, we concluded that AMPK inhibition was not responsible for the effect of compound C.
Inhibition of PDGF receptors in hESCs also failed to mimic the effects of compound C. Treatment of hESCs with PDGF receptor kinase inhibitor V slightly decreased cell proliferation (data not shown).
Compound C Inhibits Both Activin And BMP Signaling in hESCs
Two type II receptors, ActRIIA and ActRIIB, mediate Activin signaling [30] and were markedly inhibited by compound C in vitro (Fig. 3A), leading us to assess whether compound C targeted this pathway in hESCs. We found that compound C inhibited the activity of a luciferase-based Activin reporter system (pARE-GL3, [19]) in a dose-dependent manner (Fig. 3B). At a dose that effectively modulated hESC differentiation (1 µM), compound C consistently reduced the Activin reporter activity triggered by the addition of Activin A (100 ng/mL), to a similar extent to low concentration of SB431542 (250 nM) (Fig. 3B), which inhibits type I receptors including ALK4, ALK5 and ALK7 and consequently blocks the Activin signaling pathway, without affecting BMP signaling [31]. A higher dose of compound C (10 µM) was required to completely prevent the Activin reporter activity. In keeping with reduced reporter activity, compound C (1 µM) significantly attenuated the increase in the nuclear phospho-Smad2/3 levels in hESCs induced by Activin A (Fig. 3C). Thus, compound C potently inhibited the Activin pathway in hESCs. Interestingly, inhibition of Activin signaling by compound C was observed in mouse pulmonary artery smooth muscle cells (PASMCs) at higher concentrations [28]. The variability might be attributed to the differences in cell types or other undefined characteristics.
Compound C also blocked BMP signaling in hESCs. Compound C inhibited a luciferase-based inhibitor of DNA binding (ID) reporter activity (Fig. S5A). It reduced BMP-4-induced activation (phosphorylation) of Smad1/5/8 in hESCs (Fig. S5B). Thus, compound C potently inhibited the BMP-4 signaling pathway in hESCs. These results are consistent with the ability of compound C to inhibit both type I (ALK2, ALK3 and ALK6) and type II BMP receptors (ActRIIA and ActRIIB) in vitro. The effects of compound C on BMP signaling in hESCs confirm and extend previous studies in other model systems [28].
Compound C Prevents Differentiation Activities Induced By Activin A and BMP-4
It has been well established that activation of Activin signaling triggers endoderm activities in ESCs [9, 32]. Furthermore, Activin A can directly act to down-regulate PAX6 expression [33]. In RPMI medium containing low serum, Activin A induces efficient differentiation of hESCs to definitive endoderm [9]. We asked whether compound C blocked Activin A - induced endoderm activities. hESCs exhibited reduced survival in RPMI medium after the addition of compound C, prompting us to use a custom mTeSR medium instead, which lacks bFGF and TGF-β and thus causes cells to differentiate. Compound C did not cause apoptosis of cells cultured in the custom mTeSR medium. As expected, the addition of Activin A (100 ng/mL) induced up-regulation of endoderm markers including SOX17, GSC, and FOXA2 in hESCs (Fig. 3D). The presence of compound C (1 μM) reduced the up-regulation (Fig. 3D). Expectedly, SB431542, at a dose commonly used to inhibit Activin signaling (10 µM) [31], nearly completely prevented the up-regulation of the endoderm markers (Fig. 3D). In contrast, Noggin, a secreted protein that antagonizes BMP signaling, exerted little effects (Fig. 3D). Thus, compound C decreased Activin A-induced endoderm activities in hESCs, separately from its actions on BMP receptors.
BMP-4 induced mesoderm or trophoectoderm activities in hESCs, depending upon the length of stimulation [7, 8]. We thus treated hESCs with BMP-4 (20 ng/mL) for 24 h and 6 d, with or without compound C (1 μM). Short-term BMP-4 stimulation (24 h) induced a rapid up-regulation of mesoderm markers, including T, MESP1 and MIXL1 (Fig. S5C). Compound C nearly completely abolished the up-regulation (Fig. S5C). In addition, compound C prevented the up-regulation of CDX2 and down-regulation of pluripotency markers in hESCs after long-term exposure to BMP-4 (Supporting Information) (Fig. S5D and S5E). Thus, compound C inhibited BMP-4-induced mesoderm and trophoectoderm activities in hESCs.
The large-scale in vitro kinase assay showed that compound C targeted all three type I and two type II BMP receptors, which might explain its potent inhibition of BMP signaling activities in hESCs. To test this possibility, we compared the effects of depleting a single or multiple BMP receptors. Indeed, depletion of ALK2, ALK3 or ALK6 alone failed to affect BMP4-induced signals (Fig. S6A). In contrast, depletion of all three receptors nearly abolished BMP-4-induced signals including Smad1/5/8 phosphorylation, and up-regulation of the early BMP-4 responsive gene ID1 and the differentiation markers T, MESP1 and CDX2 (Fig. S6B). Moreover, addition of compound C to the receptor-depleted cells caused no additional effects on Smad1/5/8 phosphorylation (Fig. 3E). Depletion of two type I receptors also attenuated up-regulation of target genes, although the effects were less prominent than those from depleting all three (Fig. S6B). Furthermore, in cells induced to spontaneously differentiate, mRNA levels of mesoderm and trophoectoderm differentiation markers T and CGB7 were reduced in cells with triple depletion, whereas the decrease of the pluripotency and neuroectoderm markers such as POU5F1, NANOG and SOX1 was attenuated (Fig. S6C). These results suggest that inhibition of at least two receptors is necessary for robust inhibition of BMP signals. Together, the ability to target multiple TGF-β family receptors, dual inhibition of Activin and BMP signaling and suppression of Activin- and BMP-induced endoderm, mesoderm and trophoectoderm activities provide mechanistic explanations for the strong effects of compound C in hESCs.
MSX2 as A Compound C's Target Gene and a Key Intermediate of BMP Signaling in hESCs
To identify potential target genes and signaling pathway(s) of compound C in hESCs, we used Affymetrix-based whole-genome microarrays to compare global gene-expression profiles of differentiating hESCs (induced by bFGF and CM removal) with and without compound C treatment. Focusing on early times (12 and 24 h) after treatment, we sought to identify early responsive gene(s) and/or signaling pathway(s). Microarray analyses of cells at 12 and 24 h identified a small number of genes with ≥1.5-fold altered expression (Table S3). Interestingly, a subset of transcription factors, including ID2, muscle segment homeobox gene 2 (MSX2) and distal-less homeobox 2 (DLX2), were down-regulated 12 and 24 h after compound C treatment (Table S3, highlighted in yellow). Among those, IDs are shown as downstream genes of the BMP signaling in hESCs and mESCs [7, 34]. Real-time PCR confirmed and extended these findings: transcription of the ID family members (ID1-ID3) and MSX family members (MSX1 and MSX2) were markedly suppressed after compound C treatment (Fig. 4A).
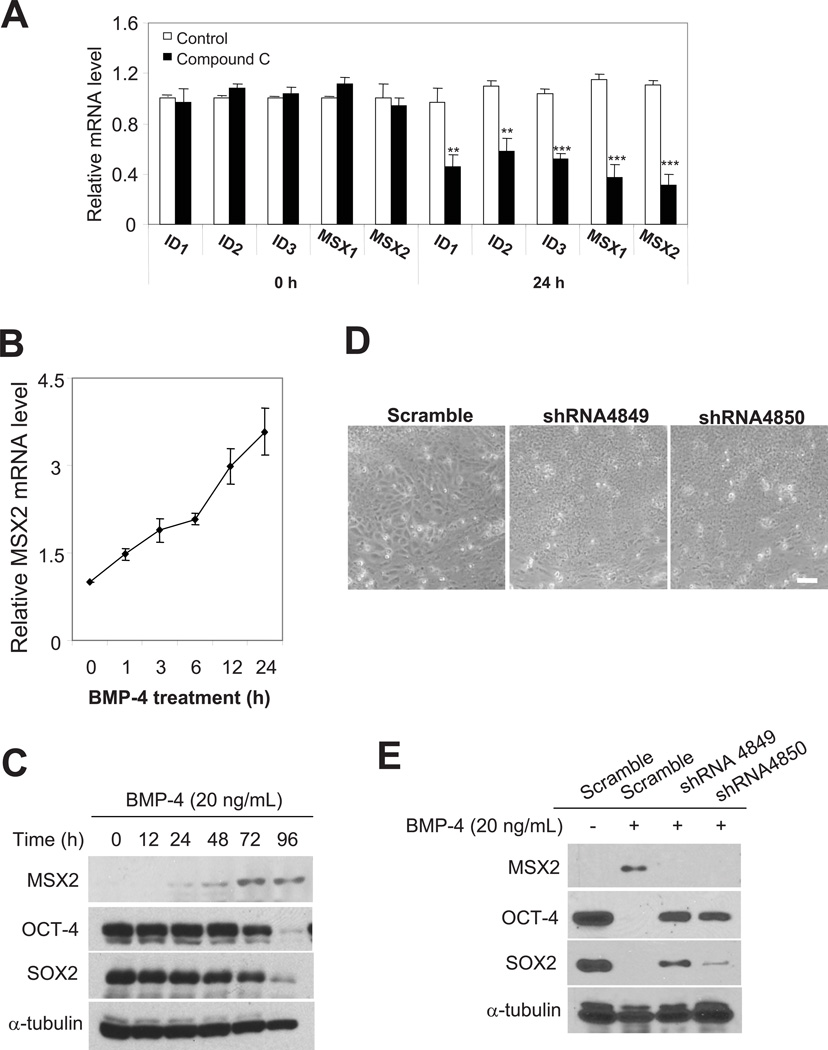
Figure 4. MSX2 as a target gene of compound C and a key intermediate of the BMP pathway.
(A) mRNA levels of ID1, ID2, ID3, MSX1 and MSX2 in H1 hESCs induced to differentiate by the removal of bFGF and CM, with or without the treatment of 1 µM compound C, assessed by real-time PCR. Four separate experiments were conducted, and quantification of four replicates of a typical experiment is shown. Bars represent mean ± SEM (error bars). All values were normalized to the mRNA level in the control cells at 0 h. Cells treated with compound C were compared to control cells treated with vehicle. **, p<0.01; ***, p<0.001. (B) The mRNA level of MSX2 in H1 hESCs after BMP-4 treatment (20 ng/mL) for various time points assessed by real-time PCR. (C) Western blot analysis of MSX2, OCT-4 and SOX2 in H1 cells after BMP-4 treatment (20 ng/mL) for various time points. (D) Phase-contrast images of H1 cells with MSX2 depletion 4 d after BMP-4 treatment (20 ng/mL). Two separate shRNAs were used to verify the effects of MSX2 depletion, while scramble shRNA was used as a control. Scale bar, 50 μm. (E) Western blot analysis of MSX2, OCT-4 and SOX2 in H1 cells with MSX2 depletion 4 d after BMP-4 treatment (20 ng/mL). Two separate shRNAs (sh4849 and sh4850) were used to verify the effects of MSX2 depletion. α-tubulin was used as loading controls for (C) and (E).
Despite the established roles of MSX family transcription factors in development, their functions have not been depicted in hESCs. We explored their potential functions in mediating BMP signaling pathway. BMP-4 stimulation rapidly up-regulated the mRNA levels of MSX1 and MSX2 in hESCs (as early as 30 min post-stimulation) (Fig. 4B). Concomitant to the increase in mRNA, MSX2 protein levels were also elevated after BMP-4 stimulation (Fig. 4C). In contrast, BMP-4 stimulation failed to alter levels of MSX1 protein, and in addition, mRNA levels of MSX1 were significantly lower than those of MSX2 in hESCs.
Depleting MSX2 prevented the morphological changes induced by BMP-4 in hESCs (Fig. 4D). Western blot analysis showed that MSX2 depletion also attenuated the downregulation of OCT-4 and SOX2 (Fig. 4E). Interestingly, the rescuing effect of MSX2 depletion on OCT-4 expression was more prominent than SOX2. In addition, down-regulation of mRNA levels of POU5F1, SOX2 and NANOG was much slower in MSX2-depleted cells than control cells after BMP-4 stimulation. These results indicate that MSX2 acts as a key component of BMP signaling in hESCs. Future experiments will dissect the potential role of the DLX family members in regulating BMP signals or other signaling pathways, pluripotency and differentiation in hESCs.
DISCUSSION
Developing conditions and factors for efficient directed differentiation of human pluripotent stem cells such as hESCs and hiPSCs is essential for their potential applications for the study of early human development, drug discovery and the treatment of diseases. Two strategies have been explored. One is to manipulate developmental pathways via the use of growth factors and their antagonists, and the other is to identify chemical compounds to direct the differentiation activities. The benefits of utilizing compounds to control stem cell fate include the elimination of animal products, better temporal and tunable control, ease of production and significant cost reduction for materials. Despite these benefits, there is no documentation to date for compounds that induce efficient differentiation from hESCs and hiPSCs to neural progenitors. Here we identified compound C, which potently suppresses mesoderm, endoderm and trophoectoderm activities and can be applied to highefficiency neural conversion in hESCs and hiPSCs. Our study provides the first example for the efficient derivation of neural progenitor cells from human pluripotent stem cells with a single chemical compound.
We found that compound C can induce nearly 90% of hESCs to form neuroectoderm in adherent cultures. Why is this compound so potent in inducing neural conversion? Our results suggest the following mechanisms. Compound C inhibits at least seven type I and type II TGF-β superfamily receptors, including two Activin receptors and five BMP receptors, thus effectively preventing Activin and BMP signals. Indeed, our experiments show that depletion of at least two BMP receptors is necessary for robust inhibition of BMP-induced signaling activities. Furthermore, dual inhibition of the BMP and the Activin pathway is necessary for efficient neural conversion in hESCs. In this scenario, inhibition of Activin and BMP signaling suppresses endoderm, mesoderm and trophoectoderm activities, thereby facilitating hESC differentiation into the neuroectoderm lineage. This notion is supported by our observation and a recent report that inhibition of BMP and Activin signaling alone improves the efficiency of neural induction, but combined inhibition (with Noggin and SB431542) leads to high-efficiency neural conversion (Fig. 2) [10]. Therefore, our results identified TGF-β superfamily receptors and the Activin and BMP signaling pathways as the main biological targets in hESCs. The ability to target multiple TGF-β family receptors, dual inhibition of Activin and BMP signaling and suppression of Activin- and BMP-induced endoderm, mesoderm and trophoectoderm activities could account for the effects of compound C on differentiation and neural conversion in hESCs.
Neural progenitors and more specified functional neural subtypes were derived previously from hESCs by using EBs as the initial step [35, 36]. While the EB-based procedures are effective and flexible in producing desired differentiated cell types, they often involve multiple steps, produce multiple differentiation lineages and require subsequent isolation/enrichment of specific differentiated cells. These limitations could hinder the acquisition of efficient neural conversion and the generation of large-scale neural precursors. In our study, we applied a single chemical compound to hESCs and hiPSCs cultivated in adherent cultures and achieved rapid, high-efficiency neural conversion in a single step. Compared with the Noggin - based protocol [10], our method produces a slightly higher efficacy of induction and also offers some other potential benefits in the long term. First, compound C costs significantly less than Noggin, thereby markedly reducing the cost for deriving neural progenitor cells from hESCs and hiPSCs, especially on a large-scale. Second, utilization of the defined medium mTeSR [37] prior to the induction of neural conversion eliminates the variability introduced by the MEFs or MEF-CM. Furthermore, xeno-free TeSR2 and KSR media can be used if more clinically-compatible culture conditions are desired. Third, compound C can be conveniently delivered and removed and is therefore highly suited for therapeutic applications. Thus, our method is highly suitable for large-scale efficient derivation of potentially therapy-grade neural precursors from hESCs and hiPSCs and should facilitate the utilization of these cells for the study of early human neural development, drug discovery, tissue repair and regeneration.
Compound C had long been used as a specific inhibitor against AMPK [24, 25]. It was recently found to inhibit the type I BMP receptors ALK2, ALK3 and ALK6 and induce dorsalization in zebrafish (thus denoted as dorsomorphin) [28]. Our large-scale proteomic analysis confirmed these known targets but discovered far more additional targets, including many other members of the TGF-β superfamily receptors, of the SNF/AMPK family and PDGF receptors. This approach served as a starting point, enabling us to identify the targets and pathways of compound C in hESCs. Our findings might explain the variability in previous studies with compound C and could provide a platform for further analysis of its potential targets in various model systems. They also suggest that experimentation with kinase inhibitors should be evaluated critically under the specific cellular and biological context. As such, our results outline a strategy that may be utilized in future studies with kinase inhibitors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Renhe Xu for providing the ID-120-luc reporter construct and Dr. Yisrael Sidis for providing the pARE-GL3 construct, Jenny Drnevich for analyzing the microarray data and members of the Wang lab for helpful discussions. Support was provided by NIH (GM-83812; to F.W.), the Illinois Regenerative Medicine Institute (IDPH 2006-05516; to F.W.), NSF CAREER award (0953267; to F.W.), the Beckman award from the University of Illinois and the National Natural Science Foundation of China (30728022; to F.W. and E.D.).
Footnotes
Author Contributions
Jiaxi Zhou: Conception and design, manuscript writing, collection and assembly of data, data analysis and interpretation
Pei Su: Collection and assembly of data, data analysis and interpretation
Dong Li: Collection and assembly of data, data analysis and interpretation
Stephanie Tsang: Collection and assembly of data, data analysis and interpretation
Enkui Duan: Conception and design, data analysis and interpretation, financial support
Fei Wang : Conception and design, financial support, manuscript writing, final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Levenstein ME, Ludwig TE, Xu RH, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 5.Xu RH, Sampsell-Barron TL, Gu F, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 7.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 9.D'Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 10.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 12.Smith JR, Vallier L, Lupo G, et al. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 15.Borowiak M, Maehr R, Chen S, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Su P, Wang L, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Rovira TCE, Massagué J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- 19.Sidis Y, Tortoriello DV, Holmes WE, et al. Follistatin-related protein and follistatin differentially neutralize endogenous vs. exogenous activin. Endocrinology. 2002;143:1613–1624. doi: 10.1210/endo.143.5.8805. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 21.Hao J, Daleo MA, Murphy CK, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daheron L, Opitz SL, Zaehres H, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 23.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 24.DG H. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerling BMVB, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 2007;581:5727–5731. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bain J, McLauchlan H, Elliott M, et al. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabian MA, Biggs WH, 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 30.Attisano LWJ. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 31.Inman GJ, Nicolas FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 32.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 33.Pituello F, Yamada G, Gruss P. Activin A inhibits Pax-6 expression and perturbs cell differentiation in the developing spinal cord in vitro. Proc Natl Acad Sci U S A. 1995;92:6952–6956. doi: 10.1073/pnas.92.15.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollnagel AOV, Heymer J, Rüther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 35.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang SCWM, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.