Abstract
Background
Although the incidence of sepsis is higher in men than women, it is controversial whether there are gender differences in sepsis-associated mortality.
Objective
To test the hypothesis that hospital mortality is higher in men compared to women with severe sepsis or septic shock and requiring intensive care.
Methods
Retrospective cohort study of 18,757 intensive care unit (ICU) patients, including 8,702 women (46%), with severe sepsis or septic shock in the Cerner Project IMPACT database.
Results
Hospital mortality was higher in women vs. men (35% vs. 33%, p = 0.006). After adjusting for differences in baseline characteristics and processes of care, women had a higher likelihood of hospital mortality than men (OR = 1.11, 95% CI = 1.04 – 1.19, p = 0.002). Women were less likely than men to receive deep venous thrombosis prophylaxis (OR = 0.90, 95% CI = 0.84 – 0.97), invasive mechanical ventilation (OR = 0.81, 95% CI = 0.76 – 0.86), and hemodialysis catheters (OR = 0.85, 95% CI = 0.78 – 0.93). Women were more likely than men to receive red blood cell transfusions (OR = 1.15, 95% CI = 1.09 – 1.22) and code status limitations (OR = 1.31, 95% CI = 1.18 – 1.47).
Conclusions
In this large cohort of ICU patients, women with severe sepsis or septic shock had a higher risk of dying in the hospital than men. This difference remained after multivariable adjustment. We also found significant gender disparities in some aspects of care delivery, but these did not explain the higher mortality in women.
Keywords: gender, sex distribution, sepsis, infection, shock, critical care
INTRODUCTION
Animal studies indicate that females have advantageous immunologic1, 2 and cardiovascular responses3 during infectious challenge. Epidemiological studies consistently report higher sepsis incidence in males compared to females, suggesting the advantageous female response to infection is also present in humans4–9. In contrast, clinical sepsis studies evaluating gender-mortality relationships are inconsistent, showing no gender difference 6, 10–12, higher risk in men13, 14, or higher risk in women9, 15. The inconsistency may result from differences in study design, including the use of billing codes for diagnosis6, 9, 10, 12, 14, limited sample size11, 13, 15, limited risk adjustment9, 10, 12, inclusion of non-ICU patients6, 9, 10, 12, 14, or inclusion of patients without severe sepsis or septic shock10, 12, 14.
The sepsis spectrum includes patients with widely varying mortality risks, and most patients who die have either severe sepsis or septic shock and require intensive care unit (ICU) admission16. Studies focused in the ICU could provide unique insights about gender-mortality relationships in critically ill patients with severe sepsis / septic shock. Identifying an association between gender and mortality in severe sepsis / septic shock would provide impetus for investigating biological and environmental causes of these disparities.
The purpose of our study was to test our hypothesis that the experimentally-demonstrated protective effects of female sex are manifested by lower mortality in women vs. men with severe sepsis / septic shock and requiring ICU admission.
MATERIALS AND METHODS
This retrospective cohort study used the Cerner Project IMPACT, Inc. (Bel Air, Maryland) database (CPI), and included patients from 98 ICUs in 71 U.S. hospitals and 4 Canadian or Brazilian hospitals. Each contributing ICU employs trained staff and standardized software to prospectively collect data on 50–100% of its ICU patients. Personnel at each site undergo training and certification to ensure uniform application of database-specific definitions and accurate data entry. The Cerner Project IMPACT software used at all participating centers has built-in checks for inconsistent or invalid entries. Only entries passing these validation checks are included in the Cerner Project IMPACT database. Data are transferred quarterly from participating sites to a central site where they are aggregated and undergo additional extensive quality control checking. The Cerner Project IMPACT database has been validated and used extensively since its creation by the Society of Critical Care Medicine in 199617–19. A random sample of admitted patients was selected for ICUs enrolling <100% of their patients.
Severe sepsis / septic shock patients ≥ 16 years of age hospitalized from mid-2003 (coincident with the release of the CPI version prospectively identifying severe sepsis cases) through 2006 were eligible. The CPI diagnostic criterion for severe sepsis was development of at least one severe acute organ dysfunction within 3 days of a presumed infection. Patients were excluded if gender, age, or hospital mortality was missing. For each patient, only data from the first ICU admission was analyzed. The University of Rochester Institutional Review Board provided exemption from informed consent.
Statistical Methods
Gender was the primary independent variable and hospital mortality was the primary outcome variable. Variable definitions are provided in the Appendix. Normally distributed variables are presented as mean ± standard deviation (S.D.) and skewed variables are presented as median (interquartile range [IQR]).
Available covariates were evaluated for associations with gender and hospital mortality using chi-square testing for categorical variables and student’s t-test or Kruskal-Wallis test for continuous variables, as appropriate. Variables associated with gender or hospital mortality (p ≤ 0.05) were included in the base logistic regression model. Interaction terms with gender were included if a variable showed > 30% departure from additive and multiplicative model predictions20.
Covariates were sequentially eliminated from the base model if the likelihood ratio test comparing nested models was insignificant (p > 0.10) and the odds ratios involving gender changed < 10% after exclusion. The model excluded 439 patients (2.3%) because of missing values. Model performance was assessed using the C statistic and Hosmer-Lemeshow test21, and model diagnostics included leverage and Pregibon’s delta beta plots22. Standard errors were calculated with robust variance estimators and ICU-level clustering, allowing for correlations between observations within ICUs23.
Two sensitivity analyses were performed. The CPI severe sepsis diagnostic criterion differs slightly from published American College of Chest Physicians / Society of Critical Care Medicine consensus conference criteria (“consensus criteria”)24. We applied consensus criteria to each patient in the dataset, then re-computed the model excluding patients who did not meet both CPI and consensus criteria. The second sensitivity analysis re-computed the model after excluding influential observations (leverage or Pregibon’s delta beta ≥ 99th percentile).
Using an expected mortality in women of 28%6, the calculated sample size was 7,903 patients of each gender to detect a ≥ 2% difference in hospital mortality with 80% power and two-sided alpha (α) = 0.05. Statistical analyses were performed using Stata/ SE version 9.2 (Stata Corp., College Station, TX).
RESULTS
18,846 patients met CPI criteria for severe sepsis. Seven subjects were excluded because age was missing or < 16 years, and 82 were excluded because of missing hospital mortality, leaving 18,757 patients (10,055 [54%] men and 8,702 [46%] women). At least 97% of patients were from U.S. hospitals. Hospitals had 496 (IQR = 350–615) beds and ICUs had 17 (IQR = 12–21) beds.
Gender comparisons are shown in Table I. Women were older and more likely to have dependent functional status at ICU admission than men. African-American race was the only racial / ethnic category associated with female gender, so the categories were collapsed into African – American race vs. other races in subsequent analyses. Men and women had similar Acute Physiology and Chronic Health Evaluation (APACHE) II scores and Simplified Acute Physiology Score (SAPS) II-predicted mortality, and similarly short delays between hospital and ICU admission (0 days, interquartile range 0–1). The most common index organ dysfunctions were cardiovascular and respiratory (see Appendix for definitions of organ dysfunction). Over 50% of patients experienced cardiovascular dysfunction, defined as refractory hypotension (see Appendix), and therefore met criteria for septic shock. There were gender differences in the frequency of specific organ dysfunctions, but the number of dysfunctional organs did not differ by gender (χ2 = 5.49, p = 0.48, Figure 1). There were gender differences in the sources of infection (Table I).
Table I.
Bivariate Association Between Other Independent Variables and Gender
| Covariate | Women (n = 8,702) | Men (n = 10,055) | OR (95% CI) | p value | |
|---|---|---|---|---|---|
|
Patient characteristics |
|||||
| Age (years) | 68 (54 – 75)* | 65 (52 – 76)* | -- | 0.0001 | |
| Dependent functional status at admission† | 44% | 36% | 1.39 (1.31 – 1.48) | <0.0001 | |
| Race / Ethnicity | |||||
| Caucasian | 74% | 75% | 0.94 (0.88 – 1.01) | 0.08 | |
| African-American | 14% | 12% | 1.18 (1.09 – 1.29) | 0.0001 | |
| Latin/ Hispanic | 5% | 6% | 0.87 (0.77 – 0.99) | 0.04 | |
| Asian or Pacific Islander | 1% | 1% | 0.93 (0.72 – 1.20) | 0.55 | |
| American Indian | < 1% | < 1% | 0.65 (0.39 – 1.08) | 0.08 | |
| Other or unknown | 5% | 5% | 1.02 (0.89 – 1.17) | 0.79 | |
| Admitted from health care facility‡ | 53% | 52% | 1.03 (1.00 – 1.06) | 0.06 | |
| Previous ICU admission this hospitalization | 4.3% | 4.7% | 0.91 (0.79 – 1.05) | 0.18 | |
| CPR within 24 hours of ICU admission | 4.8% | 5.0% | 0.96 (0.84 – 1.10) | 0.57 | |
| Medical patient (vs. surgical) | 82% | 82% | 1.00 (0.92 – 1.08) | 0.88 | |
| Medicaid or self-pay vs. other insurance § | 15% | 15% | 1.00 (0.92 – 1.08) | 0.92 | |
|
Comorbidity |
|||||
| Chronic liver disease | 3% | 4% | 0.73 (0.63 – 0.86) | 0.0001 | |
| Active cancer within 5 years | 14% | 17% | 0.81 (0.75 – 0.88) | <0.0001 | |
| Chronic cardiovascular disease | 6% | 7% | 0.80 (0.71 – 0.90) | 0.0002 | |
| Chronic renal disease | 6% | 6% | 0.90 (0.80 – 1.02) | 0.10 | |
| Chronic respiratory disease | 10% | 10% | 1.04 (0.94 – 1.14) | 0.45 | |
| Immunocompromise | 13% | 13% | 0.99 (0.91 – 1.08) | 0.80 | |
|
Severity of illness variables |
|||||
| Neurologic dysfunction | 7% | 5% | 1.32 (1.17 – 1.49) | <0.0001 | |
| Cardiovascular dysfunction | 58% | 54% | 1.20 (1.13 – 1.27) | <0.0001 | |
| Respiratory dysfunction | 43% | 47% | 0.83 (0.78 – 0.88) | <0.0001 | |
| Elevated serum lactate | 10% | 9% | 1.15 (1.04 – 1.27) | 0.0042 | |
| Acute renal failure | 20% | 18% | 1.13 (1.05 – 1.22) | 0.0012 | |
| Hepatic dysfunction | 8% | 9% | 0.87 (0.79 – 0.97) | 0.011 | |
| Hematologic dysfunction | 16% | 17% | 0.89 (0.83 – 0.97) | 0.0047 | |
| APACHE II score | 21 (15–27)* | 21 (15–27)* | -- | 0.49 | |
| SAPS II predicted mortality | 35% (15 – 64%)* | 33% (14 – 64%)* | -- | 0.19 | |
|
Source of infection |
|||||
| Urinary infection | 31% | 19% | 1.97 (1.84 – 2.10) | <0.0001 | |
| Intra-abdominal infection | 12% | 9% | 1.33 (1.21 – 1.46) | <0.0001 | |
| Chest infection | 37% | 48% | 0.63 (0.60 – 0.67) | <0.0001 | |
| Bloodstream infection | 23% | 24% | 0.94 (0.88 – 1.00) | 0.06 | |
| Unknown infection | 3.8% | 3.5% | 1.10 (0.94 – 1.28) | 0.23 | |
|
Processes of care |
|||||
| Code Status limitations ** | 9% | 7% | 1.31 (1.18 – 1.47) | <0.0001 | |
| Invasive mechanical ventilation | 63% | 68% | 0.81 (0.76 – 0.86) | <0.0001 | |
| HD catheter | 12% | 14% | 0.85 (0.78 – 0.93) | 0.0002 | |
| PRBC transfusion | 42% | 39% | 1.15 (1.09 – 1.22) | <0.0001 | |
| DVT prophylaxis | 75% | 77% | 0.90 (0.84 – 0.97) | 0.0034 | |
| Intravenous nutrition | 18% | 19% | 0.93 (0.86 – 1.00) | 0.06 | |
| FFP transfusion | 17% | 18% | 0.94 (0.87 – 1.01) | 0.10 | |
| Stress ulcer prophylaxis | 43% | 44% | 0.96 (0.90 – 1.01) | 0.12 | |
| CCM coverage | 71% | 72% | 0.96 (0.90 – 1.02) | 0.20 | |
|
Hospital Characteristics |
|||||
| Accredited residencies | 69% | 71% | 0.89 (0.83 – 0.94) | 0.0002 | |
| Accredited CCM fellowship | 28% | 30% | 0.92 (0.85 – 0.96) | 0.002 | |
| Academic hospital †† | 21% | 22% | 0.92 (0.86 – 0.99) | 0.02 | |
| Number of hospital beds (quartiles) ‡‡ | -- | -- | 0.97 (0.95 – 1.00) | 0.02 §§ | |
| Urban hospital | 60% | 62% | 0.94 (0.89 – 1.00) | 0.05 | |
| Suburban hospital | 26% | 25% | 1.06 (0.99 – 1.13) | 0.10 | |
| Rural hospital | 14% | 13% | 1.03 (0.95 – 1.12) | 0.43 | |
| Medical school | 21% | 22% | 0.94 (0.88 – 1.01) | 0.10 | |
Numbers in parentheses represent interquartile range;
206 missing values;
46 missing values;
90 missing values;
191 missing values;
4 missing values;
6 missing values;
Mantel Haenszel odds ratio per quartile increase in hospital beds.
Abbreviations: OR= odds ratio; CI = confidence interval; APACHE = acute physiology and chronic health evaluation score; SAPS = simplified acute physiology score; FFP = fresh frozen plasma; HD = hemodialysis; PRBC = packed red blood cells; DVT = deep venous thrombosis; CCM = critical care medicine
Figure 1.
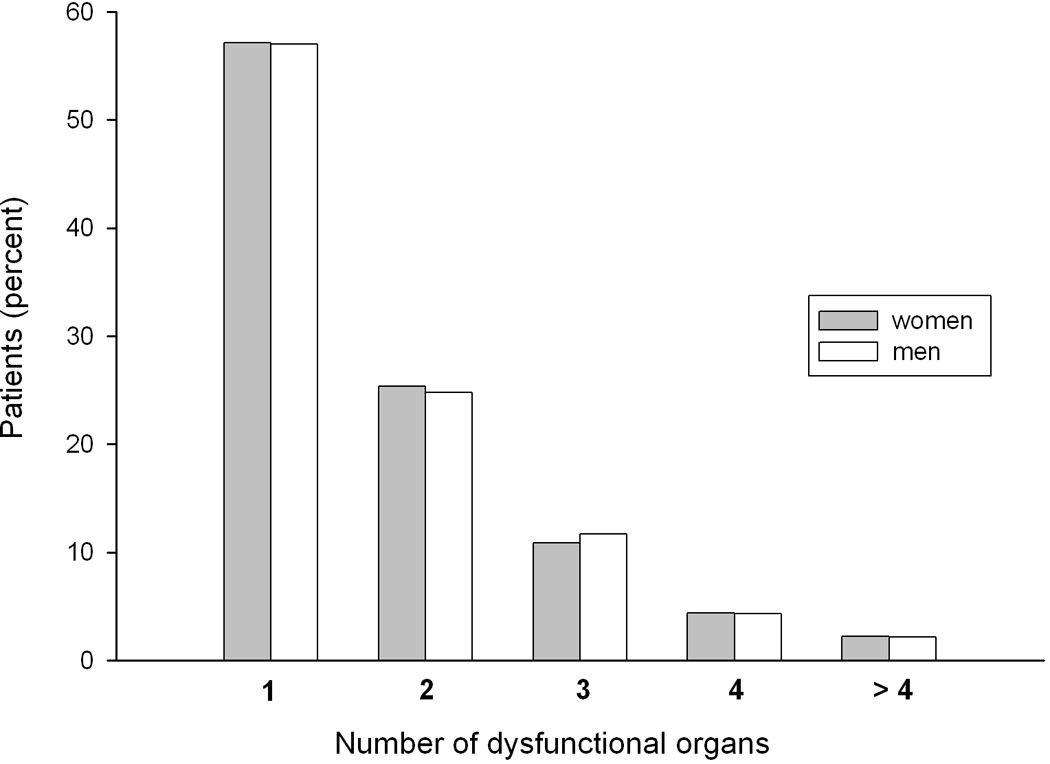
Number of dysfunctional organs in men and women
Regarding ICU processes of care, women were more likely to have code status limitations and receive packed red blood cell (PRBC) transfusions, and men were more likely to receive invasive mechanical ventilation at ICU admission, deep venous thrombosis (DVT) prophylaxis, and hemodialysis catheters (Table I).
Hospital mortality was significantly higher in women vs. men (35% vs. 33%, p = 0.006, Table II). Women also had a higher ICU mortality rate and a lower likelihood of independence upon hospital discharge when compared to men (Table II). Hospital length of stay (LOS) was shorter in women (women = 12 [IQR 7–21] days vs. men = 13 [IQR 7–23] days, p = 0.0001) even when excluding hospital non-survivors (women = 14 [IQR 9–23] days vs. men = 15 [IQR 9–25] days, p < 0.0001), so this was not simply because of shorter hospital survival in women.
Table II.
Unadjusted Outcome Differences by Gender
| Outcome | Women (n = 8,702) | Men (n = 10,055) | OR (95% CI) | p value* |
|---|---|---|---|---|
| Hospital Mortality (n = 6,359) |
35% | 33% | 1.09 (1.02 – 1.16) | 0.006 |
| ICU Mortality (n = 4,310) |
24% | 22% | 1.09 (1.02 – 1.17) | 0.01 |
| Independent on hospital discharge (n = 3,620) |
18% | 20% | 0.88 (0.82 – 0.95) | 0.0006 |
Chi-square test used for significance testing.
Many covariates were associated with hospital mortality (Table III). No variables met criteria for effect-modification on either the additive or multiplicative scale. The possible interaction between gender and age categories was of particularly importance in this regard, since previous studies in critically ill patients suggest that the higher risk of mortality in women is limited to patients > 50 years of age25, 26. In contrast, we found that the association between female gender and death was similar in subgroups of patients < 50 years (OR = 1.13, 95% CI = 0.96 – 1.32) and ≥ 50 years (OR = 1.06, 95% CI = 1.00 – 1.14), with the Mantel-Haenszel age category-adjusted OR = 1.07 (95% CI = 1.01 – 1.14, p = 0.02) and no evidence of interaction between age categories and gender in predicting mortality (p value for Breslow-Day test of homogeneity = 0.50).
Table III.
Associations Between Independent Variables and Hospital Mortality
| Bivariate analysis * |
Multivariable analysis † |
||||
|---|---|---|---|---|---|
| Covariate | OR (95% CI) | p value | OR (95% CI) | p value | |
|
Patient characteristics |
|||||
| Female gender | 1.09 (1.02 – 1.16) | 0.006 | 1.11 (1.04 – 1.19) | 0.002 | |
| Age quintiles | 1.27 (1.24 – 1.30) | < 0.001 | 1.20 (1.16 – 1.23) | < 0.001 | |
| Dependent functional status at admission | 1.53 (1.43 – 1.62) | < 0.001 | 1.30 (1.18 – 1.43) | < 0.001 | |
| African-American race | 0.95 (0.86 – 1.04) | 0.24 | 0.89 (0.77 – 1.04) | 0.137 | |
| Admitted from health care facility | 1.38 (1.30 – 1.47) | < 0.001 | 1.49 (1.37 – 1.62) | < 0.001 | |
| Previous ICU admission this hospitalization | 1.19 (1.03 – 1.37) | 0.02 | ns ‡ | ns | |
| CPR within 24 hours of ICU admission | 3.22 (2.81 – 3.70) | < 0.001 | 1.81 (1.56 – 2.11) | < 0.001 | |
| Medical patient (vs. surgical) | 1.23 (1.13 – 1.34) | < 0.001 | 1.55 (1.36 – 1.76) | < 0.001 | |
| Medicaid or self-pay vs. other insurance | 0.71 (0.65 – 0.78) | < 0.001 | ns | ns | |
|
Comorbidity |
|||||
| Chronic liver disease | 1.84 (1.57 – 2.15) | < 0.001 | 1.90 (1.55 – 2.34) | < 0.001 | |
| Active cancer within 5 years | 1.87 (1.72 – 2.02) | < 0.001 | 1.34 (1.20 – 1.50) | < 0.001 | |
| Chronic cardiovascular disease | 1.63 (1.44 – 1.83) | < 0.001 | 1.19 (1.01 – 1.40) | 0.037 | |
| Chronic renal disease | 1.48 (1.31 – 1.68) | < 0.001 | ns | ns | |
| Chronic respiratory disease | 1.16 (1.05 – 1.29) | 0.002 | 1.15 (1.04 – 1.28) | 0.009 | |
| Immunocompromise | 1.86 (1.71 – 2.04) | < 0.001 | 1.53 (1.34 – 1.75) | < 0.001 | |
|
Severity of illness variables |
|||||
| Neurologic dysfunction | 1.47 (1.30 – 1.65) | < 0.001 | 1.23 (1.05 – 1.44) | 0.01 | |
| Cardiovascular dysfunction | 1.97 (1.85 – 2.10) | < 0.001 | 1.24 (1.11 – 1.40) | < 0.001 | |
| Respiratory dysfunction | 1.26 (1.19 – 1.34) | < 0.001 | ns | ns | |
| Elevated serum lactate | 2.64 (2.39 – 2.91) | < 0.001 | 1.14 (0.99 – 1.32) | 0.067 | |
| Acute renal failure | 2.67 (2.47 – 2.88) | < 0.001 | 1.36 (1.24 – 1.50) | < 0.001 | |
| Hepatic dysfunction | 1.44 (1.29 – 1.60) | < 0.001 | 1.15 (0.97 – 1.35) | 0.104 | |
| Hematologic dysfunction | 1.60 (1.48 – 1.73) | < 0.001 | 1.36 (1.22 – 1.53) | < 0.001 | |
| APACHE II score (per 1 point increase in score) | 1.11 (1.10 – 1.11) | < 0.001 | ns | ns | |
| SAPS II score (per 1 point increase in score) | 1.06 (1.05 – 1.06) | < 0.001 | 1.04 (1.03 – 1.04) | < 0.001 | |
|
Source of infection |
|||||
| Urinary infection | 0.86 (0.80 – 0.93) | < 0.001 | 0.83 (0.76 – 0.92) | < 0.001 | |
| Intra-abdominal infection | 1.41 (1.28 – 1.55) | < 0.001 | ns | ns | |
| Chest infection | 0.94 (0.89 – 1.00) | 0.06 | 1.08 (1.00 – 1.17) | 0.06 | |
| Bloodstream infection | 1.23 (1.15 – 1.32) | < 0.001 | ns | ns | |
| Unknown infection | 1.67 (1.43 – 1.96) | < 0.001 | 1.21 (1.00 – 1.47) | 0.05 | |
|
Processes of care |
|||||
| Code status limitations | 1.84 (1.65 – 2.06) | < 0.001 | 1.74 (1.51 – 2.01) | < 0.001 | |
| Invasive mechanical ventilation | 2.37 (2.21 – 2.54) | < 0.001 | 2.19 (1.97 – 2.44) | < 0.001 | |
| HD catheter | 1.69 (1.55 – 1.84) | < 0.001 | 1.23 (1.08 – 1.40) | 0.002 | |
| PRBC transfusion | 1.32 (1.24 – 1.41) | < 0.001 | ns‡ | ns | |
| DVT prophylaxis | 0.64 (0.60 – 0.69) | < 0.001 | 0.60 (0.54 – 0.67) | <0.001 | |
| Intravenous nutrition | 1.24 (1.15 – 1.34) | < 0.001 | ns | ns | |
| FFP transfusion | 2.23 (2.06 – 2.41) | < 0.001 | 1.57 (1.41 – 1.75) | < 0.001 | |
| Stress ulcer prophylaxis | 0.54 (0.50 – 0.57) | < 0.001 | 0.60 (0.55 – 0.65) | <0.001 | |
| CCM coverage | 1.28 (1.19 – 1.38) | < 0.001 | 1.16 (1.02 – 1.31) | 0.019 | |
|
Hospital Characteristics |
|||||
| Accredited residencies | 1.02 (0.96 – 1.10) | 0.447 | ns | ns | |
| Accredited CCM fellowship | 1.23 (1.15 – 1.32) | < 0.001 | 1.20 (1.03 – 1.40) | 0.022 | |
| Academic hospital | 1.27 (1.18 – 1.36) | < 0.001 | 1.17 (1.01 – 1.36) | 0.041 | |
| Number of hospital beds (quartiles) | 1.04 (1.02 – 1.07) | 0.002 | 1.05 (0.99 – 1.11) | 0.115 | |
| Urban hospital | 1.07 (1.00–1.14) | 0.04 | ns | ns | |
| Suburban hospital | 0.98 (0.91 – 1.05) | 0.58 | ns | ns | |
| Rural hospital | 0.90 (0.82 – 0.99) | 0.025 | 0.89 (0.74 – 1.07) | 0.231 | |
| Medical school | 1.33 (1.23 – 1.43) | < 0.001 | ns | ns | |
Chi-square test;
Results of parsimonious multiple logistic regression model as described in Methods.
Covariates labeled “ns” (abbreviation for “not significant”) fell out of the logistic regression model during analysis (see Methods).
Results of the final multiple logistic regression model are shown in Table III. Female gender remained significantly associated with hospital mortality after adjustment (OR = 1.11, 95% CI = 1.04 – 1.19, p = 0.002). The model had excellent discrimination (C statistic = 0.7989) and calibration (Hosmer Lemeshow statistic = 7.14, p = 0.52). Sensitivity analyses showed minimal change in gender risk after excluding 1,401 patients (7% of the sample) not meeting both sets of diagnostic criteria (OR = 1.13, 95% CI = 1.05 - 1.22, p = 0.001), or after excluding the 797 most influential observations (OR = 1.13, 95% CI = 1.06 - 1.22, p = 0.001).
The multiple logistic regression analysis was repeated without any process of care covariates (i.e., fresh frozen plasma transfusion, mechanical ventilation, hemodialysis catheter placement, stress ulcer prophylaxis, DVT prophylaxis, code status limitations, and critical care medicine specialty coverage were removed). The gender risk was similar (OR = 1.11, 95% CI = 1.03 – 1.19, p = 0.004), reinforcing the conclusion that these care processes were not responsible for higher mortality in women.
We performed exploratory bivariate analyses to determine whether gender disparities in care processes (Table I) were explained by other clinically relevant variables (Table IV). For example, more frequent code status limitations among women might stem from their older age or more impaired functional status. However, Table IV shows that gender differences in code status persisted within strata of functional status and in the subgroup of patients > 65 years. Likewise, women were more likely to receive PRBC transfusions during the first 24 hours of ICU care when nadir hematocrit (Hct) was > 31% (a potentially deleterious practice27), less likely to receive invasive mechanical ventilation regardless of respiratory infection or dysfunction, and less likely to receive hemodialysis catheters regardless of acute renal failure.
Table IV.
Subgroup Analyses of Processes of Care in Women Compared to Men *
| Likelihood of women vs. men receiving code status limitations† | |
| Subgroups of patients with: | Stratum-specific associations OR (95% CI) |
| Impaired functional status | 1.14 (1.00 – 1.31) |
| Non-impaired functional status | 1.34 (1.09 – 1.63) |
| Age > 65 years | 1.31 (1.15 – 1.49) |
| Age ≤ 65 years | 1.02 (0.80 – 1.30) |
| Likelihood of women vs. men receiving mechanical ventilation at ICU admission | |
| Subgroups of patients with: | Stratum-specific associations OR (95% CI) |
| Chest infection | 0.88 (0.79 – 0.98) |
| Other infection | 0.92 (0.85 – 0.99) |
| Acute respiratory dysfunction | 0.83 (0.71 – 0.96) |
| Other organ dysfunction | 0.87 (0.80 – 0.94) |
| Likelihood of women vs. men receiving PRBC transfusion on 1st ICU day | |
| Subgroups of patients with: | Stratum-specific associations OR (95% CI) |
| Nadir hematocrit on 1st ICU day > 31% | 1.19 (1.06 – 1.32) |
| Nadir hematocrit on 1st ICU day ≤ 31% | 1.01 (0.93 – 1.09) |
| Likelihood of women vs. men receiving hemodialysis catheter placement | |
| Subgroups of patients with: | Stratum-specific associations OR (95% CI) |
| Acute renal failure present | 0.84 (0.72 – 0.98) |
| Acute renal failure absent | 0.82 (0.74 – 0.91) |
Each process of care is considered an outcome measure. The odds ratios refer to the likelihood of receiving the process of care in women vs. men, within the subgroup listed. For example, the odds ratio of 1.14 applies specifically to patients with impaired functional status, and indicates that women in this subgroup have a 14% higher likelihood of having code status limitations than men in this same subgroup.
191 missing values
DISCUSSION
Our retrospective cohort study of ICU patients with severe sepsis / septic shock indicates that women have approximately 10% greater risk of hospital mortality than men; this is true in both bivariate analysis and multiple logistic regression analysis controlling for numerous potential confounding variables. We also find subtle but significant gender differences in processes of care, although these do not account for the mortality difference.
Our results are derived from CPI, a database designed to measure outcomes of critically ill patients. Severe sepsis was diagnosed prospectively by staff trained to achieve consistent data collection17–19, and over 90% of patients also met published diagnostic consensus criteria24. The standardized, prospective data collection and diagnostic confirmation are important strengths of this study.
Possible mechanisms of higher severe sepsis mortality in women
Our hypothesis, that hospital mortality would be lower in women than men with severe sepsis / septic shock, was based on laboratory evidence indicating an estrogen-mediated female survival advantage during experimentally-induced sepsis1, 3. Our contrary findings suggest that this hypothesis was overly simplistic, a conclusion supported by divergent results of human studies measuring gender-specific responses to endotoxin28, 29, paradoxically higher estrogen concentrations in elderly vs. younger critically ill women, elevated estrogen concentrations in critically ill men, and the association of higher estrogen levels with higher mortality in both women and men30, 31. Elevated estrogen levels may simply be a surrogate for severity of illness, because in critical illness estradiol concentrations are primarily determined by the adrenal stress response and peripheral aromatase activity31. However, estrogens also have physiologic actions that could be detrimental in sepsis32. Unfortunately we cannot determine whether higher estrogens concentrations are associated with higher mortality in women because gonadal hormone levels are not available in this dataset.
Non-biological explanations for our findings must also be considered. We found significant gender-differences in processes of care, consistent with previous studies25, 33, 34. These differences could originate from gender differences in treatment preferences or gender bias in clinical care35. For example, gender variation in code status limitations could stem from less aggressive treatment preferences by women (or their surrogates)36, 37, or the influence of health care providers who affect end-of-life decisions yet often misperceive patient wishes38, 39. Importantly, our analysis showed that the observed disparities in care did not account for higher female mortality. Nevertheless, further investigation is required to determine whether these disparities are associated with other adverse clinical outcomes, and whether gender disparities exist in other care processes. Gender differences in numerous other observed covariates (e.g., age, sites of infection, functional status, and comorbid conditions, see Table III for full list) also cannot explain the observed mortality difference, since multivariable analysis adjusted for all of them.
Gender-based differences in symptoms40, presentation of illness41, or diagnostic bias42 are additional potential explanations for both the hospital mortality and infection site differences we observed between men and women. Gender differences in sites of infection have been observed previously12, 13, 43, but like the hospital mortality difference, it is unclear whether they originate from gender differences in biology1–3, comorbidity12, 13, or medical assessment and care40–42.
Clinical implications of higher severe sepsis mortality in women
The clinical implications of our findings will largely depend on elucidation of the underlying mechanisms. For example, novel gender-targeted therapeutic strategies could be developed if women are found to have greater aromatase activity that is associated with higher estradiol levels and excess mortality. New policies may ensure equal application of therapy if gender disparities are discovered in other care processes that affect important clinical outcomes. Finally, educational efforts may be helpful if additional work uncovers gender differences in clinical presentation of illness. By establishing the presence of gender-disparities in sepsis mortality, our findings provide a catalyst for these additional investigations.
Comparison with previous studies
Recent landmark epidemiologic sepsis studies did not find gender differences in mortality6, 10, 12. Martin et al10 identified over 10 million sepsis cases from 1979–2001 and found that septic women were older than men, but there was no mortality difference. Angus, et al6 identified nearly 200,000 severe sepsis cases from 1995 and found that men had higher mortality, but this gender difference disappeared after adjusting for age, comorbidity, and infection site.
In comparison to these studies, our discrepant findings are best explained by differences in methodology and case-mix. Both Martin et al10 and Angus et al6 used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) billing codes. While they included nested validation analyses showing that ICD-9-CM coding successfully identified sepsis in a fraction of their patients, diagnostic accuracy could not be confirmed in the majority. In contrast, over 90% of our patients met both CPI and consensus criteria for severe sepsis; we confirmed our results by repeating the analysis using only these dually-confirmed cases. In addition, Martin et al10 and Angus et al6 included substantial fractions of non-ICU patients. Indeed, approximately 70–80% of patients studied by Martin et al10 did not have any organ dysfunction. In contrast, 100% of our patients had at least one acute organ dysfunction and required ICU care, and over 50% met criteria for septic shock. We speculate that factors responsible for higher female mortality may be accentuated in a more severely ill cohort. Finally, we employed a systematic and comprehensive approach to risk adjustment, beginning with all available covariates sharing a bivariate association with either the risk factor of interest (gender) or the outcome of interest (hospital mortality). This inclusive model was pared down to the parsimonious model using objectively-defined methods. In contrast, multivariable risk adjustment was not employed by Martin et al10. In a subsequent multivariable analysis of this database the authors found that male gender was an independent predictor of mortality14. However, they were unable to include severity of illness and process of care variables in their analysis, model calibration and discrimination were not reported, and the effect of influential observations was not assessed. Angus et al6 did not provide details of their multivariable analysis.
A more recent French study by Adrie et al found 25% lower risk of hospital mortality in 608 women vs. 1,000 men with severe sepsis / septic shock matched by propensity score13. National differences in population demographics or health-care delivery, and / or differences in statistical methods may contribute to the contrary results. Our large sample size permitted multiple logistic regression as the primary statistical approach21.
Our findings support those of Dombrovskiy et al9, who found higher age-adjusted case-fatality rates in women vs. men over a 10 year time interval. Our results are also consistent with several smaller studies indicating that female gender is an independent risk factor for mortality in sepsis and infection15, 44–47.
Study limitations
This study has several limitations. First, the CPI database does not include a random sample of ICUs, potentially introducing selection bias. However, a database containing detailed clinical information from a random sample of U.S. ICUs does not currently exist. Second, if the likelihood of hospital discharge in situations of imminent or expected death (e.g., to hospice) was greater in men, the hospital mortality results could be biased. We were unable to specifically address this concern because the hospital discharge destination was unknown in approximately 40% of patients and 30- or 60-day mortality rates were not available. However, this bias seems unlikely since men had longer hospitalizations and were more likely to be independent upon hospital discharge than women (Table II). Third, we were unable to control for baseline hormonal status because menopausal status, estrous status, and information on chronic use of hormone replacement therapy were not available in this dataset. Our stratified analysis showed that women under age 50 (the approximate median age of menopause of women in the U.S.48) were not spared from the higher mortality risk, in contrast with previous studies of unselected critically ill patients25, 26. Although these data suggest that the findings are independent of menopausal status, they are clearly insufficient to fully evaluate the effect of baseline hormonal status. Finally, other non-observed covariates could confound our results. In this respect, it is notable that our processes of care analyses were limited to variables available in CPI. Future research should evaluate whether there are gender disparities in the use of validated sepsis therapies (e.g., early appropriate antibiotics 49 and goal-directed therapy 50) that could explain our results.
CONCLUSIONS
In our retrospective analysis of a large, prospectively collected dataset of ICU patients with severe sepsis / septic shock, women had significantly higher hospital mortality than men. This difference persisted after adjustment for baseline characteristics and gender differences in some processes of care. Further research should investigate the causes of gender-based differences in hospital mortality and gender disparities in care.
ACKNOWLEDGEMENT
Source of support: This research was supported by the National Heart, Lung and Blood Institute (K23 HL80077).
Additional acknowledgements: Cerner Project IMPACT provided the dataset. Steve Georas, M.D., Mary Anne Morgan, M.D., and Cynthia Mack, R.N. provided valuable critiques of this manuscript.
APPENDIX
Appendix: Definition of Variables
| Covariate | Definition |
|---|---|
| Index infection | Infection present at the time of organ dysfunction or up to 3 days before onset of organ dysfunction |
| Hospital mortality | Death in hospital before discharge |
| ICU mortality | Death in ICU |
| Independent functional status at hospital discharge | “Independent” vs. “other” functional status. “Independent” is defined when the patient is discharged home and independent in activities of daily living. “Other” functional status includes categories of partially dependent, fully dependent, or dead. |
| Hospital length of stay | Number of consecutive days in current acute care hospital |
| ICU length of stay | Number of consecutive days in ICU. Only the duration of the first ICU stay during hospitalization is counted. |
| Age | Age of the patient in years |
| Gender | Male or female |
| SAPS II* score | Calculated from the necessary variables provided in the CPI dataset according to the methods of LeGall, et al 1 |
| SAPS II predicted mortality | Calculated from the necessary variables provided in the CPI dataset according to the methods of LeGall, et al 1 |
| APACHE II score | Calculated from the variables provided in the CPI dataset according to the methods of Knaus, et al 2 |
| Origin prior to hospital admission | Admission from the community vs. a health-care associated facility, the latter including another location within the hospital or transfer from another hospital |
| Previous ICU admission | Previous ICU admission within the same hospitalization |
| Acute renal failure | Creatinine > 1.5 mg/dL evident < 48 hours before ICU admission and associated with oliguria |
| CPR within 24 hours of ICU admission | Self-expanatory |
| Functional status on hospital admission | “Independent” vs. “other” functional status. The “other” category includes partially dependent and fully dependent. |
| Code status on ICU admission | Full vs. “limited.” The “limited” category included no CPR, limited interventions/ withholding therapy, or withdrawing therapy/ comfort care code status. |
| Medicine service vs. surgery | Surgical category includes elective and emergent surgical admissions |
| Index infection | Current infection present at or up to 3 days prior to the time when acute organ dysfunction was detected. At least one of the following conditions must be met: antibiotics started for presumed infection; antibiotics administered for a known active infection (not for antibiotic prophylaxis); purulent drainage from wound or catheter site; radiological evidence of infiltrates and sputum production; white blood cells present in a normally sterile body fluid. |
| Intra-abdominal infection | Infection in the abdominal compartment and pelvis. Includes peritoneal fluid, abscess drainage, and fluid from surgical drain. |
| Bloodstream infection | Bloodstream infection not due to vascular access site |
| Chest infection | Infection of lungs, pleura, pleural fluid, or drainage around chest tube site |
| CNS infection | Infection of brain, meninges, CSF, spine, or drainage from or around invasive CNS device |
| Sinus infection | Infection of fluid in cranial or facial sinus cavity |
| Surgical infection | Infection of any surgical wound site regardless of location |
| Urinary infection | Infection of kidney, bladder, urethra, drainage around invasive device, or perinephric abscess |
| Vascular infection | Infection related to invasive vascular catheter |
| Other infection | Infection of any other known site |
| Unknown infection | Signs of infection present but unknown site (this category is not chosen if there is a clinically suspected site) |
| Index organ dysfunction | Organ dysfunction occurring within ± 3 days of a presumed infection |
| Acute cardiovascular dysfunction | Any one of the following persisting for ≥ 1 hour despite adequate fluid resuscitation: systolic blood pressure (SBP) < 90 mmHg unless known baseline SBP <90 mmHg ; SBP > 40 mmHg below baseline SBP; mean arterial pressure (MAP) < 70mmHg; vasopressor (if dopamine, > 5 mcg/kg/ min) requirement to maintain SBP > 90 or MAP > 70 mmHg |
| Elevated serum lactate | Serum lactate value above the normal range in combination with acute cardiovascular dysfunction on the same day |
| Acute respiratory dysfunction | PaO2 / FiO2 ratio ≤ 300 or PEEP requirement > 5 cm H20 in patients with acute lung injury (patients with cardiogenic pulmonary edema are excluded) |
| Acute renal dysfunction | Creatinine remains increased by > 1 mg/dL after adequate fluid resuscitation or creatinine ≥ 2 mg/dL in the absence of known baseline (patients on chronic dialysis excluded) |
| Acute hematologic dysfunction | Platelet (plt) count half of the highest value in last 3 days, or plt count <100,000mm3, or PT/PTT >1.5 times control in absence of anticoagulant |
| Acute hepatic dysfunction | Acute rise in serum total bilirubin to a level > 2 mg/dL |
| Acute neurological dysfunction | Acutely altered sensorium and all of the following: no known CNS injury, presence of sedation holiday, and Glasgow coma score (GCS) ≤ 12 |
| fresh frozen plasma transfusion | Any transfusion of fresh frozen plasma during the ICU stay |
| Packed red blood cell (PRBC) transfusion | Any transfusion of PRBCs during the first 21 days of ICU stay |
| Intravenous nutrition | Any administration of intravenous nutrition during the ICU stay |
| Stress ulcer prophylaxis | Any administration of stress ulcer prophylaxis during the ICU stay |
| Hemodialysis catheter | Placement of a hemodialysis catheter during the ICU stay |
| Deep venous thrombosis (DVT) prophylaxis | Administration of any of the following prophylactic treatments during the ICU stay: unfractionated, low-molecular weight, or synthetic heparin or spontaneous compression devices |
| Invasive mechanical ventilation | Administration of invasive mechanical ventilation upon ICU admission |
| Chronic liver disease | Any of the following: biopsy proven cirrhosis and documented portal hypertension; episodes of past upper GI bleeding attributed to portal hypertension; prior episodes of hepatic failure/ encephalopathy/ coma |
| Chronic cardiovascular disease | New York Heart Association Class IV symptoms and one or more of the following: severe coronary artery disease; severe valvular heart disease; severe cardiomyopathy |
| Chronic respiratory disease | Any of the following: chronic restrictive, obstructive or vascular disease resulting in severe mobility restriction; respiratory dependency; chronic hypoxia, hypercapnea, secondary polycythemia or severe pulmonary hypertension (>40 mmHg) |
| Chronic renal disease | A history of chronic renal compromise with most recent creatinine > 2.0 mg/dL |
| Immunocompromise | Any of the following: AIDS, immunosuppressive drugs, radiation or chemotherapy within 1 year of ICU admission, documented immuno-humoral or cellular immune deficiency state |
| Active cancer within 5 years | Any of the following in the past 5 years: solid organ tumor, hematological malignancy, lymphoma, or proven metastases |
| Race | African American / African European / Haitian) vs. “other.” The “other” category includes White / Caucasian, American Indian/ Alaska Native, Australian Aborigine, Asian/ Pacific Islander, Latin/ Hispanic, , other, or unknown. |
| Payment source | Medicaid insurance (including Medicaid managed care) or self-pay vs. other insurance. The other insurance category includes managed care, commercial/ indemnity insurance, Medicare, Medicare managed care, government insurance, national health service, or other. |
| Critical care medicine (CCM) management | A critical care medicine physician was responsible for the overall care of the patient for all or a portion of the patient’s ICU stay |
| Hospital beds | Number of licensed hospital beds |
| Academic hospital | Academic vs. “other” hospital. The “other” category includes city/ county, state, Veteran’s Administration, community/ for profit, and community/ not for profit. |
| Medical school | The hospital is the primary teaching hospital of an accredited medical school |
| CCM fellowship program | The hospital is the primary location of an accredited Critical Care Medicine fellowship |
| Residency program | The hospital is the primary location of an accredited residency program |
APPENDIX REFERENCES
1. LeGall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/ North American multicenter study. JAMA 1993;270:2957–2963.
2. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med 1985;13:818–829.
Footnotes
Author Contributions: Dr. Pietropaoli developed the research question and study design, performed the data analysis, and drafted and finalized the manuscript. Drs. Glance, Oakes and Fisher contributed to the study design, provided consultation for data analysis, assisted with manuscript revisions, and approved the final manuscript.
Financial / non-financial disclosures: The authors have indicated that they have no conflicts of interest regarding the content of this article.
REFERENCES
- 1.Nicol T, Bilbey DLJ, Cordingley CJL, et al. Oestrogen: the natural stimulant of body defense. J Endocrinol. 1964;30:277–291. doi: 10.1677/joe.0.0300277. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Wichmann MW, Ayala A, et al. Testosterone receptor blockade after hemorrhage in males: restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–1214. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 3.Kuebler JF, Jarrar D, Toth B, et al. Estradiol administration improves splanchnic perfusion following trauma-hemorrhage and sepsis. Arch Surg. 2002;137:74–79. doi: 10.1001/archsurg.137.1.74. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 5.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 8.Brun-Buisson C, Meshaka P, Pinton P, et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in the hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993–2003. Crit Care Med. 2007;35:1244–1249. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 10.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 11.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 12.Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adrie C, Azoulay E, Francais A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 14.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 15.Eachempati S, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 16.Brun-Buisson C. The epidemiology of the systemic inflammatory respone. Intensive Care Med. 2000;26:S64–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins TL, Teres D, Copes WS, et al. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 18.Cook SF, Visscher WA, Hobbs CL, et al. Project IMPACT: results from a pilot validity study of a new observational database. Crit Care Med. 2002;30:2765–2770. doi: 10.1097/00003246-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 2000. [Google Scholar]
- 22.Pregibon D. Logistic regression diagnostics. Ann Stat. 1981;9:705–724. [Google Scholar]
- 23.StataCorp. Stata statistical software: Release 9, user's guide. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- 24.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 25.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ Canadian Medical Association Journal. 2007;177:1513–1519. doi: 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romo H, Amaral AC, Vincent JL, et al. Effect of patient sex on intensive care unit survival. Arch Intern Med. 2004;164:61–65. doi: 10.1001/archinte.164.1.61. [DOI] [PubMed] [Google Scholar]
- 27.Hebert PC, Tinmouth A, Corwin HL. Controversies in RBC transfusion in the critically ill. Chest. 2007;131:1583–1590. doi: 10.1378/chest.06-1055. [DOI] [PubMed] [Google Scholar]
- 28.van Eijk LT, Dorresteijn MJ, Smits P, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;351:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 29.Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock. 2006;26:538–543. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- 30.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angstwurm MWA, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–2793. doi: 10.1097/01.ccm.0000190242.24410.17. [DOI] [PubMed] [Google Scholar]
- 32.Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentin A, Jordan B, Lang T, et al. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003;31:1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 34.Kucher N, Tapson VF, Quiroz R, et al. Gender differences in the administration of prophylaxis to prevent deep venous thrombosis. Thromb Haemost. 2005;93:284–288. doi: 10.1160/TH04-08-0513. [DOI] [PubMed] [Google Scholar]
- 35.Tilford JM, Parker JG. A gender bias in the allocation of intensive care unit resources? Crit Care Med. 2003;31:2073–2074. doi: 10.1097/01.CCM.0000069338.87489.58. [DOI] [PubMed] [Google Scholar]
- 36.Bookwala J, Coppola KM, Fagerlin A, et al. Gender differences in older adults' preferences for life-sustaining medical treatments and end-of-life values. Death Stud. 2001;25:127–149. doi: 10.1080/07481180126202. [DOI] [PubMed] [Google Scholar]
- 37.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of do-not-resuscitate orders: disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995;155 [PubMed] [Google Scholar]
- 38.Connors AF, Dawson NV, Desbiens NA, et al. A controlled trial to improve care for seriously ill hospitalized patients. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 39.Cook DJ, Guyatt GH, Jaeschke R, et al. Determinants in canadian health care workers of the decision to withdraw life support from the critically ill. JAMA. 1995;273:703–708. [PubMed] [Google Scholar]
- 40.Lee OY, Mayer EA, Schmulson M, et al. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 41.Rabeneck L, Paszat LF, Li C. Risk factors for obstruction, perforation, or emergency admission at presentation in patients with colorectal cancer: a population-based study. Am J Gastroenterol. 2006;101:1098–1103. doi: 10.1111/j.1572-0241.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- 42.Chiaramonte GR, Friend R. Medical students' and residents' gender bias in the diagnosis, treatment and interpretation of coronary heart disease symptoms. Health Psychol. 2006;25:255–266. doi: 10.1037/0278-6133.25.3.255. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree TD, Pelletier SJ, Gleason TG, et al. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 44.Leibovici L, Paul M, Weinberger M, et al. Excess mortality in women with hospital-acquired bloodstream infection. Am J Med. 2001;111:120–125. doi: 10.1016/s0002-9343(01)00771-9. [DOI] [PubMed] [Google Scholar]
- 45.Stroud L, Edwards J, Danzig L, et al. Risk factors for mortality associated with enterococcal bloodstream infections. Infect Control Hosp Epidemiol. 1996;17:576–580. doi: 10.1086/647386. [DOI] [PubMed] [Google Scholar]
- 46.Combes A, Charles-Edouard L, Trouillet JL, et al. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37:2506–2511. doi: 10.1097/CCM.0b013e3181a569df. [DOI] [PubMed] [Google Scholar]
- 47.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Critic Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 48.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 49.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 50.Rivers E, Nguyen B, Yavstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]


