SUMMARY
Two populations of Nkx2-1+ progenitors in the developing foregut endoderm give rise to the entire post-natal lung and thyroid epithelium, but little is known about these cells, as they are difficult to isolate in a pure form. We demonstrate here the purification and directed differentiation of primordial lung and thyroid progenitors derived from mouse embryonic stem cells (ESCs). Inhibition of TGFβ and BMP signaling, followed by combinatorial stimulation of BMP and FGF signaling can specify these cells efficiently from definitive endodermal precursors. When derived using Nkx2-1GFP knock-in reporter ESCs, these progenitors can be purified for expansion in culture and have a transcriptome that overlaps with developing lung epithelium. Upon induction, they can express a broad repertoire of markers indicative of lung and thyroid lineages and can recellularize a 3D lung tissue scaffold. Thus, we have derived a pure population of progenitors able to recapitulate the developmental milestones of lung/thyroid development.
INTRODUCTION
Early in embryonic development definitive endoderm progenitor cells of the developing foregut are specified into organ domains such as the primordial thyroid, lung, liver, and pancreas fields (Cardoso and Kotton, 2008; Serls et al., 2005). These primordial progenitors then give rise to all the differentiated epithelial progeny of each endodermally-derived tissue. Hence, those interested in purifying thyroid, lung, liver, or pancreatic stem or progenitor cells for disease therapies are increasingly focused on using the developing embryo as a ‘roadmap’ to derive these progenitors in vitro through the directed differentiation of pluripotent embryonic stem cells (ESCs) whose phenotype resembles the early embryo (Gadue et al., 2005). Based on this developmental approach, definitive endoderm progenitors have been efficiently derived from mouse and human ESCs using Activin A (hereafter Activin) to induce embryonic Nodal/Activin signaling (D'Amour et al., 2005; Gouon-Evans et al., 2006; Kubo et al., 2004). The definitive endoderm cells derived in this manner have been presumed to be broadly multipotent; however, the most anterior foregut endodermal lineages, such as thymus, thyroid and lung epithelia have been difficult to derive from these progenitors (Green et al., 2011), in contrast to more posterior foregut or hindgut endodermal tissues, such as hepatic and intestinal lineages (Gouon-Evans et al., 2006; Spence et al., 2011).
Although specific markers or ‘knock-in reporter cell lines’ (such as Pdx1GFP mouse ESCs) have been employed to facilitate isolation of inefficiently specified foregut progenitors, such as those of pancreatic lineage (Micallef et al., 2005), no tools have been engineered to allow the isolation of the most primordial murine lung and thyroid progenitors. Consequently lung and thyroid epithelia remain among the least studied lineages derived from ESCs in vitro to date. In heterogeneous cultures of differentiating ESCs, induction of late markers of developing lung (Ali et al., 2002; Ameri et al., 2010; Coraux et al., 2005; Qin et al., 2005; Rippon et al., 2004; Rippon et al., 2006; Roszell et al., 2009; Samadikuchaksaraei et al., 2006; Van Vranken et al., 2005; Wang et al., 2007; Winkler et al., 2008) and thyroid (Arufe et al., 2006; Arufe et al., 2009; Jiang et al., 2010; Ma et al., 2009), such as surfactant protein C (SPC) and thyroglobulin, respectively, have been reported, but their expression appears to be stochastic, and the cells expressing these markers have been difficult to expand further in culture. It is broadly accepted that prior to differentiation, all lung or thyroid epithelia must first progress through a primordial progenitor stage defined by the onset of expression of the homeodomain-containing transcription factor, Nkx2-1 (also known as thyroid transcription factor-1; Ttf1 or Titf1). However, lack of specificity of this marker has made it difficult to utilize for ESC differentiation studies, a hurdle common to many ESC-based model systems where differentiated lineages of diverse germ layers must first proceed through a progenitor state expressing a transcription factor that lacks complete specificity for that lineage.
Despite its lack of specificity, Nkx2-1 is known to be a key transcriptional regulator of lung, thyroid and forebrain development, as evidenced by Nkx2-1 knockout mice which display abnormalities in forebrain development and lung/thyroid agenesis (Kimura et al., 1996; Minoo et al., 1999). In addition, humans born with Nkx2-1 gene mutations develop pediatric lung disease, hypothyroidism and neurological impairment (Krude et al., 2002). Inability to access the presumed very rare, multipotent primordial lung and thyroid progenitors at their moment of specification within endoderm has resulted in a lack of information about their phenotype, genetic programs, or epigenetic mechanisms that control their differentiation. In turn this has limited any rational approach to try to developmentally derive their equivalents from ESCs in culture.
Here we present a novel Nkx2-1 knock-in ESC line and reporter mouse that has allowed us to develop serum-free culture protocols for the step-wise derivation of pure populations of Nkx2-1 progenitors that exhibit the differentiation repertoire of Nkx2-1+ lung/thyroid endodermal and neuroectodermal primordia known to be present in the developing embryo. We find that definitive endoderm derived from ESCs with Activin alone resists lung or thyroid lineage specification, and we propose that stage-specific inhibition of BMP and TGFβ signaling renders these endodermal progenitors competent to specify efficiently into Nkx2-1+ endodermal lung or thyroid progenitors with little if any contamination of Nkx2-1+ neuroectoderm. Following BMP/TGFβ inhibition, subsequent combinatorial induction of BMP and FGF signaling promotes initial lineage specification of endodermal Nkx2-1+ lung and thyroid progenitors. These primordial progenitors can be sorted to purity and thereafter expanded and differentiated in culture. In contrast, prolonged exposure to BMP/TGFβ inhibitors results in highly efficient derivation in culture of Nkx2-1+ neuroectodermal progeny that do not express any endodermal, lung, or thyroid markers, revealing precise control in culture over the germ layer lineage specification of cells expressing an otherwise non-specific transcriptional regulator.
RESULTS
Inhibition of TGFβ and BMP signaling renders ESC-derived endoderm competent to specify into Nkx2-1+ progenitors
In order to optimize culture conditions for the derivation of Nkx2-1-expressing candidate lung/thyroid progenitors, we generated an Nkx2-1 reporter ESC line (hereafter Nkx2-1GFP) by targeting a GFP reporter gene to the Nkx2-1 locus using homologous recombination (figure S1A, B). In Nkx2-1GFP mice generated from this cell line, the GFP reporter was expressed in the developing forebrain, lung and thyroid in the expected distribution of endogenous Nkx2-1 mRNA and protein (figure 1 and S1).
Figure 1. Inhibition of BMP and TGFβ signaling alters endodermal patterning and modulates competence of ESCs to differentiate into either endodermal Nkx2-1+ or neuronal Nkx2-1+ cells.
(A) Schematic of AP patterning of the endodermal gut tube in the developing embryo (~E8.5-9). Domains of expression of genes that demarcate prospective organ fields. (B) Kinetics of expression of knock-in reporters, Foxa2-hCD4 and Foxa3-hCD25, indicating effect of Activin stimulation alone (left panel) compared to Activin followed by exposure to the BMP and TGFβ inhibitors, Noggin and SB431542 (Nog/SB for 24 hours. (C) Nkx2-1GFP knock-in mouse embryo and lung whole mount (both at E14.5) with GFP fluorescence visible in the forebrain, thyroid and lung (thyroid in figure S2). In the lung and trachea, fluorescence appears in an epithelial pattern (Tr; arrowhead). (D) Effect of Nog/SB exposure on the competence of day 5 cells to specify to Nkx2-1+ endoderm by day 14. Representative flow cytometry dot plots and summary bar graph indicate percentages of GFP+ cells in each condition (n=4; avg±SEM; *p<0.001). qRT-PCR analysis of indicated genes comparing day 14 sorted GFP+ to GFP- cells (bars=avg fold change in gene expression±SD). (E) Effect of WFKBE with vs without FGF2 supplementation on GFP+ cell morphology. GFP fluorescence and phase contrast overlay images are shown for D12 (pre-sort) ESC-derived GFP+ cells, which occur only rarely in the absence of FGF2 (top panel) and express neuroectodermal markers, such as Pax6 (data not shown). In contrast, GFP+ outgrowth cells in presence of FGF2 (lower panel) show a different morphology and are endodermal (see markers in panel D). Size bar=100μm. (F) Prolonged Nog/SB exposure specifies Nkx2-1+ neuronal cells by day 13, as indicated by flow cytometry and kinetics of induction of neuronal markers (Olig2 and Tuj1), loss of endodermal marker, Foxa2, and loss of early thyroid marker, Pax8. [see also figures S1-S4].
To test faithfulness and specificity of the Nkx2-1GFP ESC reporter line in vitro, we first derived embryoid bodies (EBs) using published serum-free culture conditions supplemented with Activin (Christodoulou et al., 2011; Gouon-Evans et al., 2006; Kubo et al., 2004), resulting in >80% efficient definitive endoderm induction in 5 days but no detectable Nkx2-1 expression (figure S2). To induce Nkx2-1 expression, we then exposed day 5 ESC-endoderm cells to either hepatic inducing conditions, which include low levels of FGF2 (Gouon-Evans et al., 2006) or to high levels of FGF2 alone, based on previous reports suggesting FGF2 secreted by adjacent mesoderm specifies lung epithelium from endoderm (Serls et al., 2005). In either condition, we observed inefficient induction of the GFP reporter in rare cells by day 12 (~0.1% and ~1% of cells, respectively; figure S1G). Flow cytometry-based cell sorting of GFP+ cells distinguished the entirety of Nkx2-1 mRNA expressing cells, analyzed by RT-PCR (figure S1G). These results confirmed that the expression of GFP faithfully and specifically reports induction of the endogenous Nkx2-1 locus in this cell line; however, Nkx2-1 induction in Activin-exposed ESCs was inefficient. Moreover this rare GFP+ population was heterogeneous, expressing markers of neuroectoderm (Pax6 and Tuj1), very low levels of the endodermal marker Foxa2 and the early thyroid marker, Pax8, and expressing little to no differentiated thyroid (thyroglobulin) or differentiated lung (SPC or CC10) markers. (figure S3). FGF1 or Wnt3a, alternative factors previously reported to induce lung lineage within developing endoderm (Goss et al., 2009; Harris-Johnson et al., 2009; Serls et al., 2005), similarly failed to efficiently induce endodermal Nkx2-1+ lineages from ESC-derived endodermal progenitors (figure S3).
Given the capacity of ESC-derived endoderm to form hepatic lineages, but the apparently limited capacity to form Nkx2-1+ endodermal lineages, we considered the possibility that day 5 ESC-endoderm cells might display significant anterior-posterior (AP) patterning towards endodermal domains other than lung or thyroid. Indeed, interrogation of our microarray database detailing the transcriptome of day 5 ESC-endoderm (Christodoulou et al., 2011) suggested upregulation of Hex and Foxa3 (figure S4), markers that indicate AP patterning in the endodermal gut tube of embryos (Martinez Barbera et al., 2000; Monaghan et al., 1993). To explore this possibility further we employed an ESC reporter line (figure 1A,B) containing Foxa2-hCD4 and Foxa3-hCD25 knock-in reporters (Gadue et al., 2009). ESC-derived endoderm co-expressed Foxa3 in approximately 40% of Foxa2+ cells, indicating significant AP patterning of the cells in response to Activin (figure 1B). By FACS analysis, these double positive cells appeared to arise within the Foxa2+ population between days 4-5 of differentiation. This Foxa2+/Foxa3+ population has previously been demonstrated to rapidly specify into hepatic lineages in the presence of BMP4 (Gadue et al., 2009).
Given the role of Activin and BMP4 in the patterning and specification of endodermal Foxa2+/Foxa3+ cells (Gadue et al., 2009; Gouon-Evans et al., 2006), and based on reports that precise modulation of TGFβ and BMP signaling can optimize early germ layer patterning in vivo (Tiso et al., 2002) as well as in vitro in ESC-endodermal or ESC- mesodermal model systems (Green et al., 2011; Kattman et al., 2011), we tested stage-specific modulation of these pathways prior to Foxa3 induction in our system. Exposing day 4 or 5 cells to Noggin and SB431542 (hereafter Nog/SB), inhibitors of BMP and Activin/TGFβ signaling, respectively, we were able to minimize Foxa3 induction, while maintaining anterior endodermal markers, such as Sox2 and Tbx1 (figure 1B and S4).
Next we tested whether exposure to Nog/SB rendered ESC-derived endoderm more competent to specify into Nkx2-1+ endodermal cells. Using the Nkx2-1GFP ESC line, we found that only cells exposed to 24 hours of Nog/SB were competent to induce endodermal Nkx2-1GFP in 21.3±2.7% (avg±SD) of cells (figure 1). To induce Nkx2-1 following Nog/SB treatment, we exposed the competent cells to high doses of FGF2 (500ng/ml) combined with Wnt3a, FGF10, KGF, BMP4, EGF and heparin; hereafter WFKBE+F2/H. We have previously reported that WFKBE possesses endodermal ventralizing capacity in human ESC-endoderm (Green et al., 2011). However, only when supplementing the WFKBE cocktail with FGF2 in mouse ESCs did we observe efficient derivation of Nkx2-1GFP+ cells. The WFKBE+F2/H combination only induced efficient Nkx2-1GFP when ESC-endoderm cells were first exposed to TGFβ/BMP inhibition with Nog/SB (figure 1D). The sorted GFP+ cells derived with WFKBE+F2/H expressed an mRNA signature suggesting they maintained an endodermal program (Foxa2+), included thyroid competent cells (evidenced by Pax8), and were depleted of neuroectodermal Nkx2-1+ contaminants (evidenced by the absence of Pax6 and Tuj1). In contrast, when rare GFP+ cell clusters were observed in the absence of FGF2, these clusters displayed a distinct morphology (figure 1E), expressed Pax6 and Tuj1, and lacked any detectable expression of Pax8, suggesting neuroectodermal rather than endodermal Nkx2-1GFP+ cells were produced in the absence of FGF2 (data not shown).
Efficient derivation of neuroectodermal Nkx2-1+ cell fate by prolonged TGFβ/BMP inhibition
We anticipated that the duration of TGFβ/BMP inhibition would need to be brief to prevent loss of ESC-endoderm to competing neuronal cells, based on recent publications indicating that neuroectodermal derivatives may be rapidly derived from human ESCs exposed to these inhibitors (Chambers et al., 2009; Smith et al., 2008). Indeed induction of Nkx2-1+ cells of neuroectodermal lineage (Tuj1+ and Olig2+) could be achieved with 70% efficiency after extended exposure of Activin-stimulated ESCs to Nog/SB beginning on day 4 (prior to robust induction of the endodermal markers Cxcr4/ckit) and continuing for >48 hours; these conditions yielded no detectable expression of endodermal lung or thyroid differentiation (figure 1F). We concluded that stage- and time-dependent inhibition of TGFβ and BMP signaling allows precise control over whether Nkx2-1+ endoderm vs Nkx2-1+ neuroectoderm lineages are derived during in vitro directed differentiation of ESCs.
Purified Nkx2-1+ endodermal progenitors differentiate into thyroid and lung lineages
The expression of Foxa2 and Pax8 in purified Nkx2-1+ endoderm cells suggested the presence of primordial thyroid progenitors in culture prior to induction of the specific differentiation marker, thyroglobulin. In mouse endoderm development this expression pattern occurs in developing thyroid epithelial cells between E8.5-E14.5. No specific markers of lung lineage have been described in the embryo to confirm the existence of primordial Nkx2-1+ lung progenitors between E9.0, their moment of identifiable lineage specification from endoderm and E10.5, when the earliest specific lung epithelial marker, SPC, is first expressed. Two transcription factors known to be expressed non-specifically in early lung endoderm progenitors, Foxp2 and Id2 (Rawlins et al., 2009; Sherwood et al., 2009), were expressed in Nkx2-1GFP+ cells (figure S5); however, both markers were also expressed in GFP- cells.
We sought to test for the presence of potential primordial lung and thyroid progenitors within the purified Nkx2-1+ population by assessing whether, as in the embryo, these cells uniquely displayed the capacity to undergo further differentiation into more mature lung or thyroid epithelia, expressing more specific markers of lineage differentiation. We replated purified GFP+ cells to allow further expansion and differentiation (figure 2), and we withdrew Wnt3a, KGF, BMP4 and EGF from the media but maintained FGF2 and FGF10. FGF2 has been employed to specify lung progenitors in endoderm explant cultures (Serls et al., 2005), and FGF10 has been implicated as necessary for the maintenance and proliferation of a variety of endodermal lineage specified progenitors, including those of the primordial lung (Ramasamy et al., 2007). As in the developing embryo, Nkx2-1+ endodermal progenitors within 1 week of further culture continued to proliferate and upregulated a constellation of specific lung epithelial markers, SPC and SPB, as well as proximal airway patterning markers, Scgb3a2 (not shown) and the Clara cell marker, CC10 (CCSP; Scgb1a1) (figures 2 and S5). In addition markers of proximal airway basal cells (p63) and ciliated cells (Foxj1) emerged as did the cystic fibrosis transmembrane regulator, Cftr, which is expressed on a variety of endodermal cells, including ciliated cells of the airway. Thyroid maturation of endodermal Nkx2-1+ cells was also evident during this period, based on upregulation of thyroglobulin and the receptor for thyroid stimulating hormone (TSHR; figure 2 and S5). In contrast, the sorted GFP- population did not display the capacity to express a lung or thyroid program, as SPC or thyroglobulin was not detected after replating (data not shown). In cells deriving from the GFP+ sorted population, there was no evidence at any time point studied (days 15-25) of upregulation of markers of neuroectoderm (Pax6 or Tuj1), mesoderm (Myf5) or other endodermal lineages such as liver (AFP or Albumin; data not shown and figure 2). Furthermore, <0.1% of cells derived from the GFP+ sorted cells expressed Tuj1 protein by immunostaining (figure 3). Overall, these results suggest a step-wise sequential progression of ESC-derived Nkx2-1+ endodermal progenitors through differentiation stages reminiscent of the described sequence of lung and thyroid development in vivo.
Figure 2. Purified endodermal Nkx2-1GFP+ progenitors derived from ESCs proliferate in culture and express a repertoire of lung and thyroid lineage genes.
(A) Schematic of culture protocol for directed differentiation of ESCs into Nkx2-1GFP+ (B) Sort gate used to purify GFP+ cells for replating and outgrowth. (C) Expression of Nkx2-1 mRNA and indicated marker genes (D) for each cell population, quantified by real time RT-PCR. E18.5 lung and thyroid RNA extracts served as positive controls. Bars indicate average fold change in gene expression over ESCs±SEM (n=3 independent experiments). DCI+K=cells exposed to lung maturation media from D22-25 [see also figure S5].
Figure 3. Purified ESC-derived populations expressing Nkx2-1 protein co-express markers of early developing endoderm.
(A) Heterogeneous ESC-derived cultures on day 14 (D14; 1 day before GFP+ cell sorting) immunostained for Nkx2-1, Foxa2, Sox2, Sox9, and Tuj1 proteins, demonstrate co-expression of Foxa2 in the majority of Nkx2-1+ cells and expression of Sox2 or Sox9 in subsets of Nx2-1+ cells and in Nkx2-1- cells. In contrast, Tuj1+ cells do not overlap with Nkx2-1+ cells. In these wells ~16% of all cells were Nkx2-1GFP+ by flow cytometry. (B) After purification of the Nkx2-1GFP+ cells shown in A by flow cytometry on D15, outgrowth cells stained on D24 for each indicated marker show more clearly defined morphology than before sorting in panel A. Arrowhead= rare neuronal (Tuj1+) cell found after cell sorting. At this D24 time point ~60% of cells retained Nkx2-1GFP expression by flow cytometry. Size bar=100 μm. Nuclei counterstained with DAPI (blue).
To determine whether proteins known to be expressed in endodermal and early lung epithelial cells were present in the ESC-derived Nkx2-1+ population, we performed dual immunostaining for Nkx2-1 protein, and Foxa2, Sox2 or Sox9, on day 14 prior to cell sorting and on day 24 after outgrowth of purified GFP+ cells (figure 3). We found the majority of ESC-derived Nkx2-1+ cells on day 14 co-expressed the endodermal marker Foxa2. Many Nkx2-1+ cells were proliferating as indicated by Ki67 immunostaining (figure 5). In addition, a subset of Nkx2-1+ cells co-expressed either Sox2 or Sox9 (figure 3), transcription factors that in the presence of Nkx2-1 endoderm in the embryo mark lineages of the proximal airway (Que et al., 2009) or distal lung bud epithelium (Perl et al., 2005), respectively. At the completion of the protocol shown in figure 2, the GFP+ cells sorted on day 14 or 15 and replated in culture for 9-10 more days, still expressed Nkx2-1 protein in approximately half of the cell outgrowth, and Foxa2, Sox2, and Sox9 protein expression was still detected in subsets of Nkx2-1+ cells (figure 3). By day 25, approximately 40% of the sorted, replated GFP+ population had downregulated Nkx2-1GFP expression, and a portion of these Nkx2-1- cells expressed the type 1 alveolar epithelial cell (AEC1) marker, T1α (figure 4A and 6), a gene kinetic typical of late fetal AEC1 development. In order to track SPC+ cell outgrowth, we employed 2 independent techniques (figure 4B): we identified patches of cells expressing pro-SPC protein by immunostaining day 25 cells, and we assayed for SPC promoter activity using a lentiviral vector (figure S6) encoding the dsRed reporter gene expressed under regulatory control of a published 3.7kb SPC promoter fragment (Glasser et al., 1991). We observed SPC-dsRed reporter expression only in subsets of Nkx2-1GFP+ cells.
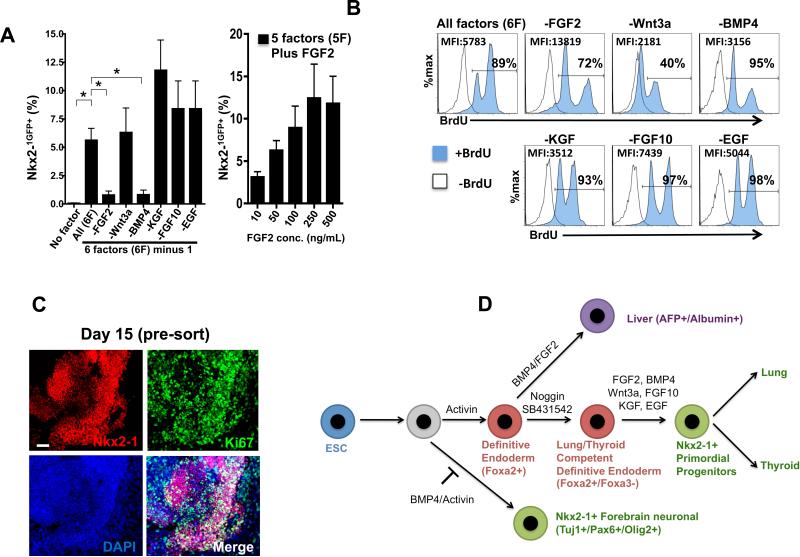
Figure 5. Combinatorial FGF and BMP signaling promotes efficient derivation of proliferating Nkx2-1GFP+ ESC-derived cells.
(A) Percentage of Nkx2-1GFP+ cells derived from ESCs on D14 when individual growth factors were withdrawn from the 6 factor (6F) ventralizingWFKBE+FGF2 cocktail. two-tailed t test *p<0.05. (B) BrdU labeling index (after 24 hours vs 0 hours of BrdU exposure) measured by flow cytometry in each condition from panel A. Cells are scored as BrdU+ based on histogram gates set on cells that did not receive BrdU. (C) Immunostaining for Nkx2-1 and Ki67 nuclear proteins in D15 ESC-derived cells. (D) Model of directed differentiation of ESCs into neuroectodermal Nkx2-1+ cells vs endodermal Nkx2-1+ cells with lung and thyroid differentiation potential.
Figure 4. Alveolar differentiation repertoire of ESC-derived Nkx2.1+ lung progenitors.
(A,B) Immunostaining for alveolar epithelial markers T1α, pro-SPC and Nkx2-1 on cells at the completion of the 25 day directed differentiation protocol. ESCs sorted on day 15 based on Nkx2-1GFP+ expression subsequently gave rise to (A) cells reminiscent of type 1 alveolar epithelial cells (AEC1) as they lost Nkx2.1 nuclear protein expression (green immunostain) while expressing T1α protein. (B) Other patches of cells appeared more reminiscent of distal SPC+ alveolar epithelial cells as they expressed punctate cytoplasmic pro-SPC protein and displayed SPC promoter activation while retaining Nkx2-1GFP expression. Arrow=SPC-dsRed and Nkx2-1GFP co-expressing cell (orange). Arrowhead= cell expressing only Nkx2-1GFP. (C) Schematic summarizing the decellularization-recellularization assay. (D) H&E stains of lung sections showing lung scaffold appearance with no recellularization (left panel) vs hypercellular sheets following reseeding with undifferentiated ESCs (middle panel) vs cells of alveolar structural morphologies after seeding with Nkx2-1GFP+ purified ESC-derived progenitors(right panel). Size bars=100μm in three left panels. Zoom of the indicated boxed region is shown in far right panel with size bar=20μm. (E) Nkx2-1+ nuclear protein (brown; arrowheads) immunostaining of engrafted cuboidal epithelial cells in the corners of alveoli, derived 10 days after re-seeding with Nkx2.1GFP+ sorted cells. Arrow= flattened nucleus of an Nkx2.1 negative cell (purple) lining the alveolar septum. Many of these flattened cells were T1α+ (F; arrowhead) Size bars=20μm. (G) Control mouse lung without decellularization showing T1α apical membrane staining pattern (brown) of mature AEC1 [see also figure S7]. (H) Ciliated airway epithelial cell (arrow) 10 days after re-seeding with differentiated/unsorted ESC-derived cells. Size bar=20μm. All nuclei counterstained with hematoxylin (purple). [See also supplemental figures S6 and S7].
Figure 6. Epigenetic changes in the Nkx2-1 and Oct4 proximal promoter regions during directed differentiation of ESCs.
(A) Schematic of cell fate decision in ESC-derived endodermal cells to commit to an Nkx2-1 descendant lineage. (B) Kinetics of Nkx2-1GFP expression during 22 days of ESC directed differentiation, following the protocol shown in figure 2. (C) Chromatin immunoprecipitation and bisulfite sequencing studies demonstrate the DNA methylation and histone trimethylation states of the proximal promoters of the Nkx2-1 and Oct4 loci in each cell population during development. Arrows and numbers indicate PCR primer binding sites relative to the ATG start site. Open circles=unmethylated DNA CpG sites, closed circles=methylated CpG sites. Lung Nkx2-1GFP+ vs Nkx2-1GFP- samples were prepared by sorting primary lung cell digests from a 3 week old mouse. MLE-15= mouse lung epithelial cell line.
ESC-derived Nkx2-1+ lung progenitors respond to fetal lung maturation media and recellularize 3D lung tissue scaffolds
A known feature of primary fetal lung epithelial cells late in development is their capacity to respond in vitro to cyclic AMP and steroid-containing media by augmenting the expression of lung epithelial specific genes, such as surfactant proteins (Gonzales et al., 2002). Hence, beginning on day 22 of our culture protocol, we started a 3 day treatment of the ESC-derived Nkx2-1GFP+ cells with dexamethasone, cyclic AMP, IBMX and KGF, hereafter “DCI+K”, a defined serum-free media previously shown to induce well-characterized global transcriptome changes in fetal lung epithelial cells. We found DCI+K treatment induced ~100 fold upregulation of lung epithelial gene expression (SPC, SPB and CC10) in the purified Nkx2-1GFP+ progeny (figure 2 and S5), but did not detectably augment thyroid marker gene expression (TSHR or thyroglobulin). Epithelial-like sheets were also evident histologically at this time, as evidenced by f-actin filament orientation after phalloidin staining (figure S5). In contrast 3 days of exposure to thyroid maturation media (TSH, IGF-1 and NaI) resulted in no increase in lung marker gene expression, but induced upregulation of the thyroid markers, thyroglobulin and TSHR (figure S5D).
Since structure and function are inextricably linked features of lung alveolar epithelial cells, we assessed the capacity of sorted day 15 Nkx2-1GFP+ progenitors to regenerate 3D alveolar lung structure (figure 4C-H and S7). The capacity of primary lung epithelial cells to seed decellularized rodent lung scaffolds is one assay recently developed to test the regenerative potential of lung epithelia (Daly et al., 2011; Ott et al., 2010; Petersen et al., 2010). Sorted day 15 Nkx2-1GFP+ ESC-derived cells, delivered by intra-tracheal instillation into decellularized murine lungs, were able to seed alveolar lung regions and adopt the morphology of lung alveolar epithelia (figure 4C-H and S7). In explant cultures of lung tissue re-seeded with GFP+ cells on day 15 and cultured for 10 more days, 71±12% (avg±SD) of engrafted cells maintained GFP fluorescence and continued to express nuclear Nkx2-1 protein (figure 4E). Some engrafted cells acquired a flattened morphology and lost expression of Nkx2-1 protein, a pattern reminiscent of developing AEC1 cells in vivo (Williams, 2003). Consistent with this pattern, the majority of these flattened cells expressed the AEC1 marker protein, T1α (figure 4F,G), but lacked expression of Nkx2-1 protein by co-staining (figure S7G). In contrast, day 0 undifferentiated cells did not form alveolar structures after intra-tracheal instillation, but rather gave rise to masses of cells in the distal lung (figure 4D) with only very rare cells (<0.1%) expressing either Nkx2-1 or T1α proteins (figure S7). Counting cell nuclei present 10 days after re-seeding revealed that lung slices re-seeded with undifferentiated ESCs contained more cellular outgrowth than slices re-seeded with differentiated cells, but the seeded cells deriving from undifferentiated ESCs remained round, and none of these undifferentiated cells developed either the morphologic or molecular features of lung epithelia (figure S7). Although ciliated cells were not observed after re-seeding with sorted cells, day 15 unsorted, differentiated cells (containing 20% GFP+ and 80% GFP- cell mixtures) were able to re-epithelialize proximal airways and gave rise to ciliated cells facing the airway lumen (figure 4H).
BMP and FGF signaling is required for efficient lineage specification of Nkx2-1+ endodermal progenitors
To identify factors essential for lung and thyroid lineage specification of endoderm, we tested the effects of removing each of the 6 growth factors from our WFKBE+F2/H cocktail. Both FGF2 and BMP4 were essential for efficient Nkx2-1+ endodermal lineage specification, as removal of either factor resulted in a significant decrease in the percentage of GFP+ cells (figure 5A, B; p<0.05). In contrast EGF, KGF and FGF10 were each dispensable for Nkx2-1 lineage specification with no significant decrement in the percentage of GFP+ cells induced in the absence of these growth factors. Removal of Wnt3a did not affect the percentage of GFP+ cells induced by day 14. However, it markedly reduced proliferation of day 14 cells, as assessed by the 24 hour BrdU labeling index which declined from 89% BrdU+ in the presence of Wnt3a to 40% BrdU+ in the absence of Wnt3a (figure 5B). Removal of EGF, KGF or FGF10 did not significantly alter the BrdU labeling index of the day 14 population. Taken together our results suggested that efficient induction of Nkx2-1 in endodermal progenitors depends on stage-specific and time-dependent inhibition of BMP and TGFβ signaling followed by re-initiation of BMP signaling together with FGF signaling.
Initial lineage commitment of Nkx2-1+ vs Nkx2-1- cells is associated with definable changes in the genetic and epigenetic programs of each population
An attractive feature of ESC-based in vitro developmental systems is the potential to use this system to learn about genetic or epigenetic programs that are otherwise difficult to study in vivo. We found that by day 14 of differentiation, cells treated with our protocol already exhibited significant restriction of cell fates, since only sorted Nkx2-1GFP+ cells at this time could go on to express lung or thyroid markers. In contrast, day 12 or day 14 sorted GFP- cells, when replated, did not express GFP, Nkx2-1 or differentiated lung/thyroid markers even after prolonged culture periods in either culture condition (WFKBE+F2/H or FGF2+10; figure 6A,B). We reasoned that the epigenetic signature of the Nkx2-1 locus might also distinguish these cell populations. We extracted DNA and chromatin from ESC populations of various developmental stages (undifferentiated, definitive endodermal prior to Nkx2-1 induction, and endodermal day 12 GFP+ vs GFP- sorted cells) as well as from primary post-natal lung cells sorted based on expression of Nkx2-1GFP+ (epithelial) vs Nkx2-1GFP- (including lung mesenchyme and endothelium) (figure 6). Regardless of developmental stage, all CpG sites in this Nkx2-1 promoter region were unmethylated. In contrast, the histone methylation signature in this promoter region in differentiated day 12 GFP- ESC-derived cells exhibited a repressive trimethylated histone modification mark (H3K27-me3), which correlated with the ESC-derived GFP- population's cell fate decision to lose endodermal lung/thyroid differentiation capacity. This same repressive mark was found in Nkx2-1GFP- sorted primary cells and contrasted with the combined active (H3K4-me3) and repressive (H3K9-me3, H3K27-me3) marks seen in populations of undifferentiated ESCs and endoderm staged cells, and the predominantly active mark (H3K4-me3) observed in ESC-derived Nkx2-1GFP+ and primary Nkx2-1GFP+ cells (figure 6C). The finding that histone modifications rather than DNA CpG methylations occur in key Nkx2-1 regulatory regions early in endoderm development is consistent with descriptions of epigenetic changes in loci encoding other key endodermal transcription factors (Zaret et al., 2008).
In contrast to Nkx2-1, the proximal promoter region of Oct4 exhibited both DNA methylation as well as repressive histone marks (H3K9-me3 and H3K27-me3) soon after endodermal differentiation (figure 6D), consistent with silencing of a locus that will not be expressed in Nkx2-1+ progenitors or their epithelial progeny.
Finally, we sought to apply our in vitro model system to define the global genetic program of the putative primordial Nkx2-1+ endodermal progenitors. We performed microarray analysis of global transcriptomes isolated from GFP+ vs GFP- day 14 ESC-derived cells. We found 1267 probes out of 29,000 were differentially expressed at FDR-adjusted p-value<0.05. As expected, Nkx2-1 was found to be the most statistically significant differentially expressed transcript (p=1.19x10-5; figure 7A-C). These 1267 differentially expressed genes provided a genetic signature that characterizes the ESC-derived Nkx2-1+ endodermal progenitor population, including 83 novel candidate cell surface markers that are upregulated in the GFP+ population (supplemental table S1). In addition this GFP+ genetic signature included additional genes known to characterize early developing thyroid and lung in vivo as well as potential ligand-receptor relationships active in signaling cascades (Notch, Wnt, RA and BMP signaling) that have been shown to play a role in early lung/thyroid development (Cardoso and Lu, 2006). Focusing on the set of 594 genes significantly upregulated in Nkx2-1GFP+ cells, we performed gene ontology analysis, revealing 5 major biological process groupings (figure 7B) represented by this gene set. The two most prominent biological processes represented in this set were related to either development or regulation of cellular processes. Within the differentially expressed gene set of the GFP- population, upregulation of Col1a1, Col1a2, Twist, Gli1, Gli3 and PDGFRα suggested the presence of mesenchymal lineages (figure 7B).
Figure 7. Differentially expressed genes in ESC-derived Nkx2-1GFP+ vs Nkx2-1GFP- cells.
(A) Heat map of 1267 genes differentially expressed between triplicate samples of Nkx2-1GFP+ vs Nkx2-1GFP- ESC-derived cells, sorted on day 14 of differentiation. (B) Gene Ontology analysis of the 594 genes upregulated in GFP+ cells (DAVID on-line analysis). (C) Selections of the 1267 differentially expressed genes demonstrate differing gene programs relating to thyroid lineage, epithelial signaling and mesenchymal programs, or (D) genes whose promoters are known targets of Nkx2-1 protein binding in E11.5 embryonic lung epithelial cells in vivo. Gene.symb=NCBI gene ID. LogFC=log2 fold change in gene expression. [see also table S1]
By comparing the transcriptome of our ESC-derived Nkx2-1+ progenitors to databases established from in vivo tissues, we found that 17% of genes (100 out of 594) upregulated in Nkx2-1GFP+ cells were also expressed in the E11.5 developing mouse lung epithelium (Lu et al., 2005). Finally, we determined which of the 594 upregulated genes in Nkx2-1GFP+ cells overlapped with published targets of Nkx2-1 protein binding in the developing lung. 12% of these genes (71 out of 594) were targets of Nkx2-1 identified previously (GEO GSE23043) (Tagne et al., 2012) by microarray analysis of chromatin (‘ChIP on chip’) immunoprecipitated from E11.5 lung epithelial cells using anti-Nkx2-1 antibodies (figure 7D).
DISCUSSION
Our results demonstrate the directed differentiation of pluripotent stem cells in serum-free culture into purified Nkx2-1+ endodermal progenitors with lung and thyroid differentiation potential. We refer to the progenitors purified in 12-15 days with this approach as ‘primordial’ as they resemble an in vivo developmental stage (approximating E8.5-10.5 in mouse development) where Nkx2-1+ endodermal progenitors have not yet expressed markers of differentiated lung or thyroid epithelium. Our protocol is the result of an approach wherein we sought to optimize the differentiation efficiency of each developmental stage prior to inducing the subsequent desired stage. We then reassessed the phenotype of the resulting cells when a stage was identified that seemed resistant to subsequent desired lineage specification. Using this approach we found definitive endoderm staged cells derived after optimization of Activin dose and timing appeared resistant to lung or thyroid lineage specification. Only after stage-specific inhibition of BMP and TGFβ signaling pathways were we able to yield these endodermal progenitors amenable to efficient lung/thyroid lineage specification. Using this strategy, the optimized protocol presented here results in approximately 160 Nkx2-1GFP+ endodermal cells per starting input ESC within 14 days of directed differentiation.
Our studies reinforce the importance of mechanisms, such as FGF and BMP signaling, that have recently been proposed as key inducers of lung lineage within foregut endoderm (Domyan et al., 2011; Serls et al., 2005). Although these pathways have been singly manipulated in vivo using genetic mouse models, our results indicate that no single pathway alone appears to be sufficient for efficient lung or thyroid lineage specification from developing endoderm. Most importantly, our results emphasize that precise inhibition of certain pathways at defined stages is as important as the addition of pathway stimulators at different developmental stages (model shown in figure 5D). The combination of the two approaches: stage-specific selective inhibition combined with induction of key signals is likely to be a common strategy that can be applied regardless of the germ layer or tissue system to be derived in vitro or in vivo (Kattman et al., 2011).
During the course of our studies we derived putative progenitors reminiscent of a developmental period (E9-10.5) that is devoid of known specific markers for lung primordial progenitors. It was useful to develop simple marker gene signatures as indicators of passage through each sequential milestone on the way to lung/thyroid differentiation. For adequate response to Activin, we monitored for the onset of robust and efficient Cxcr4/ckit expression prior to initiating 24 hours of BMP/TGFβ inhibition. During the subsequent stage of FGF and BMP stimulation we checked to verify that purified candidate Nkx2-1+ endodermal progenitors had confirmed expression of Foxa2 and absence of expression of Pax6 and Tuj1 in order to ensure that lung competent endoderm, rather than neuroectodermal Nkx2-1 progenitors had been derived. The presence of Pax8 expression at this time can also be used to verify that Nkx2-1 endoderm of thyroid lineage has been derived. Since no specific markers of lung or thyroid have yet been described to prove definitively that candidate progenitors are of lung/thyroid lineage, demonstration of subsequent induction of specific differentiated lung and thyroid epithelial markers (e.g. surfactant proteins and thyroglobulin, respectively) remains the only reliable proof of the lung/thyroid competence of the putative primordial Nkx2-1+ progenitors being derived.
A particularly striking and serendipitous outcome of our experiments is the development of a simple protocol for the highly efficient derivation of Nkx2-1+ neuroectodermal cells by prolonged inhibition of BMP/TGFβ signaling in ESCs. Although Nkx2-1 is expressed in three lineages across two germ layers in the developing embryo, stage-specific optimization of inhibition of BMP and TGFβ signaling pathways, as demonstrated here, can effectively direct the fate of the resulting Nkx2-1+ cells preferentially to either germ layer. In the ectodermal germ layer of the developing embryo, Nkx2-1 is expressed in forebrain neuronal and oligodendrocyte progenitors. Consistent with these lineages, ESC-derived neuroectodermal Nkx2-1GFP+ cells generated in our neuronal conditions expressed Tuj1, Pax6, and Olig2, however whether they also express the full constellation of forebrain markers will require further study and in vivo correlation. It is well known that neuroectoderm differentiates easily from undifferentiated ESCs, particularly when BMP and TGFβ signaling are inhibited, and it appears that Nkx2-1+ neuroectodermal progenitors may be similarly derived from undifferentiated mouse ESCs by this approach or by recently published alternate protocols for human NKX2-1 reporter ESCs (Goulburn et al., 2011).
A useful outcome for lung and thyroid research will be the utilization of this in vitro platform for the identification of new lineage markers and the study of genetic and epigenetic programs active in early lung and thyroid development. Although the gene programs and potential markers identified in vitro will require validation in vivo in the developing embryo, we have demonstrated how this system might be applied to define the genetic program of Nkx2-1+ endodermal progenitors. We found 83 new cell surface markers that potentially distinguish the Nkx2-1+ endodermal progenitors in our system, and we observed a definable epigenetic change in the Nkx2-1 promoter region that correlates with lineage commitment of Nkx2-1 negative cells away from lung or thyroid competence.
There are several issues raised by our studies. First, we acknowledge that a lack of any known genetic or epigenetic signature of authentic in vivo Nkx2-1+ endodermal progenitors of similar stage limits our ability to properly compare the phenotype or the genetic signature of the in vitro derived Nkx2-1+ endodermal population against their authentic in vivo correlates. Application of the Nkx2-1 reporter mouse introduced here for the purification of primordial Nkx2-1 endodermal thyroid and lung progenitors from developing mouse embryos will help to surmount this hurdle. Second, the relative frequency of thyroid vs lung committed progenitors within the Nkx2-1GFP+ endodermal population needs to be more precisely quantified. This will first require the discovery of new markers specific to primordial lung epithelia prior to the E10.5 time at which the earliest known marker, SPC, is expressed in the mouse developing lung. Alternatively, we have not excluded the intriguing possibility that the Nkx2-1+ progenitors derived from ESCs in vitro express bipotential features of both thyroid and lung progenitors, a differentiation repertoire that is not known to occur in vivo in endoderm development. Future work with our system, focused on testing clonogenicity and multipotency of the Nkx2-1+ population will help to address these questions. Finally, we emphasize that lung or thyroid progenitors must ultimately be defined by their capacity to give rise to functional epithelia that produce appropriate thyroid hormones or allow pulmonary gas exchange. The lack of known functional assays or reproducible in vivo engraftment models to functionally test candidate lung epithelial progenitors continues to limit progress in lung progenitor cell biology. Novel functional assays of lung epithelia, such as the recellularization of decellularized lung scaffolds employed by us and others, may provide bioartificial lungs (Ott et al., 2010) that can serve as one platform for in vitro and in vivo testing of candidate lung epithelial progenitors, such as those derived from ESCs.
In conclusion, we present here novel tools and protocols for the step-wise derivation, purification, and culture expansion of primordial lung and thyroid endodermal progenitors from pluripotent stem cells. Since primordial lung and thyroid progenitors are few in number in vivo and are thought to only transiently occur during early anterior foregut endodermal development, this in vitro system allows the capture of a developmental stage in culture that is otherwise difficult to study in vivo. Thus, when partnered with in vivo studies this in vitro system should allow high resolution study of the mechanisms and genetic/epigenetic programs of cells that to date remain virtually unstudied in developmental biology. Derivation of a potentially unlimited supply of early Nkx2-1+ lung and thyroid progenitors from ESCs should also allow the purification of progenitors for the modeling and potential treatment of the many diseases affecting lung or thyroid epithelia.
EXPERIMENTAL PROCEDURES
Derivation of Nkx2-1GFP ESCs and reporter mice
The enhanced GFP reporter gene was targeted to one allele of the endogenous Nkx2-1 locus by homologous recombination in W4/129S6 ESCs (Taconic, Hudson, NY), replacing endogenous sequences from the second ATG start site through the end of the Nkx2-1 homeobox (positions 7957-9480 in GenBank locus MMU19755), according to methods detailed in the supplement. Nkx2-1GFP ESCs were injected into C57BL/6j (Jackson Laboratory) mouse blastocysts to generate knock-in mice as detailed in the supplement. All mouse work was approved by the Institutional Animal Care and Use Committee of Boston University.
ESC culture and differentiation
The Nkx2-1GFP ESC line was maintained in the undifferentiated state in serum-free, feeder-free culture conditions using ‘2i’ media (Ying et al., 2008). Five days of definitive endoderm induction in serum-free conditions was performed using 50ng/ml Activin A as previously published (Gouon-Evans et al., 2006) and detailed in the supplement and figure S2. On day 5 EBs were plated onto gelatin-coated plates. Media was switched to Nog/SB media: cSFDM supplemented with 100ng/ml mNoggin (R&D) and 10μM SB431542 (Sigma). After 24 hours the media was switched to Nkx2-1 induction media: cSFDM supplemented with 100ng/ml mWnt3a, 10ng/ml mKGF, 10ng/ml hFGF10, 10ng/ml mBMP4, 20ng/ml hEGF, 500ng/ml mFGF2 (all from R&D) and 100ng/ml Heparin Sodium Salt (Sigma). Nkx2-1GFP+ cells were sorted and replated onto gelatin-coated dishes on the day indicated in the text (day 12-15). Replated cells were grown for 7 days in cSFDM supplemented with mFGF2 (500ng/ml), hFGF10 (100ng/ml) and 100ng/ml Heparin Sodium Salt (Sigma). On day 22, where indicated in the text, the media was switched to lung maturation media (DCI+K): Ham's F12 media +15 mM HEPES (pH 7.4) +0.8 mM CaCl2 +0.25% BSA + 5 μg/ml insulin + 5 μg/ml transferrin + 5 ng/ml Na selenite + 50 nM Dexamethasone + 0.1 mM 8-Br-cAMP + 0.1 mM IBMX + 10 ng/ml KGF. Detailed protocols are available in the supplement.
Immunofluorescent staining
Cells cultured on gelatin-coated 96-well plates were fixed with fresh 4% paraformaldehyde and immunostained with the indicated antibody according to methods detailed in the supplement.
Quantitative RT-PCR
Quantitative (real time) PCR amplification of cDNA was performed using Taqman probes. Relative gene expression, normalized to 18S control, was calculated using the 2(-ΔΔC )T method to quantify fold change in gene expression of the indicated gene compared to baseline expression (fold change=1) in ESCs. Undetectable genes were assigned a cycle number of 40. Detailed methods and primer sequences are available in the supplement.
Mouse lung recellularization and culture
B6 mouse lungs were decellularized and recellularized according to recently described methods (Daly et al., 2011; Wallis et al., 2011). Following decellularization, the right main-stem bronchus was ligated to allow intratracheal delivery of cells only to the left lung. One million cells of each population indicated in the text was mixed with 2% low-melting temperature agarose and intratracheally injected into the decellularized left lung. Each lung was sectioned sagitally into 2mm thick slices and cultured for 10 days (equivalent to days 15-25 of the protocol shown in figure 2). On day 25, paraffin sections of 4% paraformaldehyde-fixed lung slices were prepared for immunohistochemistry analyses detailed in the supplement.
Bisulfite sequencing and chromatin immunoprecipitation (ChIP)
Genomic DNA was purified and bisulfite treated as published (Cao et al., 2010) prior to nested PCR of the indicated promoter region, using primers and methods detailed in the supplement. ChIP of 500-2000bp chromatin fragments was performed using anti-H3K4me3, H3K9me3, H3K27me3 antibodies or IgG isotype control as detailed in the supplement.
Statistical and microarray methods
Unless otherwise indicated all statistical comparisons between groups were performed by two-tailed student's t test and a p-value <0.05 indicated a statistically significant difference between groups. Detailed statistical methods of analysis of Affymetrix GeneChip® Mouse Gene 1.0 ST arrays are included in the supplement. All raw data CEL files are available for free download from the Gene Expression Omnibus (GEO accession number GSE35063).
Supplementary Material
Highlights.
ESCs differentiate to lung and thyroid lineages via Nkx2-1+ endodermal progenitors
Combined BMP and FGF signaling are required to specify these Nkx2.1+ lineages
Nkx2-1+ progenitors can be purified and expanded in culture
The resulting cells can recellularize 3D lung scaffolds
Acknowledgements
We thank Paul Gadue for supplying the TV8-Puro gene targeting vector, Raul Mostoslavsky for assistance with southern blotting techniques, Susan Guttentag for DCI+K media protocols, Mary C. Williams, Wellington V. Cardoso, Bess P. Rosen, George Murphy, Gustavo Mostoslavsky and Jerome Brody for insightful manuscript editing. For technical support, we thank Dr. Yuriy Alekseyev, Sherry Zang and Dr. Gang Liu for microarray processing, Dr. Jining Lu and Avrum Spira for bioinformatics analysis assistance, and John M. Wallis for lung slice culture technical support. DNK is supported by NIH PO1 HL047049-16A1, 1RC2HL101535-01, 1R01 HL095993-01, 1R01 HL108678, a USAMRRA Award, an Alpha-1 Foundation Award and an ARC award from the Evans Center for Interdisciplinary Research at Boston University. TAL is supported by NIH training grant T32 HL007035. LI is supported by R01 HL111574 and an ATS/ChILD Foundation Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
REFERENCES
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- Ameri J, Ståhlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology. 2006;147:3007–3015. doi: 10.1210/en.2005-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arufe MC, Lu M, Lin RY. Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1. Biochem Biophys Res Commun. 2009;381:264–270. doi: 10.1016/j.bbrc.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Vo T, Millien G, Tagne JB, Kotton D, Mason RJ, Williams MC, Ramirez MI. Epigenetic mechanisms modulate thyroid transcription factor 1-mediated transcription of the surfactant protein B gene. J Biol Chem. 2010;285:2152–2164. doi: 10.1074/jbc.M109.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, Kotton DN. Specification and Patterning of the Respiratory System. 2008 StemBook wwwstembookorg doi/10.3824/stembook.1.10.1. [PubMed]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou C, Longmire TA, Shen SS, Bourdon A, Sommer CA, Gadue P, Spira A, Gouon-Evans V, Murphy GJ, Mostoslavsky G, et al. Mouse ES and iPS cells can form similar definitive endoderm despite differences in imprinted genes. J Clin Invest. 2011;121:2313–2325. doi: 10.1172/JCI43853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. AmJRespirCell MolBiol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. NatBiotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial Binding and Recellularization of Decellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells. Tissue Eng Part A. 2011;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Gouon-Evans V, Cheng X, Wandzioch E, Zaret KS, Grompe M, Streeter PR, Keller GM. Generation of Monoclonal Antibodies Specific for Cell Surface Molecules Expressed on Early Mouse Endoderm. Stem Cells. 2009;27:2103–2113. doi: 10.1002/stem.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. ExpHematol. 2005;33:955–964. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Glasser SW, Korfhagen TR, Wert SE, Bruno MD, McWilliams KM, Vorbroker DK, Whitsett JA. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am J Physiol. 1991;261:L349–356. doi: 10.1152/ajplung.1991.261.4.L349. [DOI] [PubMed] [Google Scholar]
- Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, Hatzistavrou T, et al. A Targeted NKX2.1 Hesc Reporter Line Enables Identification of Human Basal Forebrain Derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. NatBiotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Hu Y, Liu X, Wu Y, Zhang H, Chen G, Liang J, Lu X, Liu S. Differentiation of E14 mouse embryonic stem cells into thyrocytes in vitro. Thyroid. 2010;20:77–84. doi: 10.1089/thy.2008.0291. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, Tonnies H, Weise D, Lafferty A, Schwarz S, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Lu J, Izvolsky KI, Qian J, Cardoso WV. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. JBiolChem. 2005;280:4834–4841. doi: 10.1074/jbc.M410714200. [DOI] [PubMed] [Google Scholar]
- Ma R, Latif R, Davies TF. Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin A. Endocrinology. 2009;150:1970–1975. doi: 10.1210/en.2008-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. DevBiol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Tai G, Collas P, Polak JM, Bishop AE. Cell extract-derived differentiation of embryonic stem cells. Stem Cells. 2005;23:712–718. doi: 10.1634/stemcells.2004-0195. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon HJ, Ali NN, Polak JM, Bishop AE. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Roszell BR, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient Derivation of Alveolar Type II Cells from Embryonic Stem Cells for In Vivo Application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Chen TY, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagne JB, Gupta S, Gower AC, S.S. S, Varma S, Lakshminarayanan M, Cao Y, Spira A, Volkert TL, Ramirez MI. Genome-wide Analyses of Nkx2-1 Binding to Transcriptional Target Genes Uncover Novel Regulatory Patterns Conserved in Lung Development and Tumors. Plos One. 2012;7:e29907. doi: 10.1371/journal.pone.0029907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM, Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng. 2005;11:1177–1187. doi: 10.1089/ten.2005.11.1177. [DOI] [PubMed] [Google Scholar]
- Wallis JM, Borg ZD, Daly AB, Deng B, Ballif B, Allen GB, Jaworski DM, Weiss D. Comparative Assessment of Detergent-Based Protocols for Mouse Lung De-Cellularization and Re-Cellularization. Tissue Eng Part C Methods. 2011 doi: 10.1089/ten.tec.2011.0567. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. ProcNatlAcadSciUSA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- Winkler ME, Mauritz C, Groos S, Kispert A, Menke S, Hoffmann A, Gruh I, Schwanke K, Haverich A, Martin U. Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells. Cloning Stem Cells. 2008;10:49–64. doi: 10.1089/clo.2007.0075. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol. 2008;73:119–126. doi: 10.1101/sqb.2008.73.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.