Abstract
Induction of potent mucosal immune response is a goal of current vaccine strategies against mucus-infectious pathogens such as Coxsackievirus B3 type (CVB3). We previously showed that administration of lymphotactin (LTN) as an adjuvant could enhance the specific immune responses against a mucosal gene vaccine, chitosan-pVP1, against CVB3. To optimize the coadministration mode of the mucosal adjuvant, we compared the mucosal immune responses induced by chitosan-DNA vaccine with different combinations of the target VP1 antigen gene and the adjuvant LTN gene. The two genes were either cloned in separate vectors or coexpressed as a fusion or bicistron protein in the same vector before encapsulation in chitosan nanoparticles. Four doses of various adjuvant-combined chitosan-DNA were intranasally administrated to mice before challenge with CVB3. The results indicated that chitosan-formulated pVP1-LTN fusion plasmid exhibited very weak improvement of CVB3-specific immune responses. Although the bicistronic coexpression of LTN with VP1 was expected to be powerful, this combination had enhanced effects on serum IgG and systemic T cell immune responses, but not on mucosal T cell immunity. Coimmunization with VP1 and LTN as separate chitosan-DNA formulation remarkably enhanced antibody and T cell immune responses both in systemic and mucosal immune compartments, leading to the most desirable preventive effect on viral myocarditis. Taken together, how the adjuvant is combined with the target antigen has a strong influence on the mucosal immune responses induced by mucosal DNA vaccines.
Introduction
Induction of considerable mucosal immune response is the goal of many current vaccine strategies against mucus-infectious microorganisms. This is particularly true for mucosal-invading pathogens such as human immunodeficiency virus (HIV), Mycobacterium tuberculosis, and Coxsackievirus, against which induction of mucosal secretory IgA and T cell immune responses have proven critical for protection (Xu et al., 2004; Tudor et al., 2009; Jeyanathan et al., 2010; Salyaev et al., 2010). However, the overall magnitude of the immune response induced in the mucosa is relatively weak compared with that induced in the spleen and lymph nodes. Thus, the search for novel strategies maximizing mucosal immune responses remains the focus of vaccine development for HIV, Mycobacterium tuberculosis, cholera, and Coxsackievirus (Takahashi et al., 2009; Yue et al., 2009; Duerr, 2010; Hokey and Misra, 2011). This will be greatly facilitated by a better understanding of the processes that underlie induction of mucosal immunity and the special features that individual vaccine vectors have to offer.
Currently, among the most vigorously pursued approaches inducing mucosal immunity are vaccines formulated or complexed with mucosal delivery carrier, attenuated viral vector, and mucosal adjuvant. Attenuated viral vector has a number of attractive features such as potent immunogenicity and long-lasting antigen expression, but safety is a concern. Cationic polymer is a desirable mucosal vector for gene vaccines to induce immune responses with an excellent safety and immunogenicity record (Cranage and Manoussaka, 2009). Extensive studies have confirmed its better stability, low toxicity, and mucus absorbance promoting effects. Nanoparticles based on biodegradable chitosan can deliver antigen protein very efficiently through mucus (Prego et al., 2010) and have shown high efficiency as gene carriers to promote transfection of the gene at the mucosal surface (Tong et al., 2009). Our previous study showed that chitosan-DNA encoding VP1, the major structural protein of Coxsackievirus B3 type (CVB3), significantly enhanced the mucosal immune responses and conferred the improved prevention against viral myocarditis (Xu et al., 2004).
To further improve the abilities of chitosan-pVP1 to induce strong immune responses and superior immunoprotection, we introduced lymphotactin (LTN) as a mucosal adjuvant. LTN, a C family chemokine, is believed to be the most abundant chemokine produced by mucosal intraepithelial lymphocytes bearing γδ TCR upon activation, and is efficiently chemotactic toward T, B, and NK cells, and neutrophils (Hedrick and Zlotnik, 1998), suggesting that LTN plays an early, specific role during the progress of mucosal inflammation. The interaction between LTN and its receptor XCR1 is associated with mucosal inflammation and T cell–mediated mucosal pathology in Crohn's disease (Middel et al., 2001). Based on the above properties, LTN is believed to play an important role in bridging innate and adaptive immunities and could be applied as a potent adjuvant in mucosa (Lillard et al., 1999). LTN may perform immune-stimulating functions in two ways. On one hand, it could recruit more immune cells to immunization sites and increase the efficiencies of antigen uptake and presentation; on the other hand, as a Th1 cytokine, it may modulate the bias of immune responses and promote specific Th1 and cytotoxic T lymphocyte (CTL) responses (Huang et al., 2005). A recent study reported that LTN significantly increased the interleukin 2 (IL-2) and interferon gamma (IFN-γ) production by splenocytes of EG-7 tumor-bearing mice and decreased the percentages of CD4+Foxp3+ regulatory T cells and CD11b+Gr-1+ myeloid-derived suppressor cells, and, therefore, enhanced T cell cytotoxicity and increased the antitumor effects (Zhang et al., 2011). Our previous work proved that LTN significantly enhanced the mucosal secrectory IgA level when coimmunized with target antigen VP1 (Yue et al., 2009).
To maximize the mucosal immune responses induced by a DNA vaccine, combination of mucosal adjuvant and the chitosan-formulated DNA vaccine would be ideal. However, the method of coadministration of the adjuvant is a problem. Previous studies have suggested that the temporal and spatial coexpression of antigens and adjuvants seems to be critical for optimal immune priming. Yo et al. (2007) found that compared with two monocistronic plasmids, coexpression of FMS (colony stimulating factor 1 receptor)-like tyrosine kinase 3 receptor ligand and granulocyte-macrophage colony-stimulating factor genes in a bicistronic plasmid could promote a potent CD8+ T cell immunity induced by human epidermal growth factor 2 (HER2)/neu DNA vaccine against bladder tumors. Fusion plasmid encoding human papillomavirus L1 protein with regulated upon activation normal T cell expressed and secreted (RANTES) generated higher frequency of specific splenic T cells than the combination of plasmids encoding L1 and RANTES separately (Kim et al., 2003). And CpG oligodeoxynucleotides linked to, but not simply admixed with, cholera toxin B subunit was a more potent stimulator of proinflammatory cytokine responses in murine splenocytes and human peripheral blood mononuclear cells (Adamsson et al., 2006). All these studies indicated that the combination fashion of the adjuvant with the target antigen can significantly influence the magnitude of immune responses.
Therefore, in this study, to optimize the coadministration fashion of the mucosal adjuvant LTN with the antigen VP1, we compared the mucosal immune responses induced by chitosan-DNA vaccine with different combination fashions of the adjuvant LTN and target VP1. The two genes were either cloned in separate vectors or coexpressed as a fusion or bicistron protein in the same vector before encapsulated in chitosan nanoparticles. Four doses of various adjuvant-combined chitosan-DNA were intranasally administrated to mice. Then mice were challenged with CVB3 to assess the preventive effect against viral myocarditis. Mucosal immune responses were carefully examined to evaluate the influence of the combination fashion of the adjuvant with the antigen on the mucosal immune responses induced by mucosal DNA vaccines.
Materials and Methods
Animal and virus
Male inbred BALB/c (H-2d) mice 6–8 weeks of age were obtained from the Experimental Animal Centre of Chinese Academy of Science (Shanghai, China). All animals were housed in the pathogen-free mouse colonies and all animal experiments were performed according to the guidelines for the Care and Use of Laboratory Animals. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, People's Republic of China, 1998). The protocol was approved by the Ethical Committee of Soochow University. Each group contained six mice; for survival rate observation following lethal CVB3 infection, each group contained eight mice. Experiments were repeated three times.
CVB3 (Nancy strain) was a gift from Professor Yingzhen Yang (Key Laboratory of Viral Heart Diseases, Zhongshan Hospital, Shanghai Medical College of Fudan University) and was maintained by passage through HeLa cells which were grown in Roswell Park Memorial Institute (RPMI) medium 1640 (Gibco) supplemented with 10% fetal calf serum (Gibco) and 0.2% penicillin/streptomycin.
Preparation of DNA vaccines with various combination fashions of LTN and VP1
Plasmids encoding VP1 and LTN were constructed as described previously (Xu et al., 2004; Yue et al., 2009). The fusion of LTN and VP1 was created by DNA splicing through overlap extension of several synthetic nucleotide sequences, and then incorporated into pcDNA3.1 to form recombinant plasmid pVP1-LTN. To construct the bicistronic expression vector, designated as pVP1-IRES-LTN (EMCV-derived IRES was obtain from pIRES vector), VP1 and LTN were sequentially and directionally inserted into the empty plasmid. Transcription of the VP1-IRES-LTN insert was driven by a cytomegalovirus immediate-early promoter and translation of the downstream LTN gene was IRES dependent. DNA plasmids were purified with a commercially available plasmid purification kit (Qiagen).
DNA encapsulated in chitosan was generated by the following method: equal volumes of 0.02% chitosan solution and pVP1 or pLTN DNA solution were heated to 55°C, and then vigorously mixed for 30 s. Mice were mildly anesthetized and intranasally immunized with chitosan-pVP1, chitosan-pVP1 plus chitosan-pLTN, chitosan-pVP1-LTN, chitosan-pVP1-IRES-LTN, or chitosan-pcDNA3.1 (vector) for four times biweekly at a dose of 50 μg of each plasmid. For pVP1 alone, fusion plasmid bicistronic or empty plasmid immunized group, mice were received additional 50 μg pcDNA3.1 to make sure that the total DNA amount was 100 μg. Serum and fecal extracts were collected 2 weeks following the final immunization. Fecal pellets were dissolved in phosphate-buffered saline (PBS; 5% nonfat milk, 1 μg/mL aprotinin, 1 mM phenylmethanesulfonyl fluoride [PMSF]) at final concentration of 100 mg/mL. After centrifugation at 15,000 g for 10 min, supernatants were collected and stored at −70°C.
CVB3 infection and evaluation of myocarditis
Four weeks after the final immunization, mice were infected intraperitoneally with three times the 50% lethal dose (LD50) of CVB3. Seven days later, hearts were collected and fixed in 10% phosphate-buffered formalin, paraffin embedded, and sectioned and stained with hematoxylin and eosin (HE). Sections were examined by two independent investigators in a blinded manner.
Quantization of viral burden in heart tissues
Seven days after 3LD50 CVB3 challenge, hearts were collected, weighed, and frozen at −70°C in RPMI 1640 containing 10% fetal bovine serum. Samples were later thawed, homogenized, serially diluted in 10-fold increments, and incubated on confluent HeLa monolayer cells for 1 h at 37°C and 5% CO2 to allow viral attachment, and then incubated for 7 days to allow plaque formation. Virus titers were expressed as the mean plaque forming unit (PFU)/100 mg tissue±standard deviation (SD).
Enzyme-linked immunosorbent assay measurement of CVB3-specific antibody
Plates were coated with VP1 peptide237-249 (10 μg/mL) at 4°C overnight and then blocked with 1% bovine serum albumin-PBS for 2 h at 37°C. Serum samples (1:100 dilution) and fecal samples (no dilution) were added in duplicate and incubated for 2 h at 37°C. Horseradish peroxidase (HRP)–labeled goat anti-mouse IgG and IgA were added, followed by tetramethylbenzidine substrate addition. Absorption at 450 nm was measured in a microplated reader (BioLab).
T cell proliferation assay
Recombinant CVB3 VP1 protein for in vitro–specific T cell stimulation was prepared by the following method: the nucleotide sequence encoding the CVB3 VP1 was amplified with polymerase chain reaction and then cloned into the prokaryotic expression plasmid pGEX-5X-1 (GE Healthcare) to generate glutathione S-transferase (GST)-tagged VP1 protein. The recombinant fusion protein was purified by affinity chromatography using a glutathione sepharose 4B matrix and then cleaved by factor Xa to obtain recombinant VP1 without GST tag for T cell proliferation.
Two weeks following the final immunization, mice were intraperitoneally infected with 3LD50 CVB3, and spleen and mesenteric lymph node (MLN) T cells were collected 4 days later. T cells (5×105/well) were seeded in 96-well plate and stimulated for 72 h under 37°C, 5% CO2 condition with or without 10 μg/mL recombinant CVB3 VP1 protein. Then, 10 μL CCK-8 (Dojindo) was added to each well; the culture plates were maintained in the same condition for an additional 4 h, and absorption (A) value at 450 nm was measured. The stimulation index (SI) was calculated from the formula: SI=(Arestimulated–Ablank)/(Aunrestimulated–Ablank). An SI value ≥2 indicates significant CVB3-specific proliferation.
CTL activity assay
Two weeks following the final immunization, mice were intraperitoneally infected with 3LD50 CVB3. Spleen and MLN lymphocytes were collected 4 days later and were used as effector cells. Plasmid pVP1 stable–transfected autologous SP2/0 cells (H-2d) or heterologous EL-4 cells (H-2b) were used as target cells. A nonradioactive CTL assay was performed using lactate dehydrogenase cytotoxicity detection kit (Roche). In brief, effector cells were titrated in U-bottom 96-well plates at the effector cell to target cell (E:T) ratios 50:1, 25:1, and 12.5:1, and then 1×104 target cells were added. After incubating at 37°C for 4 h, 100 μL cell supernatant per well was removed and transferred into corresponding wells of a 96-well plate. Reaction mixture (100 μL) was added to each well and incubated for 30 min at room temperature. Then absorbance value at 492 nm was measured. The percentage cytotoxicity of CTL was calculated as follows: Cytotoxicity (%)=[(Effector and target cell mix – effector cell control) – low control]/(high control – low control)]×100%.
IFN-γ enzyme-linked immunosorbent plot (ELISPOT) assay
Splenocytes and MLN cells from immunized mice were isolated, plated (1×106 cells/well), and stimulated with VP1 protein (10 μg/mL) for 48 h at 37°C with 5% CO2. After sequential incubation with biotinylated detection antibody, streptavidin-HRP, and alkaline phosphatase substrate (BD PharMingen), color was developed and spot-forming cells (SFC) were enumerated with an ImmunoSpot Series 3 Analyzer (CellularTechnology Ltd.).
Western blot
293T cells were transfected with pLTN, pVP1, pVP1-LTN, pVP1-IRES-LTN, or empty plasmid by lipofectamine (Invitrogen) and cultured for 48 h. The cell lysates were electrophoresed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membrane. The membrane was probed with goat anti-murine LTN (R&D Systems) or mouse anti-enterovirus VP1 antibody (Dako) followed by HRP-conjugated anti-goat antibody (Dako) or anti-mouse antibody (SouthernBiotech). The signals were developed by chemiluminescence (Amersham Biosciences).
Chemotaxis assay
293T cells were transfected with pLTN, pVP1-LTN, pVP1-IRES-LTN, or empty plasmid by lipofectamine and cultured for 48 h. The supernatant was then collected and subjected to chemotaxis assay which was conducted using a modified 48-well Boyden chamber migration assay. Splenocytes were transferred to upper chambers (1×105 cells/50 μL). The supernatants from various plasmid-transfected 293T cells were pretreated with anti-LTN (10 μg/mL) or isotype antibody before addition to the lower chamber. After 4 h of incubation at 37°C, the filter with migrated cells (to the lower chamber) was stained and cell number was counted in five fields by microscopy set at 200×magnification. The chemotaxis index (CI) is calculated with the following formula: CI=(number of cells migrated in response to LTN)/(number of cells migrated to the medium). A CI value ≥2 indicates significant cell chemotaxis.
Statistical analysis
All data are given as mean±SD. Statistical analysis of the data was performed using the SPSS program (SPSS Inc.). Means were compared using the Student's t-test. The survival rates were analyzed with Kaplan–Meier plot. A p<0.05 was considered statistically significant.
Results
Comparative protection effects of prophylactic CVB3 DNA vaccines with various combinations of LTN and VP1 in chitosan formulation
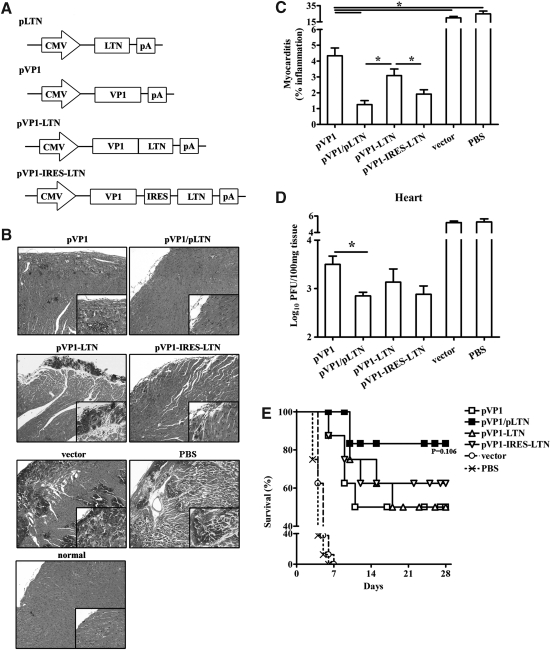
To optimize the administration of adjuvant LTN in CVB3 mucosal DNA vaccine, we constructed two monocistronic plasmids separately encoding LTN and antigen VP1 protein, a bicistronic plasmid (pVP1-IRES-LTN) containing both genes, and a fusion gene construct (pVP1-LTN) encoding fusion protein without any linker sequence (Fig. 1A). Following encapsulated with chitosan, vaccines were intranasally administrated to BALB/c with four doses biweekly. The protection effects of the various vaccines were evaluated by histological observation of the HE-stained heart tissue sections 7 days following 3LD50 CVB3 infection. As shown in Figure 1B and C, widespread myocardial infiltration and mass necrosis were seen in the heart tissue of vector and PBS group, and moderate preventive effect was showed in chitosan-pVP1 immunization group. Treatment with the bicistronic or fusion plasmid resulted in less-severe myocarditis with immune cell infiltration limited to the subendocardium but not dispersed in the myocardium. Limited inflammation was observed in mice coimmunized with chitosan-pLTN indicating the most efficient prevention of myocarditis. Consistently, 0.62 and 0.37 logs less viral load (Fig. 1D) were found in the hearts of mice treated with bicistronic or fusion plasmid compared with mice treated with chitosan-pVP1, while a 0.65 log reduction of viral load was seen in mice coimmunized with chitosan-pLTN.
FIG. 1.
Prevention of CVB3 myocarditis and protection against lethal CVB3 challenge by intranasal immunization with various combinations of LTN and VP1 genes in chitosan formulations. (A) Schematic representation of VP1 and LTN DNA constructs. (B) One representative heart section was shown for each group (magnification: 100×). Four weeks after four doses of DNA immunization, mice were intraperitoneally challenged with 3LD50 CVB3. Seven days later, heart tissues were collected and HE-stained sections were evaluated for the incidence of acute myocarditis. Heart tissues from normal mice were used as control. (C) Myocarditis was assessed as the percentage of the heart section with inflammation compared with the overall size of the heart section, with the aid of a microscope eyepiece grid. (D) Viral load in heart was determined by plaque forming unit (PFU) assays. *p<0.05. (E) The survival rate of mice was observed until day 28 following a lethal dose of CVB3 (5LD50) challenge. CVB3, Coxsackievirus B3 type; LTN, lymphotactin; HE, hematoxylin and eosin.
Parallely, the survival of mice challenged with a lethal dose of 5LD50 CVB3 was examined. All PBS- or empty vector–immunized mice succumbed to severe illness and died before 8 days (Fig. 1E). Compared with chitosan-pVP1 group, although no statistically significant difference in survival was evidenced in chitosan-pLTN coimmunization group, its cumulative survival rate tended to be higher than chitosan-pVP1 group (87.5% vs. 50%, p=0.106).
Coimmunization of monocistronic LTN plasmid significantly enhanced CVB3-specific serum IgG and fecal IgA levels compared with bicistronic and fusion plasmids in chitosan formulation
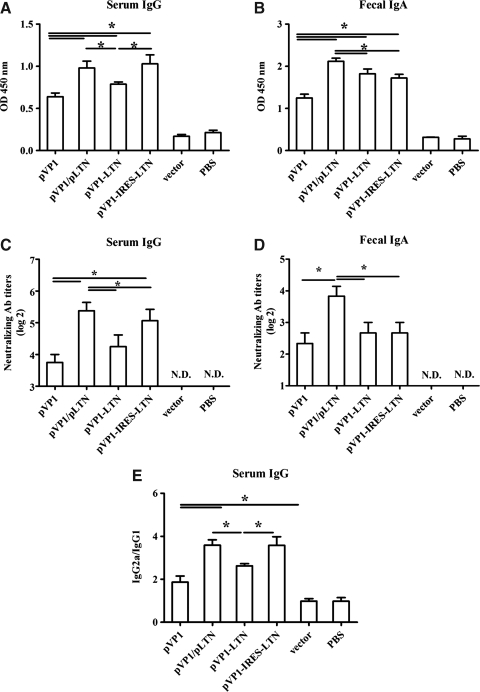
To explore the mechanism of different immunoprotection effects induced by above various genetic combinations of VP1 and LTN in chitosan formulation, the levels of CVB3-specific serum IgG and fecal IgA of immunized mice were detected at week 10 following the first immunization. Consistent with our previous study (Xu et al., 2004), chitosan-pVP1 alone elicited modest CVB3-specific serum IgG and fecal IgA responses (Fig. 2A, B). Significantly higher level of serum IgG was induced by coimmunization of chitosan-pVP1 with chitosan-pLTN or by chitosan-pVP1-IRES-LTN administration, while treatment with chitosan-pVP1-LTN caused a marginal increase. Chitosan formulation of pVP1-IRES-LTN and pVP1-LTN constructs comparably induced elevated fecal IgA level than chitosan-pVP1 alone; while coimmunization with chitosan-pLTN elicited the most profound enhancement of fecal IgA (Fig. 2B; p<0.05). Meanwhile, the highest titers of neutralizing serum IgG was detected in chitosan-pLTN coimmunization and chitosan-pVP1-IRES-LTN groups, while the highest titers of neutralizing fecal IgA was only seen in the coimmunization group. These results indicated that distinct CVB3-specific systemic and mucosal antibody responses were induced by different ways of combining LTN with VP1, among them, intranasal coimmunization with chitosan-pLTN seemed to simultaneously enhance serum IgG and mucosal IgA levels more efficiently than other vaccines.
FIG. 2.
CVB3-specific antibody responses elicited by intranasal immunization with various combinations of LTN and VP1 genes in chitosan formulations. Mice were immunized with four doses of various DNA vaccines at 2 weeks intervals. The levels of CVB3-specific serum IgG (A), fecal IgA (B), the titers of neutralizing serum IgG (C) and fecal IgA (D) as well as the ratio of serum IgG2a to IgG1 (E) were examined by ELISA at week 10 following the first immunization. ND, not detected, *p<0.05. The results are represented as the mean±SD of three separate experiments. SD, standard deviation.
Monocistronic but not bicistronic or fusion plasmid of LTN could comprehensively enhance both systemic and mucosal T cell immune responses induced by VP1 DNA vaccine
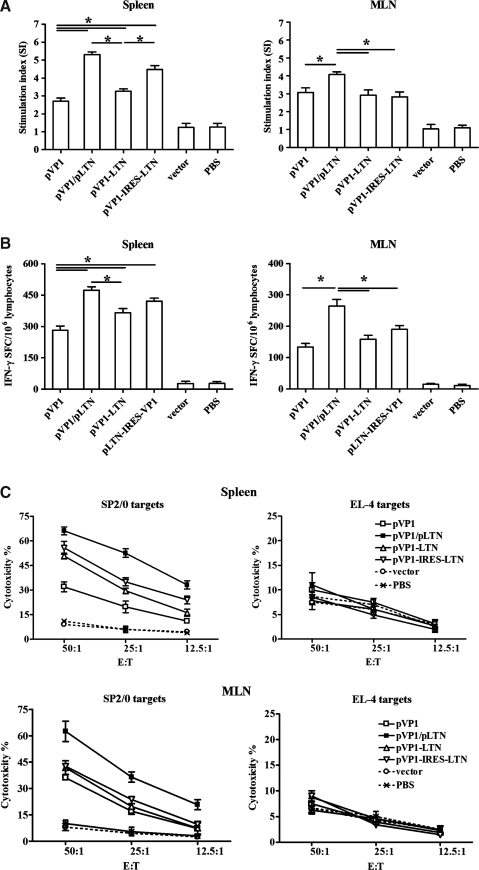
The CVB3-specific T cell immune responses induced by various vaccines were also detected in spleen and MLN. As shown in Figure 3A, compared with chitosan-pVP1 administration, coimmunization with chitosan-pLTN or immunization with chitosan-pVP1-IRES-LTN coexpression plasmid exhibited a remarkable augmentation in splenic T cell proliferation (p<0.05); much less increase was observed in the chitosan-pVP1-LTN fusion plasmid. Only chitosan-pLTN coimmunization raised a significantly elevated T cell proliferation responses in MLN compartment.
FIG. 3.
CVB3-specific T cell response elicited by intranasal immunization with various combinations of LTN and VP1 in chitosan formulations. Mice were intraperitoneally challenged with 3LD50 CVB3 2 weeks after final immunization and the spleen and MLN cells were collected 4 days later. (A) CVB3-specific T cell proliferation was assessed by CCK-8 kit after stimulation with 10 μg/mL recombinant VP1 protein and 20 U/mL IL-2 for 72 h. Results are represented as the mean±SD of three separate experiments. (B) Frequency of CVB3-specific IFN-γ–secreting lymphocytes was measured by ELISPOT assay after stimulation with CVB3 VP1 protein for 48 h. *p<0.05. (C) CVB3-specific CTL activity of splenic and mesenteric cells was evaluated by lactate dehydrogenase assays using pVP1 stable–transfected autologous SP2/0 cells or heterologous EL-4 cells as target cells. The effector/target cell ratio was between 50:1 and 12.5:1. Data represent the mean±SD using six mice in each group. MLN, mesenteric lymph node; IL, interleukin; IFN, interferon; ELISPOT, enzyme-linked immunosorbent plot; CTL, cytotoxic T lymphocyte.
IFN-γ ELISPOT assay indicated that mice coimmunized with chitosan-pLTN developed the most IFN-γ–secreting T cells in spleen and MLN, the frequencies reaching 473 and 264 SFC/106 cells, respectively (Fig. 3B). Mice receiving bicistronic plasmid developed comparable splenic (421 SFC/106) but fewer mucosal IFN-γ+ T cell frequency (190 SFC/106, p<0.05). The fusion plasmid only modestly increased the splenic IFN-γ+ T cell frequency compared with chitosan-pVP1 treatment.
In line with the ELISPOT data, the strongest CVB3-specific cytotoxicity responses were also detected in the splenic (52.5%) and mesenteric lymphocytes (36.5%) derived from mice coimmunized with chitosan-pLTN at an E:T ratio of 25:1, (Fig. 3C; p<0.05). Taken together, all these data indicated that the combination of two monocistronic plasmids was superior in inducing systemic and mucosal CVB3-specific T cell immune responses compared with the bicistronic and fusion plasmids in the chitosan formulation.
Improved systemic and mucosal Th1 immune responses were induced by coimmunization of monocistronic but not bicistronic or fusion LTN with VP1 in chitosan formulation
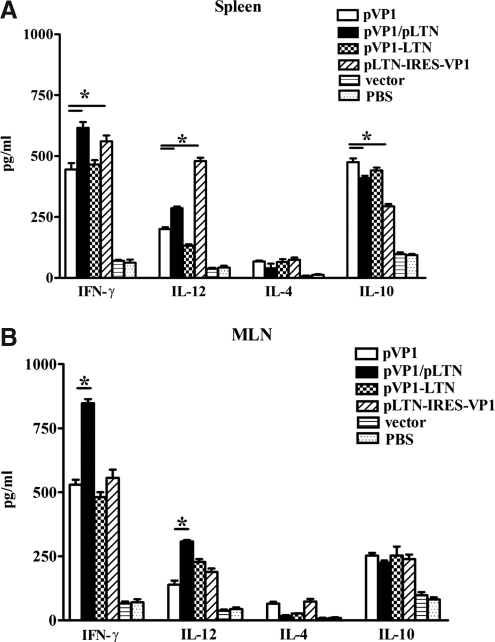
To further analyze the pattern of splenic and MLN T cell immune responses induced by various DNA vaccines, lymphocytes were stimulated with recombinant VP1 protein for 48 h and cell supernatants were then collected and subjected to Th1/Th2 cytokine enzyme-linked immunosorbent assay (ELISA). As shown in Figure 4, compared with chitosan-pVP1, immunization with chitosan-pVP1-IRES-LTN increased Th1 cytokine (IFN-γ, IL-12) in spleen but not in MLN, while coimmunization of chitosan-pLTN significantly promoted Th1 cytokine secretions both in spleen and MLNs, driving systemic and mucosal immune responses to Th1 bias. No notable changes in Th1/Th2 cytokine secretion were detected in the chitosan-pVP1-LTN–immunized group.
FIG. 4.
Th1/Th2 cytokine secretion in spleen and MLN induced by intranasal immunization with various combinations of LTN and VP1 genes in chitosan formulations. Splenocytes (A) and MLN (B) cells were stimulated with 10 μg/mL recombinant VP1 protein for 48 h, and then cell supernatants were collected and subjected to IFN-γ, IL-12, IL-4, and IL-10 ELISA. *p<0.05. The results are represented as the mean±SD of three separate experiments.
Diverse chemotactic abilities were found in various combinations of VP1 and LTN
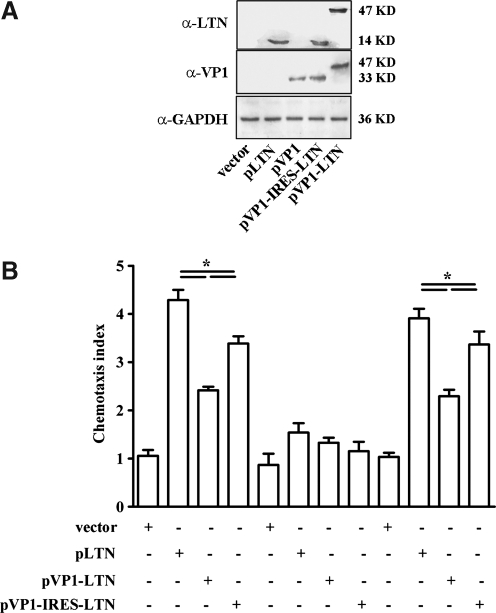
To further find out the possible reasons for the different patterns and strength of immune responses elicited by various combinations of LTN and VP1 genes, we evaluated the expression level and chemotactic ability of LTN protein expressed by various DNA vaccines. As shown in Figure 5A, a 14 kDa LTN protein and a 33 kDa VP1 protein were detected separately in the lysates of pLTN- and pVP1-tranfected cells. A fusion protein with molecular weight of about 47 kDa was also expressed following pVP1-LTN transfection and recognized by antibodies against both VP1 and LTN. The ECMV-derived IRES sequence allowed pVP1-IRES-LTN to express both the VP1 and LTN genes simultaneously. Similar expression levels of VP1 and LTN were seen in various plasmids, indicating that the different immune response strength and biases induced by various plasmids were not attributable to their different antigen and adjuvant protein expression levels. In terms of chemotactic ability, LTN expressed by the monocistronic plasmid chemoattracted the most T cells (Fig. 5B; p<0.05) with CI of 4.28 compared with that expressed by pVP1-IRES-LTN (CI=3.56) and pVP1-LTN (CI=2.41), indicating that the chemotaxis activities of expressed LTN protein differed among various DNA constructs and pLTN exhibited the most efficient chemoattractive ability.
FIG. 5.
Expression and chemotactic activity of LTN protein expressed by plasmids with various combinations of LTN and VP1 genes. (A) 293T cells were transfected with indicated plasmids with lipofectamine for 48 h, and then cell lysates were subjected to western blot analysis using anti-LTN and anti-VP1 antibodies, respectively. (B) Meanwhile, supernatants were collected and subjected to in vitro chemotaxis assays, which were conducted using a modified 48-well Boyden chamber migration assay. *p<0.05. The results are represented as the mean±SD of three separate experiments.
Discussion
Viral infection is a major cause of acute myocarditis with enteroviruses most often responsible (Calabrese and Thiene, 2003; Luo et al., 2010). There are no available preventive or therapeutic reagents to protect humans against viral myocarditis.
There are several distinct approaches to increase the potency of DNA vaccines (van Drunen Littel-van den Hurk et al., 2010; Lin et al., 2011; Wheatley et al., 2011). One is the use of adjuvants. These include proteins, small molecule compounds, or DNA plasmids encoding immunologically active proteins, such as cytokines, costimulatory molecules, and chemokines (Hirschhorn-Cymerman and Perales, 2010). Considering the abundance of LTN on mucosal surfaces and its potential to enhance mucosal and humoral immune responses, in our previous work, we used LTN as a mucosal adjuvant and found improved immunoprotection against CVB3-induced myocarditis when coimmunized with chitosan-pVP1 vaccine. In this study, we focused on the optimization of the mode of coadministration of mucosal adjuvant LTN with the target antigen VP1.
One of the important factors affecting the strength of an immune response is the amount of immunogen. That is also true for the adjuvant although other factors including dose, transfer vector, routes of administration, and formulation type are also involved (Marinaro et al., 2003; Le Guiner et al., 2007; Moreira et al., 2008). Simple mixtures of DNA vaccines with adjuvants are sometimes effective, but appropriate formulation may be required. For example, DNA vaccines mixed with aluminium phosphate enhance antibody responses, while inhibit responses if mixed with aluminium hydroxide, as a consequence of electrostatic interaction between the negatively charged DNA and positively charged adjuvant (Vajdy et al., 2004). Inappropriate delivery of adjuvant even aggravates the diseases (Hope et al., 2004). Therefore, optimization of adjuvant usage including appropriate amount and formulation is very critical.
In this study, we used chitosan to encapsulate DNA plasmid to facilitate delivery and transfection to the mucosal cells, minimize the degradation, and ensure long-term and controlled release of DNA. Also LTN is utilized to increase the immunogenicity and protection effect of chitosan-pVP1 mucosal DNA vaccine. However, how to combine the adjuvant LTN with the target VP1 is a question. There are several possible approaches. One method is to deliver the antigen and adjuvant DNA simultaneously with separate expression but a codelivery system; the second method is to deliver a coexpression DNA plasmid encoding both genes. A third approach is to mix the two plasmid DNA and then formulate them into one chitosan nanoparticle, which we ruled out because large aggregates were produced during preparation. In the first scenario, we used cationic chitosan nanoparticles to entrap the anionic adjuvant DNA, which was coadministered with chitosan-pVP1 nanoparticles. To coexpress the antigen and adjuvant genes, bicistronic and fusion plasmids were constructed and an IRES sequence facilitated the translation of both the genes from a single bicistronic mRNA.
We observed that all combined LTN DNA vaccines exhibited preventive effects against CVB3-induced myocarditis, with the most potent one being chitosan-pLTN coimmunization. To further examine the underlying mechanisms of different immunoprotection induced by various DNA vaccines, we compared the strength and patterns of both systemic and mucosal immune responses and found that although all the combined LTN DNA vaccines enhanced serum IgG and fecal IgA levels, chitosan-pLTN coimmunization was the most efficient. Regarding T cell immune responses, although coimmunization with chitosan-pLTN and immunization with chitosan-pVP1-IRES-LTN could elevate the systemic T cell immune responses compared with the chitosan-pVP1 protovaccine, mesenteric mucosal T cell immune responses were only enhanced by the coimmunization of monocistronic LTN DNA, which was in accordance with its maximal myocarditis prevention and protection effects. The activity of LTN protein expressed by fusion, nonfusion, or bicistronic plasmid is different (Fig. 5B) and may account for the different intensity of immunity induced. One reason is the conformation of LTN protein may be influenced by the different construction methods. As two native conformations of LTN have been reported, one conformation (LTN10) agonizes functional XCR1 and mediates lymphocyte migration; while another conformation (LTN40) exhibits limited chemotactic ability (Tuinstra et al., 2008). We hypothesized that the LTN expressed by monocistronic plasmid might exhibit LTN10 conformation, while that expressed by the bicistronic plasmid may display LTN40 conformation; the second possibility may lie in the different vaccine formulations. Various LTN formulations with target antigen may influence the expression duration as well as the stability of LTN and VP1. The third reason may be that VP1 antigen could be better processed in antigen-presenting cells (APCs) when DNA plasmids are administered separately. Herein, our observation was not in agreement with previous studies showing that intramuscular injection of bicistronic plasmid provoked more potential systemic immune responses than the mixture of two monocistronic plasmids encoding antigen and adjuvant (Yo et al., 2007; Tang et al., 2008). However, there is also a lot of evidence indicating that simultaneous delivery of antigens and adjuvants in nanoparticles ensures that both agents can be efficiently expressed and delivered into the same APC or T cell population and the maximal adjuvant effects could be achieved (Singh et al., 2001; Vajdy et al., 2004).
Fusion of chemokine adjuvant with antigen offers another alternative strategy to enhance the immune efficacy of DNA vaccines, as they might more effectively recruit and target antigens to APCs (Williman et al., 2008). However, in the present study, although the fusion pVP1-LTN DNA slightly enhanced the antibody level and splenic T cell activity compared with the pVP1 plasmid, the total adjuvant effect was weak which was supported by the weakest chemotactic activity of expressed LTN among the three plasmids. It can be assumed that large arm of VP1 protein at the amino-terminal might likely interfere with the interaction of LTN to its receptor leading to the compromised chemotactic capacity and weak adjuvant effects. Our findings were in compliance with other reports that fusion proteins usually have lower specific biological activities due to decreased binding stability or increased stereo-specific blockade between proteins and their receptors (Huang et al., 2007). Another explanation comes from the recent report showing less expression duration time of the fusion plasmid than the bicistronic construct (Mishra et al., 2009). In addition, as LTN is a unique protein with a known conversion between two native conformations (Tuinstra et al., 2008), it is likely that the fusion protein results in altered structures for both the VP1 and the LTN and may well lower the antigenicity of the VP1.
In the present work, we have optimized the administration of mucosal adjuvant LTN in chitosan formulation in a CVB3-induced myocarditis murine model. We found a strong influence of the combination means of adjuvant LTN formula with the antigen DNA vaccine on the spatial patterns and strength of immune responses. Learning about these processes will be helpful for the future design of more potent mucosal vaccines.
Acknowledgments
This work was funded by China NSFC grant (81072413), Jiangsu “Pan-Deng” Project (BK2010004), the National Science & Technology Key Projects during the Twelve Five-Year Plan Period of China (2012ZX10003006), Jiangsu High Level “Shuang-Chuang” Project, Major State Basic Research Development Program of China (2007CB512401), Program for Outstanding Medical Academic Leader (LJ06011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosure Statement
No competing financial interests exist.
References
- Adamsson J. Lindblad M. Lundqvist A. Kelly D. Holmgren J. Harandi A.M. Novel immunostimulatory agent based on CpG oligodeoxynucleotide linked to the nontoxic B subunit of cholera toxin. J Immunol. 2006;176:4902–4913. doi: 10.4049/jimmunol.176.8.4902. [DOI] [PubMed] [Google Scholar]
- Calabrese F. Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11–25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
- Cranage M.P. Manoussaka M. Modern mucosal vaccines, adjuvants and microbicides. Expert Rev Anti Infect Ther. 2009;7:21–23. doi: 10.1586/14787210.7.1.21. [DOI] [PubMed] [Google Scholar]
- Duerr A. Update on mucosal HIV vaccine vectors. Curr Opin HIV AIDS. 2010;5:397–403. doi: 10.1097/COH.0b013e32833d2e39. [DOI] [PubMed] [Google Scholar]
- Hedrick J.A. Zlotnik A. Lymphotactin. Clin Immunol Immunopathol. 1998;87:218–222. doi: 10.1006/clin.1998.4546. [DOI] [PubMed] [Google Scholar]
- Hirschhorn-Cymerman D. Perales M.A. Cytokine-FC fusion genes as molecular adjuvants for DNA vaccines. Methods Mol Biol. 2010;651:131–155. doi: 10.1007/978-1-60761-786-0_9. [DOI] [PubMed] [Google Scholar]
- Hokey D.A. Misra A. Aerosol vaccines for tuberculosis: a fine line between protection and pathology. Tuberculosis. 2011;91:82–85. doi: 10.1016/j.tube.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Hope M. Riding G. Menzies M. Willadsen P. A novel antigen from Anaplasma marginale: characterization, expression and preliminary evaluation of the recombinant protein. Vaccine. 2004;22:407–415. doi: 10.1016/j.vaccine.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Huang H. Bi X.G. Yuan J.Y. Xu S.L. Guo X.L. Xiang J. Combined CD4+ Th1 effect and lymphotactin transgene expression enhance CD8+ Tc1 tumor localization and therapy. Gene Ther. 2005;12:999–1010. doi: 10.1038/sj.gt.3302486. [DOI] [PubMed] [Google Scholar]
- Huang Y.S. Chen Z. Yang Z.Y. Wang T.Y. Zhou L. Wu J.B. Zhou L.F. Preparation and characterization of a potent, long-lasting recombinant human serum albumin-interferon-alpha2b fusion protein expressed in Pichia pastoris. Eur J Pharm Biopharm. 2007;67:301–308. doi: 10.1016/j.ejpb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Jeyanathan M. Heriazon A. Xing Z. Airway luminal T cells: a newcomer on the stage of TB vaccination strategies. Trends Immunol. 2010;31:247–252. doi: 10.1016/j.it.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kim S.J. Lee C. Lee S.Y. Kim I. Park J.S. Sasagawa T. Ko J.J. Park S.E. Oh Y.K. Enhanced immunogenicity of human papillomavirus 16 L1 genetic vaccines fused to an ER-targeting secretory signal peptide and RANTES. Gene Ther. 2003;10:1268–1273. doi: 10.1038/sj.gt.3301997. [DOI] [PubMed] [Google Scholar]
- Le Guiner C. Stieger K. Snyder R.O. Rolling F. Moullier P. Immune responses to gene product of inducible promoters. Curr Gene Ther. 2007;7:334–346. doi: 10.2174/156652307782151461. [DOI] [PubMed] [Google Scholar]
- Lillard J.W., Jr. Boyaka P.N. Hedrick J.A. Zlotnik A. McGhee J.R. Lymphotactin acts as an innate mucosal adjuvant. J Immunol. 1999;162:1959–1965. [PubMed] [Google Scholar]
- Lin C.C. Yu Y.L. Shih C.C. Liu K.J. Ou K.L. Hong L.Z. Chen J.D. Chu C.L. A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells. Cancer Immunol Immunother. 2011;60:1019–1027. doi: 10.1007/s00262-011-1016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. Wong J. Wong B. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc Res. 2010;85:347–356. doi: 10.1093/cvr/cvp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro M. Fasano A. De Magistris M.T. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71:1897–1902. doi: 10.1128/IAI.71.4.1897-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middel P. Thelen P. Blaschke S. Polzien F. Reich K. Blaschke V. Wrede A. Hummel K.M. Gunawan B. Radzun H.J. Expression of the T-cell chemoattractant chemokine lymphotactin in Crohn's disease. Am J Pathol. 2001;159:1751–1761. doi: 10.1016/S0002-9440(10)63022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P.J. Menon L.G. Mayer-Kuckuk P. Bertino J.R. Banerjee D. Translational modulation of proteins expressed from bicistronic vectors. Mol Imaging. 2009;8:305–318. [PMC free article] [PubMed] [Google Scholar]
- Moreira L.O. Smith A.M. DeFreitas A.A. Qualls J.E. El Kasmi K.C. Murray P.J. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine. 2008;26:5808–5813. doi: 10.1016/j.vaccine.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prego C. Paolicelli P. Diaz B. Vicente S. Sanchez A. Gonzalez-Fernandez A. Alonso M.J. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine. 2010;28:2607–2614. doi: 10.1016/j.vaccine.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Salyaev R.K. Rigano M.M. Rekoslavskaya N.I. Development of plant-based mucosal vaccines against widespread infectious diseases. Expert Rev Vaccines. 2010;9:937–946. doi: 10.1586/erv.10.81. [DOI] [PubMed] [Google Scholar]
- Singh M. Ott G. Kazzaz J. Ugozzoli M. Briones M. Donnelly J. O'Hagan D.T. Cationic microparticles are an effective delivery system for immune stimulatory cpG DNA. Pharm Res. 2001;18:1476–1479. doi: 10.1023/a:1012269226066. [DOI] [PubMed] [Google Scholar]
- Takahashi I. Nochi T. Yuki Y. Kiyono H. New horizon of mucosal immunity and vaccines. Curr Opin Immunol. 2009;21:352–358. doi: 10.1016/j.coi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Tang M. Wang H. Zhou S. Tian G. Enhancement of the immunogenicity of an infectious bronchitis virus DNA vaccine by a bicistronic plasmid encoding nucleocapsid protein and interleukin-2. J Virol Methods. 2008;149:42–48. doi: 10.1016/j.jviromet.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H. Qin S. Fernandes J.C. Li L. Dai K. Zhang X. Progress and prospects of chitosan and its derivatives as non-viral gene vectors in gene therapy. Curr Gene Ther. 2009;9:495–502. doi: 10.2174/156652309790031111. [DOI] [PubMed] [Google Scholar]
- Tudor D. Derrien M. Diomede L. Drillet A.S. Houimel M. Moog C. Reynes J.M. Lopalco L. Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- Tuinstra R.L. Peterson F.C. Kutlesa S. Elgin E.S. Kron M.A. Volkman B.F. Interconversion between two unrelated protein folds in the lymphotactin native state. Proc Natl Acad Sci U S A. 2008;105:5057–5062. doi: 10.1073/pnas.0709518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdy M. Srivastava I. Polo J. Donnelly J. O'Hagan D. Singh M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol Cell Biol. 2004;82:617–627. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S. Lawman Z. Wilson D. Luxembourg A. Ellefsen B. van den Hurk J.V. Hannaman D. Electroporation enhances immune responses and protection induced by a bovine viral diarrhea virus DNA vaccine in newborn calves with maternal antibodies. Vaccine. 2010;28:6445–6454. doi: 10.1016/j.vaccine.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Wheatley A.K. Kramski M. Alexander M.R. Toe J.G. Center R.J. Purcell D.F. Co-expression of miRNA targeting the expression of PERK, but not PKR, enhances cellular immunity from an HIV-1 Env DNA vaccine. PLoS One. 2011;6:e18225. doi: 10.1371/journal.pone.0018225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williman J. Young S. Buchan G. Slobbe L. Wilson M. Pang P. Austyn J. Preston S. Baird M. DNA fusion vaccines incorporating IL-23 or RANTES for use in immunization against influenza. Vaccine. 2008;26:5153–5158. doi: 10.1016/j.vaccine.2008.03.084. [DOI] [PubMed] [Google Scholar]
- Xu W. Shen Y. Jiang Z. Wang Y. Chu Y. Xiong S. Intranasal delivery of chitosan-DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine. 2004;22:3603–3612. doi: 10.1016/j.vaccine.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Yo Y.T. Hsu K.F. Shieh G.S. Lo C.W. Chang C.C. Wu C.L. Shiau A.L. Coexpression of Flt3 ligand and GM-CSF genes modulates immune responses induced by HER2/neu DNA vaccine. Cancer Gene Ther. 2007;14:904–917. doi: 10.1038/sj.cgt.7701081. [DOI] [PubMed] [Google Scholar]
- Yue Y. Xu W. Hu L. Jiang Z. Xiong S. Enhanced resistance to coxsackievirus B3-induced myocarditis by intranasal co-immunization of lymphotactin gene encapsulated in chitosan particle. Virology. 2009;386:438–447. doi: 10.1016/j.virol.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Zhang J. Zhou Z. Wang C. Shen J. Zheng Y. Zhang L. Wang J. Xia D. Reduced tumorigenesis of EG7 after interleukin-10 gene transfer and enhanced efficacy in combination with intratumorally injection of adenovirus-mediated lymphotactin and the underlying mechanism. Cancer Immunol Immunother. 2011;60:559–573. doi: 10.1007/s00262-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]