Abstract
Two highly efficient and convenient methods for the synthesis of functionalized and substituted allylic boronates are described. In one procedure, readily available allylic acetates are converted to allylic boronates catalyzed by Ni/PCy3 or Ni/PPh3 complexes with high levels of stereoselectivity and in good yields. Alternatively, the borylation can be accomplished with commercially available Pd catalysts [e.g. Pd2(dba)3, PdCl2, Pd/C] and starting with easily accessed allylic halides.
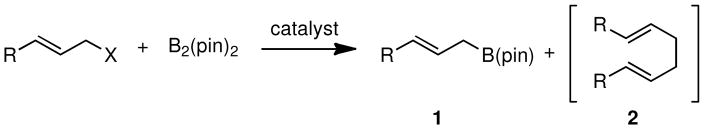
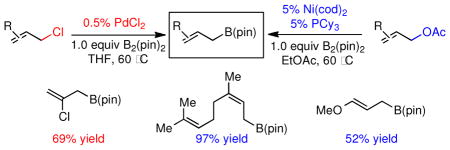
Organoboron reagents represent exceedingly versatile building blocks for organic synthesis. 1 Allylic boronates, 2 in particular, are well known for their additions to aldehydes,3 ketones4 and imines,5 generating valuable homoallylic alcohols and amines, often with high levels of stereocontrol. More recently, allylic boronates have been used as partners for cross-coupling reactions with aryl6 and allyl electrophiles.7 Due to the importance of allylboronate building blocks in synthetic organic chemistry, their synthesis has been the subject of many studies. Besides stoichiometric substitution reactions that involve allyllithium 8 or allylmagnesium 9 reagents, homologation of vinyl boronates10 has also been a practical route to many of these materials. Catalytic synthesis of allyl boronates has also received attention and is attractive because of its high level of functional group tolerance and mild reaction conditions. The preparation of allylic boronates through the cross-coupling of allyl electrophiles and boryl nucleophiles (Scheme 1), particularly B2(pin)2, was pioneered by Miyaura and represents a broadly useful strategy for construction of substituted allyl boronates. 11 While operable, the most commonly used Miyaura borylation, the C-B coupling between allyl acetates and bis(pinacolato)diboron [B2(pin)2] catalyzed by Pd(dba)2, is occasionally accompanied with dimerization that generates 1,5-diene byproducts (2, Scheme 1). More recent contributions from Szabó and Aggarwal have expanded the electrophile scope to include allylic alcohols.12 While this process is very attractive it does require the use of DMSO as a co-solvent, as well as the use excess B2(pin)2, both of which can complicate the purification. Lastly, Masuda reported that Pt(dba)2 can catalyze the coupling between allylic halides and pinacolborane [HB(pin)].13
Scheme 1.
Catalytic Borylation of Allylic Electrophiles
We have been interested in utilizing allylic boronates in allylation and cross-coupling reactions and our program required ready access to functionalized allyl boronate reagents. Ideally, the practical and efficient synthesis of allylic boronates should include the following features: (1) use commercially available catalysts and readily available electrophiles, (2) avoid using reagents in excess in order to reduce costs and simplify purification, (3) operate in a wide range of solvents such that tandem reaction sequences might be accessible. Herein, we report two practical and efficient syntheses of allylic boronates from commercially available B2(pin)2 and readily available allylic electrophiles. We demonstrate that allylic acetates are readily converted to allylic boronates by Ni-catalysis with a high level of functional group and substituent tolerance; we also demonstrate that allylboron reagents can be effectively synthesized through the borylation of allylic halides in the presence of a number of commercially available Pd sources without the assistance of a dry-box.
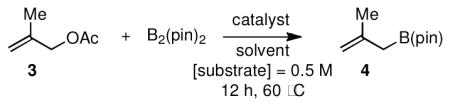
To initiate these studies, the borylation of methyallyl acetate (3) was examined (Table 1). In a preliminary experiment, conditions similar to those described by Miyaura (entry 1, Table 1) were modified: DMSO was replaced with THF, only 1 equivalent of B2(pin)2 was employed, and 10 mol% Pd was used. As depicted in entry 2 of Table 1, these changes dramatically decreased the catalyst activity with less than 5% of the starting material converted after 12 hours heating at 60 °C (vs. >99% conversion in entry 1). To improve the reaction, additives known to promote coupling reactions involving boronate nucleophiles were applied in the borylation. Cs2CO3 proved particularly beneficial and in a concentration-dependent manner. However, these modifications were not effective enough to deliver methallylB(pin) (4) with complete conversion. At this point, it should be noted that although 84% conversion (e.g. in entry 5) might provide the allyl boronate in acceptable yield, compounds 3 and 4 possess similar polarity thereby rendering their chromatographic separation a challenging task. Therefore, other catalysts were examined and we were pleased to find that Ni(cod)2,14 in the presence of phosphine ligands, provided remarkably effective catalysts delivering complete conversion as judged by 1H NMR analysis of the unpurified material (entries 8–10). Importantly, it was also found that EtOAc could replace THF as the solvent for these processes. Lastly, since it proved difficult to remove PPh3 from the allyl boronate product, PCy3 was selected as the optimal ligand for these transformations.
Table 1.
Optimization of Reaction Conditions for the Conversion of Allylic Acetates to Allylic Boronates.
 | ||||
|---|---|---|---|---|
| entry | catalyst | additive | solvent | % conva (yield)b |
| 1 | 2.5% Pd2(dba)3 | none | DMSO | 100c (62) |
| 2 | 5% Pd2(dba)3 | none | THF | <5 |
| 3 | 10% Pd2(dba)3 | none | THF | <5 |
| 4 | 5% Pd2(dba)3 | Cs2CO3d | THF | 56 |
| 5 | 5% Pd2(dba)3 | Cs2CO3e | THF | 84 |
| 6 | 5% Pd2(dba)3 | CsF | THF | 12 |
| 7 | 10% Ni(cod)2 | none | THF | 75 |
| 8f | 10% Ni(cod)2/PPh3 | none | THF | 100 |
| 9f | 5% Ni(cod)2/PPh3 | none | EtOAc | 100 (84g) |
| 10f | 5% Ni(cod)2/PCy3 | none | EtOAc | 100 (80) |
Determined by NMR analysis of unpurified reaction mixture.
Isolated yield of purified material.
Experiment employed 2.2 equiv of B2(pin)2.
Employed 1.2 equiv of additive.
Employed 3 equiv of additive.
Employed 1:1 Ni/ligand.
Contaminated with PPh3.
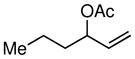
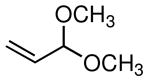
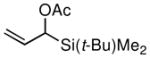
The scope of the Ni-catalyzed allylic borylation was examined using a series of allylic acetates. As depicted in Table 2, the reaction was effective with a range of substrates, generally delivering terminal allylic boronate products with excellent selectivity towards trans-olefins (except entries 8 and 9). A variety of substitution and functional groups were tolerated and it should be noted that both internal and terminal allylic acetates (both E and Z isomers) could be used interchangeably in the reaction. Reactions of aromatic and aliphatic substrates proceeded in good yields, while more functionalized substrates can be converted smoothly into allylic boronates bearing oxygen (entry 7) and silicon (entries 8 and 9) groups on γ-carbon. Noteworthy, is that acetals are reactive substrates for the coupling, affording the E γ-oxygenated allyl boronate in excellent selectivity (entry 7).
Table 2.
Ni-Catalyzed Allylic Boronate Synthesis from Allylic Acetates.
Isolated yield of purified material. Value is an average of two experiments.
Contains ca. 5% impurity.
Employed 10% catalyst and PPh3.
Employed 10% catalyst.
Product is a 2:1 ratio of E:Z isomers as established by NMR.
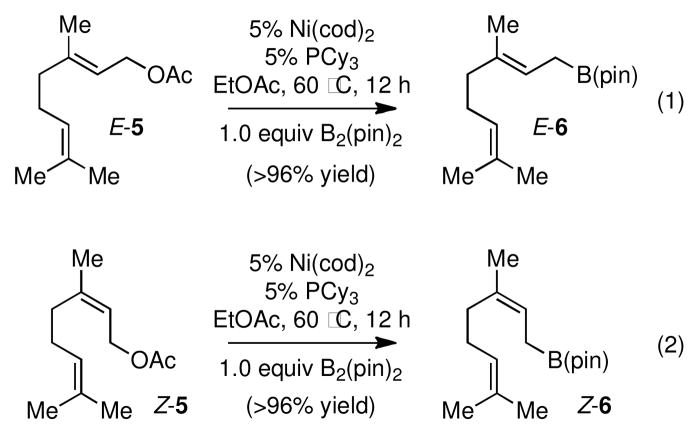
Presumably due to isomerization of the Ni-π-allyl complex during the borylation reaction, terminal boronates bearing trans olefins are the favored product even when cis allylic acetates are employed (see entry 5, Table 2). However, it was found that when trisubstituted allylic acetates (e.g. E-5 and Z-5 in Scheme 2) were used as substrates, the olefin geometry was unchanged over the course of the reaction. As shown in Scheme 2, both E and Z allylic boronates were synthesized in excellent yield and in very high stereoisomeric purity from the corresponding geraniol and nerol derivatives.
Scheme 2.
Ni-Catalyzed Synthesis of Trisubstituted Allylic Boronates with Retention of Configuration.
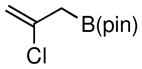
Considering the ready availability of allylic chlorides, we considered whether these substrates might also be converted to the derived allyl boronates. While Ni catalysts proved less effective it was found that Pd complexes were highly efficient in these transformations (Table 3). Attractive features of this Pd-catalyzed borylation are: (1) multiple commercially available Pd sources (e.g. Pd2(dba)3, PdCl2, Pd/C) can be applied as catalysts equally; (2) the catalysts can give excellent turnover numbers such that only 0.5 mol % of catalyst was used in this reaction; (3) the reaction can be set up without the assistance of a dry box and can operate with a minimum of solvent ([substrate] = 2 M in THF), which is important for larger scale syntheses. It was also found that both allylic chlorides and bromides can be used as substrates in this reaction (entry 1), although the transformation using the bromide is not as clean as that of the corresponding chlorides. Importantly, as shown in entry 5 of Table 3, 2-chloro-allylB(pin) was prepared from 2,3-dichloropropene wherein the vinylic chloride was untouched during the allylic borylation. Lastly, it was found that executing the reaction on 10 mmol scale (entry 4), was equally effective as smaller scale experiments.
Table 3.
Pd-Catalyzed Allyl Boronate Synthesis from Allylic Halides.a
 | ||||
|---|---|---|---|---|
| entry | substrate | product | Pd2(dba)3 yield (%) | PdCl2 yield (%)b |
| 1 |
|
|
90 (85c) | 97 |
| 2 |
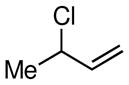
|
|
77d | 81d |
| 3 |
|
|
70d | 70d |
| 4 |
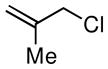
|
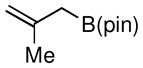
|
67 | 70e |
| 5 |
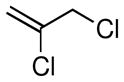
|
|
69f | 69f |
| 6 |
|
|
79(74g) | 80 |
Yields are isolated yields of purified material. Value is an average of two experiments.
Experiments with PdCl2 were executed without the aid of a drybox; all reagents were weighed in air.
Employed cinnamyl bromide as starting material.
Product is a 6:1 mixture of E:Z isomers.
Yield of 71% obtained on 10 mmol scale.
Employed 1 equiv of KOAc.
Employed Pd/C 10% wt as catalyst.
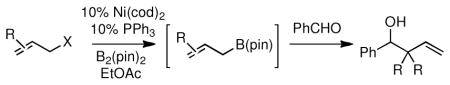
Ready access to functionalized and substituted allylboron reagents can facilitate the preparation of useful organic building blocks. As one example, benzaldehyde was directly added to unquenched Ni-catalyzed borylation mixtures. As depicted in Table 4, this strategy directly furnished allylation products in a highly diastereoselective fashion and in good yields.
Table 4.
One-pot Ni-Catalyzed Borylation/Allylboration.
 | |||
|---|---|---|---|
| entry | substrate | product | % yield (dr)a |
| 1 |
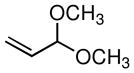
|
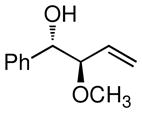
|
80 (20:1) |
| 2 |
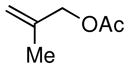
|
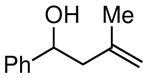
|
88 |
| 3 |
|
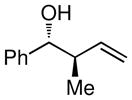
|
80 (20:1) |
| 4 |
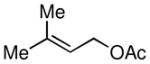
|
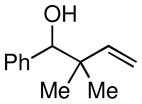
|
50 |
| 5 |
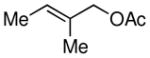
|
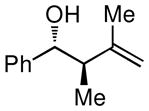
|
70 (15:1) |
Isolated yield of purified material. Diastereomer ratio determined by 1H NMR analysis.
In conclusion, we have presented Ni- and Pd-catalyzed allylic borylation reactions. Both processes employ readily available starting materials, catalysts, and furnish products in a readily usable form.
Supplementary Material
Acknowledgments
Support by the NIGMS (GM-64451) and the NSF (DBI-0619576, BC Mass. Spec. Center) is gratefully acknowledged. PZ is grateful for LaMattina and AstraZeneca Fellowships. We thank AllyChem for a donation of B2(pin)2.
Footnotes
Supporting Information Available. Complete experimental procedures and characterization data (1H and 13C NMR, IR, and mass spectrometry). This material is free of charge via the internet at http://pubs.acs.org.
References
- 1.For representative reviews: Brown HC, Singaram B. Pure & Appl Chem. 1987;59:879.Miyaura N, Suzuki A. Chem Rev. 1995;95:2457.Miyaura N. Bull Chem Soc Jpn. 2008;81:1535.Thomas SP, French RM, Jheengut V, Aggarwal VK. The Chemical Record. 2009;9:24. doi: 10.1002/tcr.20168.
- 2.For represenative reviews: Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.Christmann M, Braese S. Asymmetric Synthesis. 2. Wiley-VCH; Weinheim: 2008. Hugo H, Hall D. Organic Reactions. 2008;73:1.
- 3.For representative examples: Kobayashi S, Endo T, Ueno M. Angew Chem, Int Ed. 2011;50:12262. doi: 10.1002/anie.201106433.Jain P, Antilla JC. J Am Chem Soc. 2010;132:11884. doi: 10.1021/ja104956s.Rauniyar V, Zhai H, Hall DG. J Am Chem Soc. 2008;130:8481. doi: 10.1021/ja8016076.Gravel M, Lachance H, Lu X, Hall DG. Synthesis. 2004;8:1290.Hoffmann RW. Angew Chem Int Ed. 1987;26:489.
- 4.For a review, see: Marek I, Sklute G. Chem Commun. 2007:1683. doi: 10.1039/b615042j.For representative examples: Fandrick KR, Fandrick DR, Gao JJ, Reeves JT, Tan ZT, Li W, Song JJ, Lu B, Yee NK, Senanayake CH. Org Lett. 2010;12:3748. doi: 10.1021/ol101301s.Lou S, Moquist PN, Schaus SE. J Am Chem Soc. 2006;128:12660. doi: 10.1021/ja0651308.Wada R, Oisaki K, Kanai M, Shibasaki M. J Am Chem Soc. 2004;126:8910. doi: 10.1021/ja047200l.
- 5.For a review, see: Ramadhar TR, Batey RA. Synthesis. 2011;9:1321.For representative examples: Viera EM, Snapper ML, Hoveyda AH. J Am Chem Soc. 2011;133:3332. doi: 10.1021/ja200311n.Lou L, Moquist PN, Schaus SE. J Am Chem Soc. 2006;129:15398. doi: 10.1021/ja075204v.Wada R, Shibaguichi T, Makino S, Oisaki K, Kanai M, Shibasaki M. J Am Chem Soc. 2006;128:7687. doi: 10.1021/ja061510h.Wu TR, Chong JM. J Am Chem Soc. 2006;128:9646. doi: 10.1021/ja0636791.Itsuno S, Watanabo K, Ito K, El-Shehawy AA, Sarhan AA. Angew Chem Int Ed. 1997;36:109.
- 6.For representative examples: Yamamoto Y, Takada S, Miyaura N. Chem Lett. 2006;35:704.Yamamoto Y, Takada S, Miyaura N. Chem Lett. 2006;35:1368.Yamamoto Y, Takada S, Miyaura N. Organometallics. 2009;28:152.Gerbino DC, Mandolesi SD, Schmalz H, Podestá JC. Eur J Org Chem. 2009:3964.Dai Q, Xie X, Xu S, Ma D, Tang S, She X. Org Lett. 2011;13:2302. doi: 10.1021/ol2005616.
- 7.(a) Zhang P, Brozek LA, Morken JP. J Am Chem Soc. 2010;132:10686. doi: 10.1021/ja105161f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang P, Le Hai, Kyne RE, Morken JP. J Am Chem Soc. 2011;133:9716. doi: 10.1021/ja2039248. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brozek LA, Ardolino MJ, Morken JP. J Am Chem Soc. 2011;133:16778. doi: 10.1021/ja2075967. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Flegeau EF, Schneider U, Kobayashi S. Chem—Eur J. 2009;15:12247. doi: 10.1002/chem.200902221. [DOI] [PubMed] [Google Scholar]; (e) Jiménez-Aquino A, Flegeau EF, Schneider U, Kobayashi S. Chem Commun. 2011;47:9456. doi: 10.1039/c1cc13348a. [DOI] [PubMed] [Google Scholar]
- 8.For representative examples: Brown HC, Rangaishenvi MV. Tetrahedron Lett. 1990;31:7113.
- 9.For representative examples: Roush WR, Walts AE, Hoong LK. J Am Chem Soc. 1985;107:8186.Brown HC, Racherla US, Pellechia PJ. J Org Chem. 1990;55:1868.
- 10.For representative examples: Matteson DS, Majumdar D. J Am Chem Soc. 1980;102:7588.Sadhu KM, Matteson DS. Organometallics. 1985;4:1687.Brown HB, Singh SM, Rangaishenvi MV. J Org Chem. 1986;51:3150.Althaus M, Mahmmod A, Suárez JR, Thomas SP, Aggarwal VK. J Am Chem Soc. 2010;132:4025. doi: 10.1021/ja910593w.
- 11.Ishiyama T, Ahiko T, Miyaura N. Tetrahedron Lett. 1996;37:6889. [Google Scholar]
- 12.Dutheuil G, Selander N, Szabó KJ, Aggarwal VK. Synthesis. 2008;14:2293. [Google Scholar]
- 13.Murata M, Watanabe S, Masuda Y. Tetrahedron Lett. 2000;41:5877. [Google Scholar]
- 14.For Ni-catalyzed allylic borylations, see: Sumida Y, Yorimitsu H, Oshima K. Org Lett. 2008;10:4677. doi: 10.1021/ol801982d.Crotti S, Bertolini F, Macchia F, Pineschi M. Org Lett. 2009;11:3762. doi: 10.1021/ol901429g.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.