Abstract
Cancer stem cells (CSCs) are recognized as contributors to cancer progression and therapeutic resistance in liquid and solid malignancies. We analyzed a panel of human colon cancer cell lines for CSC populations by side population and aldehyde dehydrogenase activity. IGF-1 enriches these putative colon CSC populations in a β-catenin-dependent manner. Chemical inhibition of Akt depletes SP cells, and conversely, the overexpression of a constitutively active mutant version of Akt is sufficient to enrich CSC populations. CP-751,871, a fully human antibody with specificity to the IGF-1 receptor, is currently being tested in clinical trials for a variety of solid tumors. CP-751,871 reduces CSC populations in colon cancer cell lines in vitro and reduces tumor growth in vivo. We have identified a novel role for IGF-1 in the enrichment of chemoresistant CSC populations. Our results suggest that CP-751,871 has preferential activity against putative CSC populations and, therefore, may complement current standard chemotherapeutic regimens that target cycling cells.
Key words: IGF-1, cancer stem cell, colon cancer, figitumumab
Introduction
The cancer stem cell (CSC) model of carcinogenesis proposes that cancers develop from and are maintained by a small population of cells with self-renewing tumorigenic potential. Originally defined in acute myelogenous leukemia, the cancer stem cell model is increasingly being recognized as a determinant of tumorigenicity, therapeutic resistance and metastasis in solid tumors. The identification of CSCs is based on cell surface marker expression or functional assays, such as side population (SP) analysis and aldehyde dehydrogenase-1 (ALDH1) activity.1–3 Cellular antigen expression is linked with the degree of differentiation and has proven useful in the identification of CSCs from AML (CD34+/CD38−, CD96+), breast cancer (CD44+/CD24−) and glioblastoma (CD133+) primary specimens.4–7 Although the CSC populations of solid tumors originating in the breast and brain have been identified and thoroughly validated, the presence of a colon-specific CSC antigen remains less clear. CD133 was initially identified as the marker for colon tumor-initiating cells, yet conflicting reports have suggested that CD133 expression does not define the CSC population, and both CD133+ and CD133− cells are tumorigenic.8–12 More recent evidence has extended the colon CSC phenotype to include the markers LGR5, CD44, CD166, Musashi-1, EpCAM and CD26.13–18
SP and Aldefluor assays take advantage of functional characteristics of drug efflux and increased ALDH1 activity, respectively, to identify populations enriched with putative cancer stem cells.3,19–21 Increased efflux has long been recognized as a protective characteristic of stem cells and other sensitive populations.22 SP analysis exploits this function for the identification of a rare population of drug-resistant cells from both normal and transformed primary samples as well as established cell lines. We and others, have identified SP cells in a number of established human cancer cell lines and found the SP fraction is enriched following treatment with chemotherapeutic agents.23–25 ALDH1 is a stem cell marker in normal and malignant cells, and ALDH1 levels correspond to early metastasis and decreased survival in breast cancer patients.26,27 Both SP and ALDH1 assays have proven useful in the identification of putative CSCs, especially from established cell lines that may not have maintained inherent cell surface antigen expression profiles.
The insulin-like growth factor 1 (IGF-1) is expressed ubiquitously and exhibits autocrine, paracrine and endocrine chemical signaling activity. IGF-1 primarily binds and activates IGF1R, a tyrosine-kinase receptor frequently overexpressed in cancer.28,29 IGF1R activation initiates a signaling cascade involving the mitogen-activated protein kinase (MAPK) and PI3K/Akt pathways culminating in both the promotion of cell growth and survival and the inhibition of apoptosis.30 IGF-binding proteins, such as IGFBP-3, bind and sequester the vast majority of IGF-1 ligand. This sequestration allows excess tissue and serum IGF-1 levels to be maintained in an inactive state, preventing non-specific and/or constitutive signaling. Epidemiological evidence suggests that higher circulating IGF-1 and lower IGFBP-3 levels independently correlate with an increased risk of developing colon, breast, prostate and lung cancer.31,32 In addition, patients with acromegaly (excessive levels of IGF-1 and growth hormone) have an increased risk of developing both benign and malignant colorectal tumors.33 Such studies have shed light on a contributing role for IGF-1 in colon malignancies with regards to cancer progression, metastasis and resistance to therapy.31,34–36 Taken together, these findings suggest a role for IGF-1 signaling in the progression of colon cancer and have lead to the development of specific IGF-1 inhibitors, including the fully human monoclonal IGF1R antibody, CP-751,871 (figitumumab). Here, we examine the role of IGF-1 signaling and IGF1R inhibition by CP-751,871 in the context of colon CSCs.
Results
Human colon cancer cell lines possess putative CSC populations.
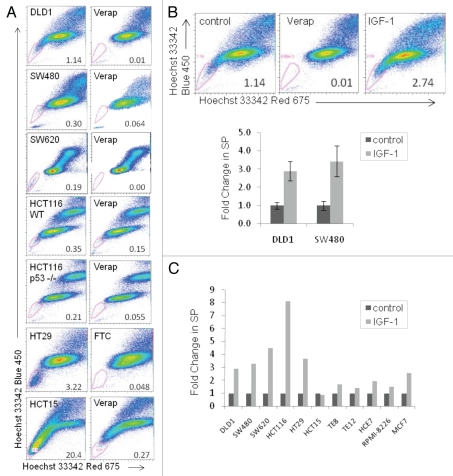
We employed the SP and ALDH1 assays for the identification of CSCs across a panel of human colon cancer cell lines. Almost all of the cell lines possessed both SP and ALDH1+ populations of slightly varying percentages (Fig. 1A and Sup. Fig. 1A). One of nine colon cancer cell lines tested (RKO) lacked an SP (data not shown). All cell lines expressed high levels of P-glycoprotein (Sup. Fig. 2A). To examine the tumorigenicity of the CSCs in vivo, cells were sorted for SP and either non-SP or parental populations and subcutaneously injected at limiting dilution (1 × 102–1 × 105 per flank) in immunocompromised mice. Tumor growth was monitored and considered positive in animals with tumors reaching 200 mm3 in 90 days or less (Table 1). We found an increased percentage of tumor growth in SP populations compared with non-SP or parental populations in five colon and esophageal cancer cell lines tested.
Figure 1.
Human colon cancer cell lines possess a side population enriched by IGF-1. (A) side population analysis of a panel of human colon cancer cell lines (verapamil or FTC treatment is used to establish SP gating parameters), (B) side population analysis of DLD1 and SW480 cells serum starved overnight followed by treatment with IGF-1 at 100 ng/ml for 24 hours (upper panel DLD1 SP profiles), and (C) side population analysis of a variety of human cancer cell lines serum starved overnight followed by treatment with IGF-1 at 100 ng/ml for 24 hours. All error bars represent the SEM of at least three independent experiments.
Table 1.
Tumorigenicity by limited dilution in human colon and esophageal cell lines
| Tumors / No. Injections | |||||
| Cells injected per flank | 1 × 102 | 1 × 103 | 1 × 104 | 1 × 105 | |
| DLD1 | SP | - | 5/6 | 8/8 | - |
| Parental | - | 3/4 | 4/4 | 4/4 | |
| SW480 | SP | 0/4 | 1/4 | 4/4 | - |
| Parental | - | 1/4 | 1/4 | - | |
| SW620 | SP | 4/4 | 3/4 | - | - |
| Non-SP | 0/4 | 1/4 | - | - | |
| TE8 | SP | 2/2 | 2/2 | - | - |
| Non-SP | 0/2 | 2/2 | - | - | |
| TE12 | SP | 2/2 | 2/2 | 2/2 | - |
| Non-SP | - | 0/2 | 3/4 | - | |
Although cellular antigen expression has proven useful for the identification of colon CSCs in primary specimens,8 we found expression varied greatly among established human cell lines. In a panel of eight cell lines tested, 0.28–47.0% of cells were positive for surface expression of CD133 (Sup. Fig. 2B), and many low surface expressing lines still expressed significant levels of cytoplasmic CD133 (data not shown). In analyzing potential overlap between CD133 and SP populations, we found DLD1 SP cells express more CD133 (both cell surface expression and total cell extract) than non-SP cells (Sup. Fig. 2C). Although this implies some overlap between SP and CD133+ populations, we performed the remainder of our studies using SP and ALDH1 measures, since, in contrast to CD133 expression, the functional assays provide a relatively consistent measure across the panel of cell lines.
CSC maintenance and enrichment by IGF-1 depends on Akt and β-catenin.
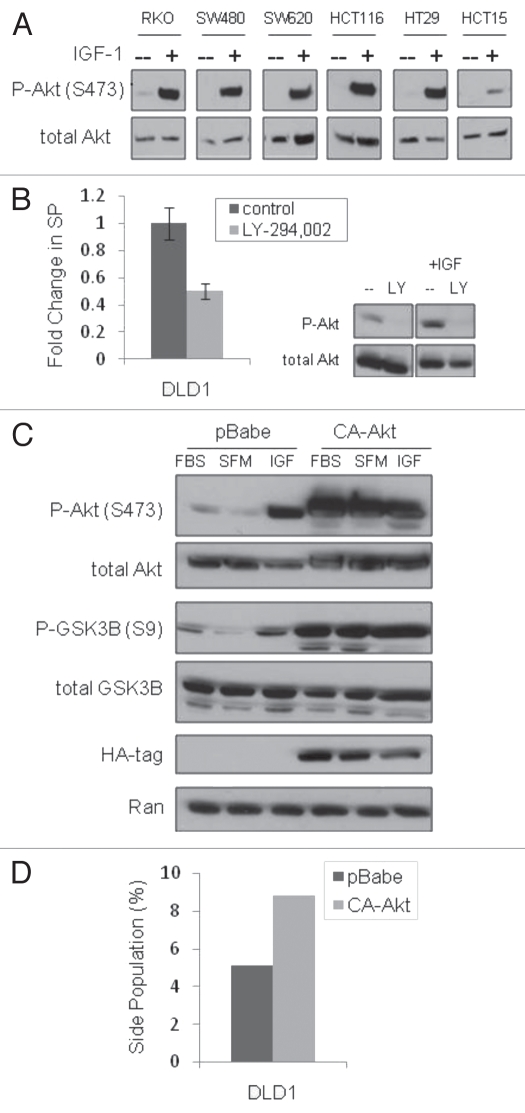
Our primary interest lies in the role of IGF-1 in the propagation and/or survival of colon CSCs. To this end, we analyzed SP and ALDH1+ populations in serum-starved cells stimulated with IGF-1. Human cancer cell lines responded to IGF-1 treatment with an approximately three-fold enrichment in the percentage of SP cells (Fig. 1B and C). Interestingly, colon cancer SP cells showed a greater enrichment by IGF-1 than cells originating in other tissues (TE8, TE12, HCE7, RPMI-8226) (Fig. 1C). The effect of IGF-1 on the enrichment of ALDH1+ populations was more modest (Sup. Fig. 1B). We confirmed IGF1R expression and IGF-1-induced Akt activation across the panel of colon cancer lines (Fig. 2A and Sup. Fig. 2A). Published reports have demonstrated that Akt activation induces drug efflux activity and, therefore, increases the SP fraction.37 Here, we report that IGF-1 treatment enriches the SP without altering the activity of the drug pump protein, ABCG2, as measured by the ability of the cells to efflux mitoxantrone (Sup. Fig. 2D). In fact, it appears as though serum starvation alters the drug pump activity, and stimulation of starved cells with IGF-1 does not further alter ABCG2 function. This suggests that the effect of IGF-1 on the percentage of SP cells is not merely a side effect of altered drug pump activity, but likely a result of the differential cellular response to IGF-1 between SP and non-SP cells.
Figure 2.
Akt activation is important in the maintenance of the SP. (A) immunoblot analysis of Akt phosphorylation (Ser473) in response to IGF-1 (100 ng/ml for 10 minutes) treatment of serum starved cells, (B) side population analysis of DLD1 cells treated with the PI3K inhibitor LY-294,002 (10 µM for 24 hours) and an immunoblot panel confirming LY-294,002 inhibition of IGF-1-induced P-Akt, (C) immunoblot analysis of downstream signaling in pBabe (control) and CA-Akt (constitutive active Akt) DLD1 cells (FBS, cells in culture with serum; SFM, serum free media overnight; IGF, serum starved cells stimulated with 100 ng/ml IGF-1 for 10 minutes) and (D) side population analysis of pBabe and CA-Akt DLD1 cells.
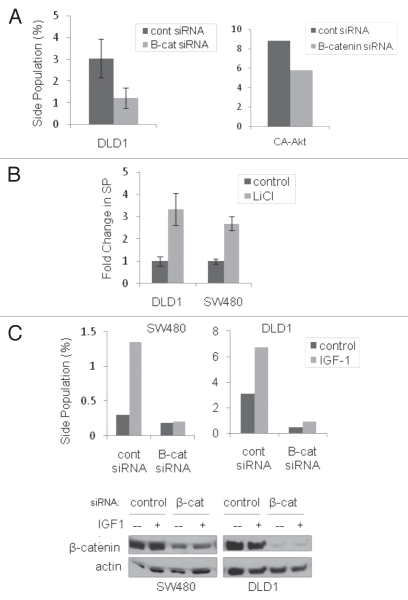
To further clarify the role of Akt in SP enrichment by IGF-1, we analyzed the SP in response to chemical and genetic manipulation of Akt activity. Inhibition of Akt activation by treatment with the PI-3K inhibitor, LY-294,002, decreased the percentage of SP cells, suggesting Akt activation plays a role in the maintenance of the SP phenotype (Fig. 2B). To test the effect of activated Akt on SP cells, DLD1 cells were transfected and selected for expression of a constitutively active Akt mutant (CA-Akt), as confirmed by phosphorylation of Akt (Ser473) and the downstream effector GSK3β (Ser9) (Fig. 2C). The CA-Akt cells maintained an increased SP approximately twice the size of control cells (Fig. 2D). Although nuclear β-catenin was observed in unstimulated cells (presumably due to colon cancer-associated Wnt signaling mutations), IGF-1 treatment resulted in increased translocation of β-catenin to the nucleus (data not shown), a finding that suggests the potential for additional β-catenin activation in these cells, as already described by others.15 Knockdown of β-catenin by siRNA resulted in a more than 50% depletion of SP cells and decreased the SP of CA-Akt cells to the level found in control DLD1s (Fig. 3A). The latter result links Akt and β-catenin in the regulation of the SP phenotype. Additionally, activation of β-catenin with the GSK3β inhibitor, lithium chloride, resulted in a three-fold increase in the percentage of SP cells in both DLD1 and SW480 cells (Fig. 3B). These findings point to Akt activation as a positive regulator of the SP CSC phenotype.
Figure 3.
β-catenin is required for SP enrichment by IGF-1. (A) side population analysis of DLD1 cells (left panel) and CA-Akt DLD1 cells (right panel) with β-catenin knockdown by siRNA, (B) side population analysis of DLD1 cells treated with lithium chloride (20 mM for 24 hrs) and (C) side population analysis (top panels) of DLD1 and SW480 cells with β-catenin knockdown in response to IGF-1 treatment. (Bottom panel: immunoblot confirming β-catenin knockdown). All error bars represent the SEM of at least three independent experiments.
We next sought to determine whether β-catenin knockdown affects the ability of IGF-1 to enrich SP cells. DLD1 and SW480 cells were transfected with β-catenin siRNA and serum starved, followed by stimulation with IGF-1. As expected, β-catenin knockdown reduced the SP to less than half that of control cells (Fig. 3A and C). Furthermore, the absence of β-catenin precluded the SP cells from enrichement by IGF-1 (Fig. 3C). The same experiment was performed in the CA-Akt DLD1 cells with constitutively active Akt. Control pBabe cells respond to IGF-1, with more than a two-fold increase in the percentage of SP cells; however, in the absence of β-catenin the SP levels remained similar with or without IGF-1 stimulation (Sup. Fig. 3A). In addition, there was a minimal effect of IGF-1 on the CA-Akt cells regardless of the presence or absence of β-catenin. This result was expected, since IGF-1 should have no additional effect on the SP in cells with already active Akt or in cells lacking β-catenin. Conversely, IGF-1 enrichment of the SP was minimally altered in the presence of the MEK inhibitor, PD-098,059 (Sup. Fig. 3B). These results highlight a specific role for Akt activation in the maintenance of the CSC populations and the regulation of their response to IGF-1 stimulation.
IGF-1R inhibition decreases CSC populations and colon tumor growth.
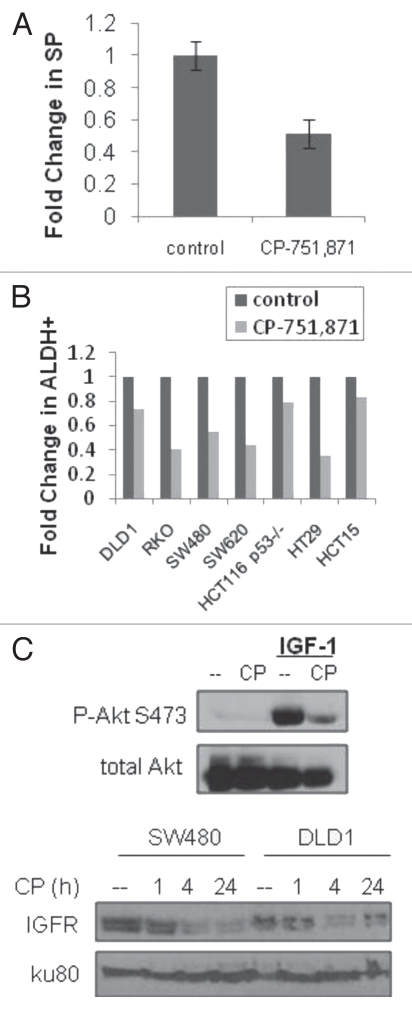
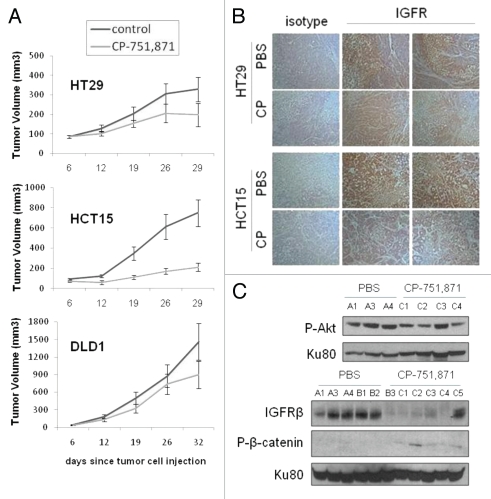
The role of IGF-1 in the enrichment of putative cancer stem cell populations lends credence to the therapeutic potential of targeting CSCs with novel small molecules and biologics. One such therapeutic currently undergoing clinical testing is CP-751,871 (figitumumab), a fully human monoclonal antibody with specificity for IGF-1R (Pfizer, Inc.). In vitro treatment of the panel of colon cancer cell lines with CP-751,871 showed a decrease in both SP and ALDH1+ populations (Fig. 4A and B). As expected, CP-751,871 prevented IGF-1-induced activation of Akt and downregulated IGF1R protein levels (Fig. 4C). In a subcutaneous xenograft tumor model in athymic nude mice, CP-751,871 treatment decreased tumor growth by approximately 50% in three different human colon cancer cell lines with the largest effect on HCT15 tumors (Fig. 5A). Immunohistochemical analysis revealed the in vivo decrease of IGF1R protein levels in CP-751,871 treated tumors (Fig. 5B) and the presence of human IgG within the tumor tissue, confirming the in vivo delivery of figitumumab (Sup. Fig. 4A). Immunoblot analysis of lysed DLD1 tumor tissue revealed a decrease in both total IGFR protein and phosphorylated Akt (Fig. 5C). Of the cell lines tested, CP-751,871 produced the largest decrease in the ALDH population in HT29 cells (Fig. 4B), which also showed a marked decrease in ALDH1 staining in vivo (Sup. Fig. 4B). Additionally, the tumors from figitumumab treated animals were significantly less vascularized upon visual inspection, an observation consistent with reports of IGF-1-induced angiogenesis,38–40 as well as the anti-angiogenic activity of CP-751,871 in the absence of adjuvant chemotherapy.41
Figure 4.
CP-751,871 decreases putative CSC populations. (A) side population analysis of DLD1 cells treated with CP-751,871 (100 ug/ml for 24 hrs; error bars represent the SEM of three independent experiments), (B) Aldefluor analysis of colon cancer cell lines treated with CP-751,871 (100 ug/ml for 48 hrs) and (C) immunoblot analysis of Akt activation (upper panel) and IGF1R-β levels (lower panel) in cells treated with CP-751,871 (100 ng/ml).
Figure 5.
CP-751,871 inhibits in vivo colon xenograft growth. (A) in vivo subcutaneous tumor growth analysis of HT29, HCT15 and DLD1 cells in response to CP-751,871 (weekly injections of 200 µg per mouse), (B) immunohistochemical analysis of IGF1R-β levels in tumors from control mice and mice treated with CP-751,871 and (C) immunoblot analysis of DLD1 tumor lysates for P-Akt (Ser473) and IGF1R-β.
Discussion
The application of marker expression to established human cancer cell lines in culture has proven inconsistent. In the interest of identifying novel CSC-specific cell signaling pathways and subsequently screening CSC-specific therapeutics, we and others have employed the SP and ALDH functional assays with promising results. Although it is unexpected that a single cellular antigen or functional assay will exclusively identify a CSC population, these assays provide the groundwork by which the CSC theory is tested, and the signaling intricacies of rare populations can be identified and exploited for therapeutic gain.
The identification of CSCs by SP analysis takes advantage of increased drug efflux activity, a characteristic of stem cells and a contributor of chemoresistance.24 Our interest lies in IGF-1 signaling in human colon cancer. Due to the documented relationship between IGF-1 and colon cancer chemoresistance, we hypothesized that IGF-1 enriches colon CSC populations. Both SP and ALDH analyses revealed 50–800% enrichment by IGF-1, a finding we attribute primarily to Akt signaling. Akt activation has previously been shown to induce ABCG2 drug pump activity in brain CSCs,37 but we ruled this out as a cause of the increased colon CSC populations based on a lack of IGF-1 induced mitoxantrone efflux in colon cancer cells. In addition, only one cell line (HT29) from the panel of human colon cancer cells was dependent on ABCG2 function, requiring the specific ABCG2 inhibitor fumitremorgin C as an SP gating control, whereas the remaining lines were gated based on the non-specific calcium channel inhibitor, verapamil. Therefore, the effect of IGF-1 on SP is not likely to be merely a result of altered ABCG2 activity but, rather, a preferential sensitivity of SP cells to IGF-1-induced cell survival or proliferation.
Colon cancer progression requires aberrant signaling of Wnt pathway co-factors, most often by inactivation of APC or activating mutations in β-catenin.42 Despite already activated Wnt signaling, many groups have identified the further activation of β-catenin in colon cancer, especially in the context of sub-populations of cells including CSCs, where putative CSCs have higher levels of β-catenin, decreased β-catenin phosphorylation, increased phosphorylation of GSK3β and increased levels of downstream targets, such as cyclin D1 and c-Myc.15,43 These findings both substantiate our demonstration that β-catenin activation is linked to the CSC phenotype and complement the evidence, supporting a role of c-Myc in putative CSCs and induced pluripotent cells (iPCs).25,44 The requirement of β-catenin for SP enrichment by IGF-1 further corroborates a role for β-catenin activation in CSCs. We have not ruled out the possibility that other Akt-dependent or Akt-independent IGF1R downstream targets are important for the survival and propagation of CSCs. Although inhibition of the MAPK pathway with PD-098,059 did not prevent IGF-1 enrichment of the SP, a more thorough analysis is required to rule out contribution from MAPK. Considering CP-751,871 treatment and β-catenin knockdown did not completely eliminate the SP fraction, β-catenin-independent pathways are expected to be at least partially involved in colon SP maintenance. However, it appears as though Akt activation is sufficient for inducing the SP fraction, and β-catenin is necessary for IGF-1-dependent enrichment of the SP. Interestingly, the cell line with the least SP enrichment, HCT15, also maintained the largest percentage of SP cells and was the most responsive to CP-751,871 treatment in vivo. We believe the already large SP has precluded the HCT15 cells from any additional effect of IGF-1; however, the large SP fraction increased the portion of the tumor with particular sensitivity to IGF1R inhibition. IGF1R inhibition is not believed to CSC-specific, but a decrease in the percentage of SP cells suggests an increased IGF1R dependence of SP cells over their non-SP neighboring cells. The modest effect of CP-751,871 in HT29 and DLD1 cells is not discouraging, since the mechanism of CP-751,871 activity is the downregulation of IGF1R and proliferation, not the induction, of cell death. Considering that the SP represents a chemo-resistant population, we expect the combination of figitumumab and chemotherapy (and/or radiation) to provide the best advantage in tumor control, effectively targeting both CSCs and the more proliferative chemo-sensitive non-SP cells. Our findings highlight the use of IGF1R targeting therapeutics, such as CP-751,871 in the treatment of IGF-1 responsive tumors, such as colon carcinomas. We propose a role for CSCs in IGF-1-associated resistance of cancer cells to various chemotherapeutic agents that may be targeted by figitumumab in colorectal carcinoma and other cancer patients.
Materials and Methods
Cell culture, drug treatments and siRNA.
All cells were obtained from American Type Culture Collection and maintained in Dulbecco's modified Eagle medium, McCoy's 5A or RPMI-1640 (Invitrogen) containing 10% fetal bovine serum and penicillin/streptomycin. Human recombinant IGF-1 was purchased from Millipore (used at 100 ηg/ml), and CP-751,871 (figitumumab, used at 20–100 µg/ml) was obtained from collaboration with Pfizer, Inc. The following chemicals were obtained from Sigma: PD-098,059 (used at 10 µM), LY-294,002 (used at 10 µM), lithium chloride (used at 20 mM), verapamil (used at 50 µM) and fumitremorgin C (used at 10 µM). β-catenin and non-targeting siRNA (Santa Cruz Biotechnology) were transfected with the Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's protocol.
Side population, aldefluor activity and mitoxantrone assays.
Flow cytometry was performed using an Elite ESP flow cytometer (Beckman-Coulter). To analyze the SP population, a solid state 355 nm UV laser (Lightwave Electronics) was used to excite the Hoechst 33,342, and the dual emission was captured with a 450/20BP (Hoechst Blue) and a 675/40BP (Hoechst Red) separated with a 550LP dichroic. Forward scatter and PI (675/40BP) were captured from a 488 nm argon laser 40 vsec upstream of the UV laser. Cells were harvested and incubated (1 × 106/ml) in HBSS (Gibco) containing Hoechst-33342 (Invitrogen, 5–10 µg/ml depending on the cell line) for 90 minutes at 37°C. A negative control sample was treated with verapamil (Sigma, 50 µM) or Fumitremorgin C (Sigma, 10 µM) for 15 min at room temperature prior to the addition of Hoechst-33342. Dead cells were gated out based on positive staining with propidium iodide (2 µg/ml). The mitoxantrone assay was performed similarly to the side population but with mitoxantrone (3 µM), and flow cytometry was performed at 635 nm thru a 670 bandpass filter. The Aldefluor assay (Stem Cell Technologies) was performed according to the manufacturer's protocol.
Immunoblots and immunohistochemistry.
For immunoblotting assays, cells were harvested in a buffer containing NP-40 and protease and phosphatase inhibitors (Complete Mini, Roche and Halt Phosphatase Inhibitor Cocktail, Thermo Scientific) and PMSF (Sigma). Proteins were resolved on SDS-PAGE gels and, following western transfer, the PVDF membranes were blocked in TBS-tween containing 5% milk and incubated with the following primary antibodies overnight: Akt (#9272), P-Akt (Ser473, #9271), IGF-1R-β (#3027), β-catenin (#9562) and P-GSK3β (Ser9, #9336), all obtained from Cell Signaling Technologies. GSK3β (#612312) was purchased from BD Transduction Labs, and CD133 (clone 1) was purchased from Miltenyi Biotec. All membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Thermo), and immunoreactive bands were detected using ECL Plus chemiluminescence (Amersham Biosciences). Immunoblots with tumor tissue were performed as already described, with the exception that tumors were lysed by sonication in a buffer containing Triton-X-100 (1.0%), 50 mM HEPES, sodium chloride, magnesium chloride, EGTA, Complete Mini (Roche), Halt Phosphatase Inhibitor Cocktail (Thermo Scientific) and 10% glycerol. Immunohistochemistry was performed according to standard protocols on paraffin-embedded tissues that were sectioned by the Morphology Core at the Center for Molecular Studies in Digestive and Liver Diseases, University of Pennsylvania School of Medicine. Briefly, slides were deparaffinized and hydrated, antigens were unmasked in citrate buffer, and endogenous peroxidases were quenched with hydrogen peroxidase. Slides were blocked with goat serum (5% in TBS-tween), followed by primary antibody incubation (IGF-1R-β, #3027 Cell Signaling Technologies, human IgG or ALDH1, BD Transduction Labs, each at 1:100) overnight at 4°C. Horse-radish peroxidase-conjugated secondary antibodies (Jackson Laboratories, 1:100) were used for 1 hour at 37°C. Peroxidase signal was retrieved with DAB substrate (ImmPACT DAB, Vector Laboratories), and slides were counterstained with hematoxylin, dehydrated, and coverslips were mounted with Permount (Fisher).
Animal experiments.
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. For tumorigenicity experiments, cells were sorted for SP and non-SP or parental populations at the Flow Cytometry and Cell Sorting Facility at the University of Pennsylvania. Cells were injected at limited dilution (in a 1:1 PBS:Matrigel mixture) subcutaneously on the flanks of athymic nude mice (Taconic Farms, Inc.). Mice were monitored three times a week for tumor growth. For experiments involving CP-751,871 treatment, cells (1 × 106) were injected on either flank of athymic nude mice (Taconic Farms, Inc.). Caliper measurements were made for an estimate of tumor volume according to the following equation: volume = ½ (length × width2). Mice were treated with CP-751,871 (200 µg/mouse in 100 µL) or PBS (100 µL) once a week for the duration of the experiment beginning on the day of tumor cell injection.
Acknowledgments
The authors would like acknowledge Training Grant #T32 DK00780 for supporting L.S.H. We would also like to thank to the Flow Cytometry and Cell Sorting Facility and Morphology Core at the Center for Molecular Studies in Digestive and Liver Diseases (both at the University of Pennsylvania) for their technical support. This work was supported in part by NIH grant CA135273 (W.S.E.D.), CA094214 (C.K.) and by the Littlefield-AACR grant in metastatic colon cancer research (W.S.E.D.). W.S.E.D. is an American Cancer Society Research Professor.
Abbreviations
- CSC
cancer stem cell
- SP
side population
- ALDH1
aldehyde dehydrogenase 1
- IGF-1
insulin-like growth factor 1
- MAPK
mitogen-activated protein kinase
Supplementary Material
References
- 1.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. PNAS. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde Dehydrogenase 1 Is a Marker for Normal and Malignant Human Colonic Stem Cells (SC) and Tracks SC Overpopulation during Colon Tumorigenesis. Cancer Research. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosen N, Park C, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha M, Benito-Hernandez A, Morrison S, Clarke M. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami S, Ueno S, Arigami T, Uchikado Y, Setoyama T, Arima H, et al. Prognostic Impact of CD133 Expression in Gastric Carcinoma. Anticancer Res. 2010;30:2453–2457. [PubMed] [Google Scholar]
- 10.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, et al. The AC133 Epitope, but not the CD133 Protein, Is Lost upon Cancer Stem Cell Differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 12.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gires O, Klein CA, Baeuerle PA. On the abundance of EpCAM on cancer stem cells. Nat Rev Cancer. 2009;9:143. doi: 10.1038/nrc2499-c1. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar S, Yu Y, Nautiyal J, Patel B, Majumdar A. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang R, Law WL, Chu ACY, Poon JT, Lam CSC, Chow AKM, et al. A Subpopulation of CD26+ Cancer Stem Cells with Metastatic Capacity in Human Colorectal Cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 18.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, et al. Aldehyde Dehydrogenase Expressing Colon Stem Cells Contribute to Tumorigenesis in the Transition from Colitis to Cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho MM, Ng AV, Lam S, Hung JY. Side Population in Human Lung Cancer Cell Lines and Tumors Is Enriched with Stem-like Cancer Cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 21.Chu P, Clanton DJ, Snipas TS, Lee J, Mitchell E, Nguyen ML, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. International Journal of Cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- 22.Goodell M, McKinney-Freeman S, Camargo F. Isolation and characterization of side population cells. Methods Mol Biol. 2005;290:343–352. doi: 10.1385/1-59259-838-2:343. [DOI] [PubMed] [Google Scholar]
- 23.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 24.Tavaluc R, Hart L, Dicker D, El-Deiry W. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle. 2007;6:2554–2562. doi: 10.4161/cc.6.20.4911. [DOI] [PubMed] [Google Scholar]
- 25.Sussman R, Ricci M, Hart L, Sun S, El-Deiry W. Chemotherapy-resistant side-population of colon cancer cells has a higher sensitivity to TRAIL than the non-SP, a higher expression of c-Myc and TRAIL-receptor DR4. Cancer Biol Ther. 2007;6:1490–1495. doi: 10.4161/cbt.6.9.4905. [DOI] [PubMed] [Google Scholar]
- 26.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Human Pathology. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 29.Weber MM, Fottner C, Liu SB, Jung MC, Engelhardt D, Baretton GB. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002;95:2086–2095. doi: 10.1002/cncr.10945. [DOI] [PubMed] [Google Scholar]
- 30.Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giovannucci E. Insulin, Insulin-Like Growth Factors and Colon Cancer: A Review of the Evidence. J Nutr. 2001;131:3109–3120. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 32.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the Insulin-Like Growth Factor Axis and Mortality in Patients With Nonmetastatic Colorectal Cancer. Journal of Clinical Oncology. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins PJ, Fairclough PD, Richards T, Lowe DG, Monson J, Grossman A, et al. Acromegaly, colonic polyps and carcinoma. Clinical Endocrinology. 1997;47:17–22. doi: 10.1046/j.1365-2265.1997.1911029.x. [DOI] [PubMed] [Google Scholar]
- 34.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, et al. Chemoresistant Colorectal Cancer Cells, the Cancer Stem Cell Phenotype and Increased Sensitivity to Insulin-like Growth Factor-I Receptor Inhibition. Cancer Research. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimberg A. Mechanisms by which IGF-1 may promote cancer. Cancer Biology & Therapy. 2003;2:630–635. [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating Insulin-like Growth Factor-I Levels Regulate Colon Cancer Growth and Metastasis. Cancer Research. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 37.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt Pathway Regulates the Side Population Phenotype and ABCG2 Activity in Glioma Tumor Stem-like Cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol-3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 39.Bustin SA, Dorudi S, Phillips SM, Feakins RMJPJ. Local expression of insulin-like growth factor-I affects angiogenesis in colorectal cancer. Tumor Biol. 2002;23:130–138. doi: 10.1159/000064029. [DOI] [PubMed] [Google Scholar]
- 40.Reinmuth N, Fan F, Liu W, Parikh AA, Stoeltzing O, Jung YD, et al. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth and metastasis of colon cancer. Lab Invest. 2002;82:1377–1389. doi: 10.1097/01.lab.0000032411.41603.c2. [DOI] [PubMed] [Google Scholar]
- 41.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The Insulin-like Growth Factor-1 Receptor-Targeting Antibody, CP-751,871, Suppresses Tumor-Derived VEGF and Synergizes with Rapamycin in Models of Childhood Sarcoma. Cancer Research. 2009;69:7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-Catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.