Abstract
Although mass extinctions probably account for the disappearance of less than 5% of all extinct species, the evolutionary opportunities they have created have had a disproportionate effect on the history of life. Theoretical considerations and simulations have suggested that the empty niches created by a mass extinction should refill rapidly after extinction ameliorates. Under logistic models, this biotic rebound should be exponential, slowing as the environmental carrying capacity is approached. Empirical studies reveal a more complex dynamic, including positive feedback and an exponential growth phase during recoveries. Far from a model of refilling ecospace, mass extinctions appear to cause a collapse of ecospace, which must be rebuilt during recovery. Other generalities include the absence of a clear correlation between the magnitude of extinction and the pace of recovery or the resulting ecological and evolutionary disruption the presence of a survival interval, with few originations, immediately after an extinction and preceding the recovery phase, and the presence of many lineages that persist through an extinction event only to disappear during the subsequent recovery. Several recoveries include numerous missing lineages, groups that are found before the extinction, then latter in the recovery, but are missing during the initial survival–recovery phase. The limited biogeographic studies of recoveries suggest considerable variability between regions.
However much one may mourn the passing of trilobites, conodonts, ammonoids, richtofenid brachiopods, and even dinosaurs, there is no denying the profound evolutionary impetus mass extinctions have provided to the history of life. Mass extinctions create new evolutionary opportunities and redirect the course of evolution. During the past two decades, paleontologists have focused great effort on the patterns, rates, and causes of various mass extinctions. Our understanding of these events has improved greatly, but postextinction rebounds have received far less attention. This lack of attention is unfortunate, for the available detailed empirical studies of recoveries have revealed great complexity to postextinction rebounds, raising questions about the applicability of many models of evolutionary dynamics. Analysis of these extensive biotic disturbances provides detailed information about how ecosystems respond to perturbations and the processes underlying diversification, and insights into what we might plausibly expect from our current biodiversity crisis. In this paper, I will place recovery studies within the context of models of biodiversity dynamics, review the results of both modeling work and empirical studies of specific postextinction recoveries, consider the general patterns that can be derived from a comparative study of recoveries, and close with a discussion of the evolutionary significance of biotic recoveries.
Recoveries and Biodiversity Dynamics
Paleontological discussions of postextinction recoveries have been heavily influenced by models of evolutionary dynamics, particularly competition-driven models governed by the Lotka–Volterra equations and the equilibrial models from MacArthur and Wilson's theory of island biogeography (ref. 1, reviewed in ref. 2). Coupled logistic models have been applied to the dynamics of clades from the fossil record and the patterns of recoveries after mass extinctions (3–6). The models suggest that recoveries will follow a sigmoidal increase to a new equilibrium as survivors radiate into a now-empty ecospace. The sigmoidal shape of such a pattern will produce an apparent lag before an exponential increase, with paleontologists noting the exponential phase as the onset of recovery. The duration of the lag should be proportional to the magnitude of the diversity drop (3, 4). Empirical studies have recognized that many mass extinctions are followed by a survival interval, of variable duration, during which little or no diversification is evident, followed by rapid diversification during a recovery phase (7).
Such equilibrium models give rise to the most common definition of postextinction recoveries: the interval of exponential growth immediately after the end of the extinction, and ending with a decline in origination rates to normal levels as a new equilibrium is approached (7–9). Other definitions have been used, however. Paleoecologists focus on the reappearance of apparently normally functioning ecosystems and emphasize community diversity, structure, and complexity (10). Geochemists have invoked carbon isotopes as a proxy for ecosystem behavior (11). Additionally, different clades may recover at different rates during the same event, and the same clade may recover at different rates in different regions. This ecological and biogeographic texture of biotic recoveries robs many definitions and models of their generality but underscores the complexity of the phenomenon.
Although most analyses of biotic recoveries have focused on individual events, a recent paper involves a time series analysis of the offset between origination and extinction peaks and suggested an approximately 10 million-year lag between the two, irrespective of the magnitude of extinction (12). This lag was found even when the five great mass extinctions were excluded from the analysis. Defining recovery as the interval between a peak in extinction intensity and the subsequent peak in origination is novel, and a lag of this magnitude is not immediately evident after any of the great mass extinctions. The time series analysis is plagued by a number of potential problems, however, and the results will have to be confirmed by future work. The time scale used was not updated with recent information, and hence the 10 million-year lag should best be interpreted as a delay of one stratigraphic time unit before the onset of diversification (13). A delay in the onset of recovery of about 5 million years (myr) has long been apparent in the Early Triassic, after the end-Permian mass extinction, and Sepkoski (14) noted the same pattern after other mass extinction events. He suggested several possible explanations, including preservational artifacts, an artifact resulting from mixing clades with different intrinsic rates of origination (although he discounted this), or a delay in the reestablishment of ecological communities. Variability in origination rates between clades could also produce a synergistic effect in the data (14).
If the results of (12) are valid, they suggest the recovery involves positive feedback, and the active creation of ecospace (12, 13), similar to that recently proposed on the basis of a recent analysis of the delayed recovery of forests in the Early Triassic after the end-Permian mass extinction (15). This pattern of positive feedback is a likely feature of biotic recoveries, irrespective of the validity of ref. 12.
Postextinction Recoveries: Case Studies
In describing extinction–recovery events, I have found it useful to distinguish between rapid perturbations to the system during which no adaptive response is possible and longer-term perturbations during which some lineages may experience adaptive evolution. By analogy with some ecological discussions of disturbance, the former is termed a pulse extinction and the latter a press extinction (16). Sepkoski (2) noted that if perturbations are sufficiently rapid, recovery may begin before the ecosystem fully relaxes to the new expected equilibrium diversity; this pattern is also likely during press extinctions. Continuing perturbations will allow at least some groups to accommodate and potentially diversify while other groups may still be declining (e.g., ref. 17). Several mass extinction episodes, particularly during the Late Devonian, fall into this category.
Five great mass extinction have traditionally been recognized by paleontologists, although there is growing evidence for at least one more event in the Early Cambrian. Recoveries from several other less significant biodiversity crises have also been studied.
Early Cambrian marine faunas are quite distinct from Middle and Late Cambrian assemblages and, although still poorly defined, there is a significant extinction in the latest Early Cambrian (18), possibly in two pulses (19, 20). The reef-building archaeocyath sponges were virtually eliminated, along with calcareous algae and many of the small shelly fossils. Although metazoans do not again play a prominent role in reef formation until the Ordovician, algal and cyanobacterial reefs are common in the Middle and Late Cambrian (21), suggesting that reef ecosystems rebounded relatively quickly but without a significant metazoan component. Other aspects of recovery have not been studied.
Three or four smaller biotic crises during the Late Cambrian and earliest Ordovician are associated with the elimination of many shallow-water trilobites (as well as brachiopods and conodonts) followed by rapid incursions and diversifications of trilobites lineages from deeper waters. Although debate continues over the relative importance of falls in sea level, temperature changes, and other possible causes, the earliest recovery phase is dominated by clades with broad environmental distribution but low relative diversity (number of taxa). Recovery to diverse and specialized faunas occurs within 3 myr (22), with an associated increase in morphologic breadth (23). To the extent that deep-water environments are viewed as refugia, the pattern is consistent with repopulation from refugia followed by diversification.
The end-Ordovician mass extinction [439 million years ago (Ma)] was the second largest of the Phanerozoic but had a far less significant ecological impact than several smaller events. Glaciation and drop in sea level during the first phase of this two-part extinction produced a low-diversity, eurytopic, cool-adapted assemblage that was in turn wiped out during the second phase of the extinction (24). The refined biostratigraphy of this extinction has yielded detailed data on patterns of recovery, emphasizing the independent histories of different clades. Conodonts began expanding from deep water environments, which again served as a refuge, onto the shelf during the interregnum between extinction pulses and formed a low diversity assemblage the earliest Silurian (25). Low-diversity, high-abundance assemblages with broad geographic range are found in the earliest Silurian among graptolites, corals, brachiopods, and some other benthic marine clades (24, 26–28). A number of brachiopod, cystoid, trilobite, and other genera have no fossil record during and immediately after the extinction but then reappear later in the Early Silurian (28–31). These “Lazarus taxa” (32) reveal the persistence of many groups at small population sizes through the extinction and may be significant contributors to the survival fauna. They also serve as a cautionary reminder of the often fragmentary nature of the fossil record of postextinction recoveries. A broad diversification occurs among most groups later in the Early Silurian (24, 26–29). Surprisingly, despite the number of families and genera that disappeared, the extinction had limited ecological effect on reef ecosystems (33).
The Late Devonian extinctions extend from the Givetian through the Devono-Carboniferous boundary, although the major event has been associated with the Frasnian–Fammenian (Late Devonian) extinction (33–36). These repeated extinction pulses complicate the pattern of recovery during this interval, but the general pattern of survival interval followed by diversification appears to hold true for most groups. Sponges, corals, and brachiopods evidently survived in deeper and temperate waters, from which they rediversified (33, 34). Rugose corals are virtually absent from most localities during the early Famennian, followed by a mid-Famennian radiation. A few deep-water Lazarus genera have been recognized, but the new Famennian forms are distinct from those of the underlying Frasnian and their origins obscure. Rugose corals suffer another extinction in the late Famennian but reappear quickly in the earliest Carboniferous and were widespread but of low diversity through much of the Tournasian (37).
There is considerable biogeographic complexity to the recovery (21, 38, 39). A lengthy reef gap evident in North America and western Europe (33) is missing in Asia and Australia, where Famennian reefs were initially dominated by microbes rather than coral or sponges (38). In the Canning Basin of Australia, Wood has described a diverse early Famennian reef composed of surviving calcimicrobes, bryozoans, brachiopods, and an array of sponges (40), casting further doubt on the existence of a reef gap in the aftermath of this extinction. An exquisitely preserved echinoderm fauna from the Fammennian Hongegulung Formation of northwestern China demonstrates the rapid, extensive innovation among blastoids and crinoids (39). These groups did not migrate into Europe and North America until the early Cabroniferous; the delayed migration, probably influenced by the Devono-Carboniferous extinction, produced what appeared to be a long lag before recovery. Such biogeographic studies suggest that apparent delays often reflect biogeographic differences in postextinction habitats, and claims of a global survival–recovery pattern should be approached with caution.
The end-Permian mass extinction (251 Ma) provides perhaps the classic example of a delay before the onset of biotic recovery (41, 42). Paleoecological studies reveal that other than ammonoids, conodonts, and some bivalves, most of the Early Triassic is characterized by low-diversity assemblages of opportunistic forms. Not until the end of the Early Triassic, perhaps 5 myr after the end of the extinction, did signs of broad recovery appear (42–45). Lazarus taxa are particularly notable during the Early Triassic, including up to 30% of the gastropod lineages (16). Gastropods illustrate that surviving the mass extinction is not sufficient to assure continued success. Several lineages, including bellerophontids and subulitids, survived the extinction with little difficulty but quickly disappeared as origination rates increased and other Lazarus forms reappeared. Thus survivorship alone may reveal little about success during the recovery.
The Lazarus taxa return in the latest Early Triassic and Middle Triassic, coincident with diversification among other clades. The Early Triassic recovery lag is the longest documented for any mass extinction, but the causes remain unclear. A continuation of harsh environmental conditions (44–46) (the “environmental damping” of ref. 47), ecological disturbance, and preservation failure have all been implicated (46, 47). The formation of extensive sea-floor carbonate cements into the late Early Triassic supports claims of environmental damping (46), yet the return of stable isotopes and the presence of stenotopic echinoids in shallow waters earlier in the Triassic suggest the lag may in part be ecologic. A potential explanation of delayed recovery that has not been widely explored in the context of biotic recoveries is ecosystem function. One might propose that a prerequisite for recovery would be the rebuilding of sufficient within-trophic-level biodiversity and other aspects of ecosystem function. The relationship between biodiversity and ecosystem function is actively debated, although a recent metanalysis found little support for the idea (48).
Recovery of plants followed a pattern similar to marine groups. The weedy lycopsid Isoetes diversified rapidly and dominated many Early Triassic assemblages (49). Looy et al. (15) documented a long period of dominantly opportunistic lycopsid pollen into the Spathian stage, when a rapid diversification occurs in Europe. The recovery of this equitorial conifer assemblage corresponds to the recovery of higher latitude peat forests, ending the “coal gap” (50, 51). Retallack (51) has suggested that a pervasive short-lived greenhouse climate could explain the data from plants and paleosols; it may also explain the apparent anoxia in shallow marine settings (35, 45, 46).
The end-Triassic mass extinction (200 Ma) is one of the most significant during the Phanerozoic for both marine and terrestrial groups, but the recovery has been poorly documented. Bivalves, ammonites, brachiopods, crinoids, foraminifera, and ostracodes in Europe show no survival interval but simply a steady diversification over several myr (52), although qualitative data for reefs suggest an early Jurassic interval with missing reefs (21).
Several smaller biotic extinctions and recoveries during the Mesozoic and Cenozoic have received attention, including a recent comparative study of the Early Jurassic Toarcian event and the Late Cretaceous Cenomanian–Turonian bioevent (53). Both extinctions are press extinctions of similar magnitude and involved marine anoxia during relatively high sea level and a greenhouse climate. The biotic recoveries share many characteristics as well: planktic and nektonic clades experienced little extinction and display only limited postextinction diversification. Epifaunal bivalves were well adapted to the anoxic conditions responsible for the extinction and were relatively unaffected. Although there is a clear survival interval after the Cenomanian–Turonian biotic crisis followed by a recovery interval, almost 80% of the species during the recovery interval represent surviving lineages, so there is no evidence for an initial dominance by opportunists (54) except among foraminiferal assemblages in Spain (55). A detailed study of the Andean Basin in South America suggests that the extinction may be exaggerated by a pulse of short-lived endemic taxa (56). Detailed δ13C data show a drop during the anoxic episodes, with the end of the excursions closely correlated with the onset of the recovery interval (ref. 53, but see ref. 55 for a different interpretation). The stratigraphic acuity possible for this event has allowed a detailed reconstruction of the recovery of the pelagic food chain, which has not been possible for other events (57). The calcareous nanoplankton reappear quickly, followed by pelagic foraminifera then benthic foraminifera and dinoflagellates.
The catastrophic nature of the Cretaceous–Tertiary (K/T) extinction (65 Ma) and the abundant early Paleogene sections have yielded an excellent record of biotic recovery. Low-diversity high-abundance opportunists dominate the early record of planktonic foraminifera. A single species of Guembelitria is the only species found in the earliest Danian, and all younger planktonic foraminifera are derived from this and one other species. As the recovery progressed, Guembelitria gave rise to a number of other opportunistic forms as environmental conditions ameliorated (58, 59). The radiation into diverse habitats is still not well understood (59). Benthic foraminifera from the El Kef section in Tunisia shows a pattern similar to the pelagic forams. The immediate postextinction assemblage is low diversity with shallow-water affinities. This brief survival interval is followed by a gradual increase in the species diversity of the assemblage (60), although this scenario is not accepted by those who question the role of impact in causing the extinction (e.g., ref. 61).
Benthic organisms experienced considerable extinction at the K/T boundary but diversified quickly during the Paleogene. At the Nye Kløv locality in Denmark (62), the first several meters of post-Cretaceous deposits are virtually barren of most groups of fossils other than bourgueticrinid crinoids (this interval corresponds to the very low diversity foram assemblage zone described above). Gradually a more diverse faunal assemblage appears, including many bryozoans and some other echinoderms, and the relative importance of the crinoids wanes. A similar burst of opportunistic molluscan clades has been described from the earliest Danian of the Gulf Coastal Plain (63). But extension of such studies to three other well-studied regions reveals no opportunistic forms at all (64), emphasizing the extreme geographic variability in recovery patterns. Because the level of extinction is similar in all four regions, heightened extinction does not explain the higher number of opportunists in the Gulf Coastal Plain.
Although cheilostome bryozoans gradually replace cyclostomes during the Cretaceous and Tertiary, this long-term pattern is briefly reversed by the greater resilience of cyclostomes to the effects of the K/T mass extinction (65). This resilience appears to reflect not ecological opportunism but a difference in the response to the extinction, which is evident only through analysis of abundance data, rather than simply taxonomic diversity.
Plants have received considerable attention (66–69). A barren interval is found immediately above the extinction horizons in terrestrial sections in western North America, followed by abundant fern spores. Angiosperm-dominated floral assemblages gradually recover over the succeeding 1.5 myr, but an increase in precipitation and a decline in temperature at the boundary complicates analysis of the recovery. Extinction is less apparent in the southern hemisphere, with fewer changes during the recovery.
The complexities of interpreting carbon isotopic studies are evident from recent work on the K/T extinction (11). In contrast to most earlier mass extinctions, the presence of planktic and benthic foraminifera provides a ready means of determining both deep- and shallow-water isotopic signals. The collapse in the differential between the two signals indicates a productivity crisis during the extinction interval. The differential does not appear fully in marine settings until 3 myr after the extinction, but the delayed isotopic recovery evidently does not mean that productivity was reduced for this entire interval. Instead, the evidence suggests that marine productivity recovered within a few hundred thousand years, but the flux of organic material to the deep sea was reduced because of a reorganization in the open ocean ecosystems. The formation of a new ecosystem with multiple trophic levels marked the final recovery of the ecosystem and the final reappearance of the isotopic differential. Analysis of the organic carbon isotope record of C3 plant cuticles, in contrast, has shown that recovery of the terrestrial carbon cycle (and thus atmospheric carbon as well) occurred within about 130,000 years (69).
Postextinction Recoveries: General Results
Several generalities emerge from this review of postextinction rebounds. Initial postextinction faunas often are of low diversity, with abundant eurytopic taxa. This pattern has been documented among late Cambrian trilobites (20), earliest Silurian corals (26), and other groups (27, 28), Late Devonian corals (37), various Early Triassic groups (15, 42, 43, 49), and a number of early Tertiary groups, including pelagic and benthic foraminifera (58–60), some benthic forms (62), and molluscs from the Gulf Coastal plain (63). No apparent survival interval or low-diversity opportunistic assemblages are documented for the end-Triassic mass extinction or the smaller early Jurassic and Cenomanian–Turonian events [with the exception of one locality in Spain (55)]. Jablonski's biogeographic analysis of earliest Tertiary benthic molluscs demonstrates that the opportunistic bursts in one area should not be interpreted as a global signal (64). The ecological and evolutionary influence of the various mass extinctions differ considerably, with no clear connection between the magnitude of extinction and impact. Guild structures were dramatically reduced during the Permo-Triassic extinction (43), and, at least for open-ocean ecosystems, during the Cenomanian–Turonian (57) and end-Cretaceous extinctions (11). A change in guild structure is not evident during the late Cambrian events, although this absence likely reflects a limited understanding of guild structure in late Cambrian ecosystems (22).
Support for the intuitively attractive hypothesis that mass extinctions preferentially remove morphologically complex forms comes from a recent analysis of trends toward increased sutural complexity in Paleozoic ammonoids (70). Sutural complexity increased steadily during this interval, but this trend was reset during the Late Devonian and Permo-Triassic mass extinctions. The simple surviving forms then resumed the trend toward increased complexity. A significant trend among post-Paleozoic molluscs is toward the acquisition of predator-resistant morphologies. In contrast to the ammonoid study, Hansen et al. (71) found no evidence for resetting of trends toward less predator-resistant morphologies during the K/T extinction or three other Cenozoic extinctions.
Reefs have been a central focus of much work on mass extinctions and subsequent recoveries. A recent review of reef evolution (21) proposes that the apparent greater susceptibility of reef ecosystems to mass extinctions may actually reflect the greater susceptibility of carbonate platform ecosystems to perturbation. In this view, the apparent lag in reef recovery may reflect a delay in reestablishing an appropriate carbonate platform environment rather than an inherent lag in reef ecosystems. This view is sure to be controversial, in part because not all reefs are found on carbonate platforms, and there may be great practical difficulty in distinguishing between the emergence of carbonate platforms and reef ecosystems. The Frasnian–Fammenian mass extinction and recovery does provide an example of this phenomenon, with diverse reefs reappearing quickly in Canning Basin, Australia but a reef gap is present in North America and Europe (40). This study, and a recent analysis of corals across the K/T event (72), have raised questions about the existence of the widely discussed postmass extinction “reef gaps.” Moreover, they suggest that the formation of reef communities is more individualistic than often supposed, and thus any apparent gap is not because of an ecologically imposed delay in recovery.
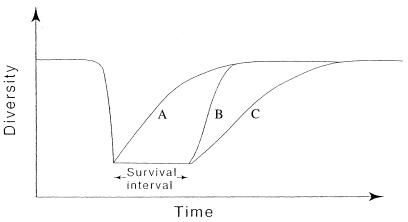
These results can be compared with proposed models of the recovery process (10, 73). The detailed studies of individual recovery events have demonstrated the variety of roads to success, and these models have explored the possible range of ecologic strategies that could aid in survival and trigger the recovery process. Although both empirical and modeling studies usefully emphasize that all survivors are not eurytopic, generalized, opportunistic taxa (54, 73), the range of proposed survival patterns in the models goes beyond what can be reliably determined from the fossil record. More importantly, such pattern-based models provide few insights into the processes driving the survival and recovery process. There is also substantial doubt about the applicability of even multiphase logistic growth models as explanations for evolutionary recoveries. Although the existence of equilibria is an important issue in diversity dynamics (3–6, 74), it is beyond the scope of this contribution. The apparent inapplicability of logistic growth models to postextinction recovery indicates the need for development of a new class of process-based models involving the synergistic interaction between components of the ecosystem (Fig. 1). In such models, the creation of new species would trigger the creation of new opportunities, producing a positive feedback process.
Figure 1.
Expectations from different models of the recovery process. (A) A logistic increase in diversity beginning immediately after the end of the mass extinction. (B) A postextinction rebound with a lag, followed by positive feedback. (C) A logistic diversity increase after a lag survival phase before the onset of recovery.
Clear directions for future research are evident from this overview. Recoveries are still poorly known from almost all of the mass extinctions, and detailed carbon isotope records, useful as a proxy of the health of the carbon cycle, are available for only a few events. There is also a need to expand the repertoire of biogeochemical and environmental proxies for biotic recovery. Nitrogen isotopes, biomarkers, and techniques used by modern ecologists are all worth exploring. Collecting data on the biogeographic structure of recovery is tedious but critical to the development and testing of general recovery models, and the limited biogeographic data clearly illustrate the great spatial variation in recoveries. Virtually absent are detailed phylogenetic studies through extinction, survival, and recovery intervals, yet these are vital to understanding the role and fate of survivors and the locus of recovery. Harries and Little's (53) study of early Jurassic and Late Cretaceous mass extinctions is the only detailed comparative study of biotic recoveries available. Finally, most studies either are paleoecologic or focus on taxonomic diversity. The analysis of ammonid sutural complexity (70) and Foote's studies of crinoids (75) are among the few to explore the changes in morphospace associated with biotic recoveries.
Evolutionary Significance of Postextinction Recoveries
That some mass extinction events have changed the course of evolution is clear, but it is equally obvious that there is no apparent relationship between the magnitude of an extinction and its ecological or evolutionary impact. The end-Permian extinction produced a complete transformation of marine communities, yet even the elimination of perhaps 95% of all marine species did not result in a complete resetting of the evolutionary clock (76). Simulation studies confirm that 80% of the phylogenetic structure can survive a 95% species loss (77). Thus the primary significance of mass extinction may lie in the new ecological patterns that arise during recovery events.
Acknowledgments
This research was funded by grants from the Exobiology Program and Astrobiology Institute of the National Aeronautics and Space Administration and by the Santa Fe Institute through grants from the E. C. Thaw Charitable Trust. The general research program of the Santa Fe Institute is supported by core funding from the John D. and Catherine T. MacArthur Foundation, the National Science Foundation (PHY9600400), and the U.S. Department of Energy (DE-FG03–94ER61951), and by gifts and grants from individuals and members of the Santa Fe Institute's Business Network for Complex Systems Research. I thank Scott Wing and Charles Marshall for discussions and Gunther Eble and Andy Knoll for reviews.
Abbreviations
- Ma
million years ago
- myr
million years
- K/T
Cretaceous–Tertiary
Footnotes
This paper was presented at the National Academy of Sciences colloquium, “The Future of Evolution,” held March 16–20, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.MacArthur R H, Wilson E O. The Theory of Island Biogeography. Princeton, NJ: Princeton Univ. Press; 1967. [Google Scholar]
- 2.Sepkoski J J., Jr . In: Evolutionary Paleobiology. Jablonski D, Erwin D H, Lipps J, editors. Chicago: Univ. Chicago Press; 1996. pp. 211–255. [Google Scholar]
- 3.Carr T R, Kitchell J A. Paleobiology. 1980;6:427–443. [Google Scholar]
- 4.Sepksoki J J., Jr Paleobiology. 1984;10:246–267. [Google Scholar]
- 5.Benton M J. Trends Ecol Evol. 1997;12:490–494. doi: 10.1016/s0169-5347(97)84410-2. [DOI] [PubMed] [Google Scholar]
- 6.Hewzulla D, Boulter M C, Benton M J, Halley J M. Philos Trans R Soc London B. 1999;354:463–469. doi: 10.1098/rstb.1999.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwin D H. Trends Ecol Evol. 1998;13:344–349. doi: 10.1016/s0169-5347(98)01436-0. [DOI] [PubMed] [Google Scholar]
- 8.Benton M J. In: The Unity of Evolutionary Biology. Dudley E C, editor. Portland, OR: Dioscorides; 1991. pp. 89–102. [Google Scholar]
- 9.Walliser O H. In: Global Events and Event Stratigraphy. Walliser O H, editor. Berlin: Springer; 1995. pp. 7–19. [Google Scholar]
- 10.Harries P J, Kauffman E J. In: Extinction Events in Earth History. Kauffman E G, Walliser O H, editors. Berlin: Springer; 1990. pp. 277–298. [Google Scholar]
- 11.D'Hondt S, Donaghay P, Zachos J C, Luttenberg D, Lindinger M. Science. 1998;282:276–279. doi: 10.1126/science.282.5387.276. [DOI] [PubMed] [Google Scholar]
- 12.Kichner J W, Weil A. Nature (London) 2000;404:177–180. doi: 10.1038/35004564. [DOI] [PubMed] [Google Scholar]
- 13.Erwin D H. Nature (London) 2000;404:129–130. doi: 10.1038/35004679. [DOI] [PubMed] [Google Scholar]
- 14.Sepkoski J J., Jr Philos Trans R Soc London B. 1998;353:315–326. doi: 10.1098/rstb.1998.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looy C V, Brugman W A, Dilcher D L, Visscher H. Proc Natl Acad Sci USA. 1999;96:13857–13862. doi: 10.1073/pnas.96.24.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erwin D H. In: Evolutionary Paleobiology. Jablonski D, Erwin D H, Lipps J, editors. Chicago: Univ. Chicago Press; 1996. pp. 398–418. [Google Scholar]
- 17.Budd A F, Johnson K G. Paleobiology. 1999;25:188–200. [Google Scholar]
- 18.Brasier M D. In: Global Events and Event Stratigraphy. Walliser O H, editor. Berlin: Springer; 1995. pp. 113–138. [Google Scholar]
- 19.Zhuravlev A Y. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 79–96. , Special Publication 102. [Google Scholar]
- 20.Zhuravlev A Y, Wood R A. Geology. 1996;24:311–314. [Google Scholar]
- 21.Wood R. Reef Evolution. Cambridge, U.K.: Cambridge Univ. Press; 1999. [Google Scholar]
- 22.Westrop S R, Cuggy M B. J Paleontol. 1999;73:337–354. [Google Scholar]
- 23.Sundberg F A. Paleobiology. 1996;22:49–55. [Google Scholar]
- 24.Sheehan P M, Coorough P J. In: Palaeozoic Palaeogeography and Biogeography. McKerrow W S, Scotese C R, editors. London: Geology Society Memoir 12; 1990. pp. 181–187. [Google Scholar]
- 25.Armstrong H A. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 105–107. , Special Publication 102. [Google Scholar]
- 26.Elias R J, Young G A. Palaios. 1998;13:98–112. [Google Scholar]
- 27.Kaljo D. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 127–133. , Special Publication 102. [Google Scholar]
- 28.Harper D T, Rong J Y. Mod Geol. 1995;20:83–100. [Google Scholar]
- 29.Fortey R A. Philos Trans R Soc London B. 1989;325:327–355. [Google Scholar]
- 30.Rong J Y, Harper D A T. Geol J. 1999;34:321–348. [Google Scholar]
- 31.Rong J Y, Zhan R B. Science in China, Ser D. 1999;42:553–560. [Google Scholar]
- 32.Jablonski D. In: Dynamics of Extinction. Elliot D K, editor. New York: Wiley; 1986. pp. 183–229. [Google Scholar]
- 33.Cooper P. Proc 8th Int Coral Reef Symp. 1997;2:1623–1630. [Google Scholar]
- 34.McGhee G. The Late Devonian Mass Extinction. New York: Columbia Univ. Press; 1996. [Google Scholar]
- 35.Hallam A, Wignall P W. Mass Extinctions and Their Aftermath. Oxford, U.K.: Oxford Univ. Press; 1997. [Google Scholar]
- 36.Caplan M L, Bustin R M. Palaeogeog Palaeoclimatol Palaeoecol. 1999;148:187–207. [Google Scholar]
- 37.Poty E. Palaeogeog Palaeoclimatol Palaeoecol. 1999;154:11–26. [Google Scholar]
- 38.Webb G E. Geology. 1999;26:951–954. [Google Scholar]
- 39.Lane N G, Waters J A, Maples C G. Paleo Soc Mem. 1997;47:1–43. [Google Scholar]
- 40.Wood R. Palaeontology. 2000;43:671–703. [Google Scholar]
- 41.Hallam A. Hist Biol. 1991;5:257–262. [Google Scholar]
- 42.Erwin D H. The Great Paleozoic Crisis. New York: Columbia Univ. Press; 1993. [Google Scholar]
- 43.Schubert J K, Bottjer D J. Palaeogeog Palaeoclimatol Palaeoecol. 1995;116:1–39. [Google Scholar]
- 44.Twitchett R J, Wignall P B. Palaeogeog Palaeoclimatol Palaeoecol. 1996;124:137–151. [Google Scholar]
- 45.Twitchett R J. Palaeogeog Palaeoclimatol Palaeoecol. 1999;154:27–37. [Google Scholar]
- 46.Woods A D, Bottjer D J, Mutti M, Morrison J. Geology. 1999;27:645–648. [Google Scholar]
- 47.Erwin D H. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 223–229. , Special Publication 102. [Google Scholar]
- 48.Schwartz M W, Brigham C A, Hoeksema J D, Lyons K G, Mills M H, van Mantgem P J. Oecologia. 2000;122:297–305. doi: 10.1007/s004420050035. [DOI] [PubMed] [Google Scholar]
- 49.Retallack G J. J Paleontol. 1997;71:500–521. [Google Scholar]
- 50.Veevers J J, Coagham P J, Shaw S E. Geol Soc Am. 1994;288:187–196. [Google Scholar]
- 51.Retallack G J. GSA Bulletin. 1999;111:52–70. [Google Scholar]
- 52.Hallam A. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 223–229. , Special Publication 102. [Google Scholar]
- 53.Harries P J, Little C T S. Palaeogeog Palaeoclimatol Palaeoecol. 1999;154:39–66. [Google Scholar]
- 54.Harries P J. Cret Res. 1993;14:563–583. [Google Scholar]
- 55.Peryt D, Lamolda M. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 245–258. , Special Publication 102. [Google Scholar]
- 56.Aberhan M, Fürsich F T. J Geol Soc London. 2000;157:55–60. [Google Scholar]
- 57.Hart M B. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 265–277. , Special Publication 102. [Google Scholar]
- 58.Olsson R K, Hemleben C, Berggren W A, Liu C A. J Foram Res. 1992;22:195–213. [Google Scholar]
- 59.Koutsoukos E A M. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 319–335. , Special Publication 102. [Google Scholar]
- 60.Speijer R P, Van der Zwaan G J. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 343–371. , Special Publication 102. [Google Scholar]
- 61.MacLeod N. In: Cretaceous–Tertiary Mass Extinctions. MacLeod N, Keller G, editors. New York: Norton; 1996. pp. 85–138. [Google Scholar]
- 62.Håkansson E, Thomsen E. Palaeogeog Palaeoclimatol Palaeoecol. 1999;154:67–85. [Google Scholar]
- 63.Hansen T A. Paleobiology. 1988;14:37–51. [Google Scholar]
- 64.Jablonski D. Science. 1998;279:1327–1330. doi: 10.1126/science.279.5355.1327. [DOI] [PubMed] [Google Scholar]
- 65.McKinney F K, Lidgard S, Sepkoski J J, Jr, Taylor P D. Science. 1998;281:807–809. doi: 10.1126/science.281.5378.807. [DOI] [PubMed] [Google Scholar]
- 66.Nichols D J, Fleming R F. Geol Soc Am. 1996;247:445–455. [Google Scholar]
- 67.Tschudy R H, Tschudy B D. Geology. 1986;14:667–670. [Google Scholar]
- 68.Wolfe J A, Upchurch G R. Nature (London) 1986;324:148–152. [Google Scholar]
- 69.Arens N C, Jahren A H. Palaios. 2000;15:314–322. [Google Scholar]
- 70.Saunders W B, Work D M, Nikovaeva S V. Science. 1999;286:760–763. doi: 10.1126/science.286.5440.760. [DOI] [PubMed] [Google Scholar]
- 71.Hansen T A, Kelley P H, Melland V D, Graham S E. Geology. 1999;27:1139–1142. [Google Scholar]
- 72.Rosen B R. In: Biotic Responses to Global Change. Culver S J, Rawson P F, editors. Cambridge, U.K.: Cambridge Univ. Press; 2000. pp. 164–180. [Google Scholar]
- 73.Harries P J, Kauffman E G, Hansen T A. In: Biotic Recovery from Mass Extinction Events. Hart M B, editor. London: Geology Society of London; 1996. pp. 41–60. , Special Publication 102. [Google Scholar]
- 74.Alroy J. In: Biodiversity Dynamics. McKinney M L, Drake J A, editors. New York: Columbia Univ. Press; 1998. pp. 232–287. [Google Scholar]
- 75.Foote M. Science. 1996;274:1492–1495. doi: 10.1126/science.274.5292.1492. [DOI] [PubMed] [Google Scholar]
- 76.Erwin D H, Valentine J W, Sepkoski J J., Jr Evolution (Lawrence, Kans) 1987;37:1177–1186. [Google Scholar]
- 77.Nee S, May R M. Science. 1997;278:692–694. doi: 10.1126/science.278.5338.692. [DOI] [PubMed] [Google Scholar]