Abstract
Accurate perception of chemical signals from the environment is critical for the fitness of most animals. Drosophila melanogaster experiences its chemical environment through families of chemoreceptors that include olfactory receptors, gustatory receptors and odorant binding proteins. Its chemical environment, however, changes during its life cycle and the interpretation of chemical signals is dependent on dynamic social and physical surroundings. Phenotypic plasticity of gene expression of the chemoreceptor repertoire allows flies to adjust the chemosensory window through which they “view” their world and to modify the ensemble of expressed chemoreceptor proteins in line with their developmental and physiological state and according to their needs to locate food and oviposition sites under different social and physical environmental conditions. Furthermore, males and females differ in their expression profiles of chemoreceptor genes. Thus, each sex experiences its chemical environment via combinatorial activation of distinct chemoreceptor ensembles. The remarkable phenotypic plasticity of the chemoreceptor repertoire raises several fundamental questions. What are the mechanisms that translate environmental cues into regulation of chemoreceptor gene expression? How are gustatory and olfactory cues integrated perceptually? What is the relationship between ensembles of odorant binding proteins and odorant receptors? And, what is the significance of co-regulated chemoreceptor transcriptional networks?
Key words: olfaction, gustation, odorant binding proteins, chemosensation, Drosophila, genetics, transcript profiling, gene expression, gene regulation, phenotypic plasticity
Throughout the life cycle of a fly, chemical signals serve critical roles. When larvae hatch, they depend on chemosensory information for feeding behavior and growth. Following pupation and eclosion, different chemical signals guide the behaviors of each sex. To identify mating partners, males depend on chemical cues to distinguish males from females, and to distinguish conspecific receptive females from non-receptive females. During mating, transfer of proteins from the male's accessory gland with his sperm into the female profoundly changes her physiology and behavior. A change in receptiveness towards other males and stimulation of egg production and oviposition gives rise to dependence on different chemosensory cues (Fig. 1).1 The dynamic interplay between changes in the chemical environment and changes in the perceived importance of chemical signals under different developmental, social and physiological conditions requires adaptation of chemosensory recognition and behavior to maximize the chance for survival and reproduction, i.e., individual fitness. Whereas experiencedependent changes in olfactory behavior have been well-documented in laboratory studies and are mediated by central pathways that include the mushroom bodies, genome-wide transcriptional regulation of chemoreceptors under different developmental, social and physiological conditions has been studied only recently. Here, we first provide a brief overview of the organization of the olfactory and gustatory systems in Drosophila. We then summarize recent findings that illustrate the extensive phenotypic plasticity of expression of the chemosensory gene repertoire. Finally we discuss as yet unsolved fundamental questions which arise from this phenotypic plasticity.
Figure 1.
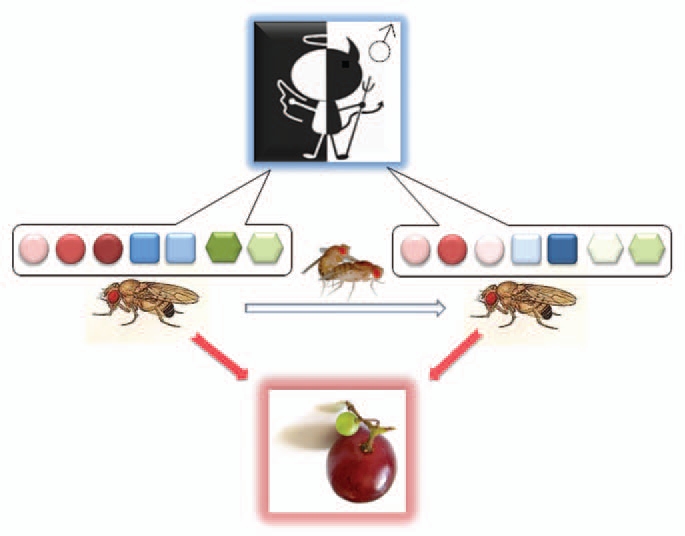
Phenotypic plasticity of chemoreceptor gene expression. Female flies change their chemosensory perception after mating, being less attracted to males and focusing on oviposition site selection and egg laying. These behavioral changes are accompanied by modulation of the expression of different combinations of chemoreceptor genes, Obps, Ors and Grs, represented by circles, squares and hexagons, respectively, with color intensities indicating relative expression levels. Following mating, females show a reduction in transcript levels of four Gr and 12 Or genes, as well as changes in transcript levels of 23 Obp genes.18
Chemoreception in Drosophila
Chemoreception in Drosophila is mediated by multigene families of odorant receptors, gustatory receptors and odorant binding proteins (Table 1). Chemoreceptor proteins are expressed in the antennae, maxillary palps, gustatory tissues, including taste cells in the proboscis and cibarial organs, tarsi, wing margins and, in females, at the ovipositor of the abdominal plate.2,3 Odorant receptors are expressed by olfactory sensory neurons housed in sensilla of the third antennal segments and the maxillary palps. Sensilla in the antennae fall in three morphologically distinct categories, basiconic, coeloconic and trichoid sensilla, whereas the maxillary palps contain only basiconic sensilla.
Table 1.
Functional subdivisions of the chemoreceptor system and the chemoreceptor repertoire of drosophila
| Odorant Receptors and Gustatory Receptors | Antenna | Basiconic sensilla | Recognition and discrimination of volatile food odors | Ors/Or83b, Gr10a |
| Detection of CO2 vapor, leading to avoidance behaviors | Gr21a, Gr63a | |||
| Trichoid sensilla | Detection of the courtship pheromone 11-cis-vaccenyl acetate | Or67d/Or83b | ||
| Recognition and discrimination of volatile food odors | Or35a/Or83b | |||
| Coeloconic sensilla | Detection of amines, carboxylic acids and food odors | IRs | ||
| Detection of water | Unknown | |||
| Maxillary palp | Basiconic sensilla | Recognition and discrimination of volatile food odors | Ors/Or83b | |
| Detection of sugars, leading to acceptance behavior | Gr5a, Gr61a, Gr64a, Gr64f | |||
| Proboscis, legs, wing margins, vaginal plate | Taste hairs and taste pegs | Detection of toxic compounds, leading to avoidance behavior | Gr66a, Gr22b, Gr22e, Gr22f, Gr28b, Gr32b, Gr47a, Gr59a, Gr59b, Gr93a | |
| Detection of carbonation | Unknown | |||
| Detection of pheromone | Gr32a, Gr68a | |||
| Odorant Binding Proteins | Olfactory sensillar lymph | Odorant transportation or removal | Obps, A5, A10 (mostly hypothetical; evidence from association studies only for the Obp99a-d cluster) | |
| Detection of the courtship pheromone 11-cis-vaccenyl acetate and short chain alcohols | Lush | |||
| Gustatory sensillar lymph | Detection of the host plant odors hexanoic acid and octanoic acid | Obp57d, Obp57e | ||
Two distinct odorant receptor families have been identified, Or receptors, which contain seven transmembrane segments,2,3 and Ir receptors, which resemble ionotropic glutamate receptors and are expressed in the coeloconic sensilla.4 Or receptors are expressed in basiconic sensilla and—at least in the case of Or67d,—trichoid sensilla. Each neuron expresses one, or in few cases two, unique odorant receptors from among a repertoire of about 60, along with the common Or83b receptor. The latter forms a dimer with each uniquely expressed receptor that is essential both for transport to and correct insertion in the chemosensory membrane5 and forms an ion channel which mediates signal transduction upon odorant binding.6,7
Olfactory sensory neurons that express the same receptors converge bilaterally on single glomeruli, spherical structures of neuropil in which convergent primary afferents synapse with local interneurons and central projection neurons. The nature and quantity of the olfactory stimulus is encoded in the activation pattern of 43 individually identifiable glomeruli in the antennal lobes. Projection neurons convey olfactory information to the mushroom bodies and the lateral horn of the protocerebrum, where it is processed, integrated and channeled into an appropriate behavioral response.2
Elegant electrophysiological experiments in which spike frequencies were recorded from neurons expressing Or transgenes under the promoter of an endogenous receptor, which due to a deletion was no longer expressed (the “empty neuron” paradigm), elucidated the molecular response profiles of a wide range of both larval and adult Or receptors to a broad spectrum of odorants.8 Ir receptors respond to specific classes of odorants, including amines, but the integration of their projection patterns with those of Or receptors to the antennal lobes has not yet been completely defined.4
In addition to the Or genes, the Drosophila genome encodes a distantly related, diverse, similar size family of gustatory receptor (Gr) genes. Gustatory receptors are expressed in the proboscis, cibarial organs, the antennae and the tarsi.9 The Gr21a and Gr63a receptors are expressed in ab1C antennal neurons that project to a distinct antennal lobe glomerulus and mediate the perception of CO2.10 The Gr68a receptor is expressed in chemosensory cells on the tarsi and contributes to contact pheromone recognition during the courtship ritual that precedes mating.11 Gustatory receptors that mediate perception of sugars, e.g., the Gr5a receptor, and gustatory receptors that recognize compounds that are perceived as bitter in humans are expressed in distinct cell types that project to separate projection areas in the subesophageal ganglion.12 In addition, axons from taste cells expressed in different body parts project to topographically distinct regions of the subesophageal ganglion.13
The Drosophila genome also encodes a family of about 50 Odorant binding protein (Obp) genes. Obps are secreted by support cells in olfactory sensilla into the aqueous perilymph that surrounds olfactory dendrites and are thought to facilitate solubilization and transport of hydrophobic odorants, thereby either promoting or limiting access of odorants to their receptors. Specific Obps have been implicated in pheromone recognition14 and host plant selection.15 Association analyses between polymorphisms in genes of the Obp99a-d cluster and phenotypic variation in behavioral responses to two closely related odorants, benzaldehyde and acetophenone, showed that these odorants interact with overlapping sets of Obps, but that different SNPs are associated with odorant-specific individual variation.16 These results suggest that dual olfactory recognition where Obps regulate odorant access to receptors may enhance olfactory discrimination. Whole genome transcriptional profiling experiments have shown that some Obp genes undergo altered expression following mating,17,18 during exposure to starvation stress,19 during the development of alcohol tolerance after exposure to alcohol,20 and as a correlated response to artificial selection for divergent levels of copulation latency21 and aggression.22 These observations indicate that Obps serve important functions that broadly affect fly physiology and behavior.
Evolution of Chemoreceptor Families
The evolution of large chemoreceptor gene families for long-range and short-range chemical detection and the development of specialized functional classes of chemoreceptors within the Gr and Or families has resulted in a highly sophisticated system exquisitely adapted to regulate the dependence of flies on their chemical environment. Chemosensory receptor genes are thought to evolve rapidly through gene duplication events that result from unequal crossing over during meiosis and give rise to clusters of chemoreceptor genes in the genome.23 Processes of gene duplication and deletion can occur at random by a process that has been coined “genomic drift,” and are not necessarily adaptive.23 Gene duplications result in copy number variation of chemoreceptors, which is especially prominent in vertebrate genomes, and inherently leads to redundancy that is resilient to the emergence of pseudogenes, as lost function of a pseudogenized gene can be partially or completely compensated for by redundant functions of other members of the gene family. Daughter genes of duplication events can accumulate mutations on which adaptive selective forces can act, resulting in functional diversification, and positive selection on a subset of chemoreceptor genes can occur to provide optimal fitness under specific environmental conditions.
Plasticity of Chemoreceptor Expression
Whereas the evolutionary history of the chemoreceptor gene repertoire provides the organism with a diverse “chemosensory toolkit”, changing environments during the animal's lifetime and changes in its physiological state require modulation of the expression of chemoreceptor genes (Fig. 1).18 Drosophila melanogaster is an ideal model organism in which we can address the question of how flexible the expression of chemoreceptor genes is in the face of rapidly changing conditions encountered during an individual's lifetime, since both the genetic background and environmental rearing conditions can be controlled precisely. Whereas posttranslational modifications could regulate the functions of gene products, such modifications have not been documented for insect olfactory or gustatory receptors and would not affect odorant binding proteins once they are excreted into the external environment. Thus transcriptional regulation is likely to be the primary mechanism by which flies can align their chemosensory receptor repertoire with changes in the external chemical environment. By measuring expression levels of all chemosensory genes simultaneously, transcripts were identified with altered expression at different developmental stages, during aging, in males and females, following mating, and under different social conditions. These experiments showed that chemosensory genes that are located in close proximity to one another on the chromosome are often regulated independently at different developmental stages and between the sexes. Of special interest are the Gr22d and Gr22e genes, which are highly expressed in larvae, but undetectable in adults, and likely serve a larval specific feeding function. Dramatic changes in expression levels of Or, Gr and Obp genes occur during aging and are more extensive in males than in females. After mating, four Gr genes and 12 Or genes show altered expression, only in females, whereas members of the Obp gene family undergo altered regulation in both sexes.
Different complements of Obp transcripts respond most prominently to changes in social environment, i.e., flies reared in isolation versus flies reared in single sex groups. When single flies are separated from same or opposite sex groups by a cheesecloth barrier, which allows olfactory signals to be exchanged but prevents tactile stimulation, changes in chemoreceptor gene expression are few when isolated flies are paired with same sex groups, but more extensive when they are exposed to odor of the opposite sex. Again, modulation of Obp transcripts stands out, and, in this case, there is no overlap between Obp transcripts that show altered regulation when single males are exposed to female group odor and when single females are exposed to male group odor. The extensive sexual dimorphism and often antagonistic regulation, in which certain transcripts are upregulated in males and downregulated in females, or vice versa, under different conditions, suggests that a large segment of the chemoreceptor gene repertoire is utilized differently in males and females to extract information from the chemical environment. Moreover, much of the adjustment of the chemosensory window appears to be due to altered regulation of expression of Obp genes.
Environmental plasticity of chemoreceptor expression is heterogeneous, with certain members of the chemoreceptor ensemble responding more frequently to environmental changes than others. This raised the question whether some chemosensory genes might show correlated transcriptional regulation across all experimental conditions. This question was answered with a statistical analysis using the modulated modularity clustering method, an unbiased, self-organizing paradigm based on correlations of transcript abundance levels between different conditions, which sorts transcripts into modules such that transcript abundance levels among members within each module are more closely correlated than with members outside that module.24 This analysis organized the chemoreceptor repertoire in 20 covariant ensembles, revealing an underlying modular organization of the phenotypic plasticity of the chemosensory receptor repertoire. There are indications that this modular organization reflects biological significance. For example, four odorant receptors that all respond electrophysiologically to alcohols and aliphatic esters are contained within the same module, as are Obp76a [a.k.a. Lush] and Or67d, which encode an odorant binding protein and an odorant receptor, respectively, that respond to the pheromone 11-cis-vaccenyl acetate.14,25 The finely pixilated modular organization of the chemosensory subgenome, which consists of a large number of relatively small, albeit highly correlated modules, enables fine tuning of the expression of the chemoreceptor repertoire in response to ecologically relevant environmental and physiological conditions.
Mechanism of Plasticity of Chemoreceptor Expression
The mechanism by which external cues modulate sensory systems is a question of fundamental biological importance. In a broad sense, phenotypic plasticity of the chemoreceptor repertoire in Drosophila is reminiscent of modulation of brain circuits during the acquisition of birdsong. Here, expression of the immediate early gene transcription factor ZENK is induced by auditory experience of a tutor song.26 Similarly, the mechanisms that translate environmental cues into regulation of chemoreceptor gene expression must involve environment-dependent regulation of transcription factors. The identity of these transcription factors and their mode of regulation, remain, however, unknown. Elegant studies by Carlson and colleagues have demonstrated that the POU domain transcription factors acj6 and pdm3 control expression of odorant receptors in subsets of olfactory sensory neurons.27 The acj6 transcription factor is essential for expression of Or genes in the pb1A neurons in the maxillary palps, which respond to the odorant E2-hexenal, and interacts genetically with pdm3.27 Flies that carry a pdm3 mutation do not express the Or42a receptor in antennal ab1A sensory neurons, whereas three other neurons contained within the same sensilla appear to be unaffected.27 Combinations of transcription factors acting on cis-regulatory elements either promote or inhibit the expression of specific Or genes.28 Such transcription factors include lozenge and Scalloped, respectively, but it is reasonable to predict that a wide range of additional as yet unannotated transcription factors may contribute to controlling the expression of chemoreceptor genes. An examination of enrichment of known transcription factor binding motifs among transcriptionally coregulated chemoreceptor genes did not yield readily interpretable results, attesting to the complexity of transcriptional regulation of chemoreceptor genes during the animal's lifetime not only within but also among classes of chemoreceptor genes.
It is possible that differences in chemoreceptor gene expression between the larval and adult stages are largely controlled by hard-wired genetic programs, but that variation in chemoreceptor gene expression as a function of environmental experience is due to upregulation of chemoreceptor genes in neural circuits that are more frequently activated, while chemoreceptor genes that are expressed in neurons that undergo less frequent stimulation are downregulated. This mechanism of phenotypic plasticity would empower neurons that are most important for sensing relevant chemical information at a particular place and time. If such an adaptive mechanism indeed occurs it begs the question as to whether peripheral signal transduction at the level of the olfactory sensory neuron is sufficient to modulate transcriptional regulation of chemoreceptor gene expression, or whether central feedback plays a role, which would suggest that perception of the chemical milieu at the level of the mushroom bodies or lateral protocerebrum might regulate the sensitivity of peripheral chemosensory organs.
A second fundamental biological question is how gustatory and olfactory cues are integrated perceptually? The perception of carbon dioxide via receptors expressed in the antenna or receptors expressed in the proboscis results in different behavioral responses. Antennal perception of carbon dioxide is interpreted as an alarm signal and generates an avoidance response,29 whereas the perception of carbon dioxide via taste receptors on the proboscis is interpreted as the presence of a fermenting food source and elicits an attractant response.30 These observations and behavioral responses to pheromones would suggest that inputs from specialized chemosensory systems are processed via distinct specialized neural pathways. Nevertheless, one cannot exclude that input from olfactory receptors and gustatory receptors might often be integrated, a notion that is supported by covariant transcriptional modules that contain both Gr and Or transcripts.18
A similar issue concerns the relationship between Obps and Ors. On the one hand distinct odorant response profiles can be documented electrophysiologically for a wide range of Or genes expressed in the same “empty” neuron, suggesting that the role of Obps is of limited importance.8 On the other hand, behavioral studies have shown that Obps are critical for activation of the Or67d receptor which is stimulated by the Lush odorant binding protein after it binds the courtship pheromone 11-cis-vaccenyl acetate.24 Furthermore, a 4 bp insertion/deletion in Obp57e determines differences in host plant specificity between D. sechellia and D. melanogaster. Inactivation of the Obp57e and Obp57d genes abolishes repulsion by hexanoic and octanoic acid, toxins produced by Morinda citrifolia, the host plant for D. sechellia.15 In addition, SNPs in the Obp99a-d cluster contribute to phenotypic variation in olfactory behavior in response to benzaldehyde and acetophenone in a population of wild-derived D. melanogaster lines.16
Whereas the connection between Lush and Or67d might suggest a simple relationship between distinct Obps and Ors, there appear to be no similarities in expression patterns between Obp and Or genes in chemosensory tissues. One can speculate that odor recognition in insects is a two step process, in which combinatorial recognition proceeds sequentially through two moderate-size chemoreceptor gene families, the Obp and Or families. Such a system might achieve similar discrimination power as the much larger vertebrate odorant receptor gene families, which consist of hundreds of genes. One might predict that multiple Obps in the sensillar perilymph would provide distinct, yet broad odorant binding specificities that serve as a filter to modulate interactions with Ors that have more circumscribed ligand binding characteristics. How covariant regulation is coordinated between Obp, Or and Gr transcripts in response to environmental or physiological changes is a question of profound importance that cannot be answered at present. Thus, the remarkable phenotypic plasticity where males and females adjust their chemosensory windows by modulating expression of different subsets of chemoreceptor genes illustrates the complexities of the genes-brain-behavior paradigm and the many unanswered questions of the mechanisms by which environmental effects modulate gene expression even in as “simple” an organism as a fly.
Acknowledgements
Work on chemosensation in the authors' laboratories is supported by grants GM059469 and GM045146 from the National Institutes of Health.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/11627
References
- 1.Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered. 2009;100:399–410. doi: 10.1093/jhered/esp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 4.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 8.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin JD, Ha TS, Jones DN, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 2007;5:118. doi: 10.1371/journal.pbio.0050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Lyman RF, Mackay TFC, Anholt RRH. Natural variation in odorant recognition among odorant binding proteins in Drosophila melanogaster. Genetics. 2009 doi: 10.1534/genetics.109.113340. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Stone EA, Mackay TFC, Anholt RRH. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster. PLoS Genet. 2009;5:1000681. doi: 10.1371/journal.pgen.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbison ST, Chang S, Kamdar KP, Mackay TFC. Quantitative genomics of starvation stress resistance in Drosophila. Genome Biol. 2006;6:36. doi: 10.1186/gb-2005-6-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morozova TV, Anholt RRH, Mackay TFC. Transcriptional response to alcohol exposure in Drosophila melanogaster. Genome Biol. 2006;7:95–s. doi: 10.1186/gb-2006-7-10-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay TFC, Heinsohn SL, Lyman RF, Moehring AJ, Morgan TJ, Rollmann SM. Genetics and genomics of Drosophila mating behavior. Proc Natl Acad Sci USA. 2005;102:6622–6629. doi: 10.1073/pnas.0501986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards AC, Rollmann SM, Morgan TJ, Mackay TFC. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:154. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 24.Stone EA, Ayroles JF. Modulated modularity clustering as an exploratory tool for functional genomic inference. PLoS Genet. 2009;5:1000479. doi: 10.1371/journal.pgen.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tichy AL, Ray A, Carlson JR. A new Drosophila POU gene, pdm3, acts in odor receptor expression and axon targeting of olfactory neurons. J Neurosci. 2008;28:7121–7129. doi: 10.1523/JNEUROSCI.2063-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray A, van der Goes van Naters W, Carlson JR. A regulatory code for neuron-specific odor receptor expression. PLoS Biol. 2008;6:125. doi: 10.1371/journal.pbio.0060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 30.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]


