Abstract
The metadata of 10 published studies and 3 vaccine trial reports comprising of 19 vaccine cohorts from four countries conducted over a period of 23 years (1986–2009) was used for metaanalysis. The vaccines studied were purified chick embryo cell vaccine (Rabipur, India and Germany), purified vero cell rabies vaccine (Verorab, France; Indirab, India) and human diploid cell vaccine (MIRV, France). The potency of these vaccines varied from 0.55 IU to 2.32 IU per intradermal dose of 0.1 ml per site. The vaccines were administered to 1,011 subjects comprising of 19 cohorts and using five different ID regimens. The immunogenicity was measured by assays of rabies virus neutralizing antibody (RVNA) titres using rapid fluorescent focus inhibition test (RFFIT) [15 cohorts] and mouse neutralization test (MNT) [4 cohorts]. The statistical analysis of the data was done by Karl Pearson's correlation coefficient to measure the relationship between antigenicity and immunogenicity. It was revealed that, there was no significant linear relationship between antigenicity and immunogenicity of rabies vaccines when administered by intradermal route (p > 0.230 and p > 0.568).
Key words: rabies vaccines, intradermal route, antigenicity, immunogenicity, metaanalysis
Introduction
Approximately 55,000 people die from rabies each year, the vast majority of these deaths occur in Asia and Africa. Annually, more than 10 million people, mostly in Asia, receive post-exposure vaccination against rabies.1 For many years, safe and highly efficacious rabies vaccines produced in various cell cultures and embryonated egg, have been commercially available. In some countries enzootic for rabies, cell culture vaccines (CCVs) are in short supply and/or unaffordable. However, intradermal rabies vaccination (IDRV) using selected CCVs has been established as an efficacious and economic alternative to the standard intramuscular (IM) regimens. IDRV has been successfully introduced for post-exposure prophylaxis (PEP) in developing countries such as India, Philippines, Srilanka and Thailand.
Currently, three types of cell culture vaccines are available for application by intradermal (ID) route for prevention of human rabies. These are human diploid cell vaccine (HDCV), purified chick embryo cell vaccine (PCECV) and purified vero cell rabies vaccine (PVRV). As per the recommendations of World Health Organization (WHO), these vaccines shall have an antigen content as measured by potency of at least 2.5 international units (IU) per IM dose. This potency is expected to produce a rabies virus neutralizing antibody (RVNA) response of ≥0.5 IU per ml in the vaccinees, which is considered as adequate for protection against rabies and this holds good for ID regimens too. However, WHO has neither prescribed an upper limit of potency for CCVs by intramuscular route nor recommended the potency per ID dose. Whereas, the national regulatory authorities for procurement of rabies vaccines for ID route in Thailand and Sri Lanka advocate a minimum potency of 0.7 IU in 0.1 mL per ID site2 and in Philippines 0.5 IU in 0.1 mL per ID site.
The dose of HDCV and PCECV is 1 mL by IM route, whereas the dose of PVRV is 0.5 mL. However, the ID dose of all the three vaccines is 0.1 mL per ID site, irrespective of the volume by IM route. Besides, the use of different ID regimens viz. 8 site, 4 site, 2 site, etc., result in different antigenic loads i.e., the total amount (in IU) of antigen injected in the vaccinees, as the volume of vaccine administered varies according to the number of sites injected.
In this context, the authors having conducted previously a meta-analysis assessing the relationship between antigenicity and immunogenicity of human rabies vaccines by IM route,3 this time embarked on a similar study of human rabies vaccines administered by ID route.
Results
A scatter diagram of nineteen cohorts drawn by plotting antigenic loads versus geometric mean concentrations (GMCs) of RVNA to measure the relationship between antigenicity (antigenic load by day 7) and immunogenicity (GMC by day 14) using Karl Pearson's correlation coefficient revealed a poor linear relationship between antigenicity and immunogenicity and this was not significant (r = 0.289 with r2 = 0.084, p > 0.230) (Fig. 1). A similar relationship was observed between antigenicity by day 28 and immunogenicity by day 90 (r = 0.140 with r2 = 0.020, p > 0.568) (Fig. 2).
Figure 1.
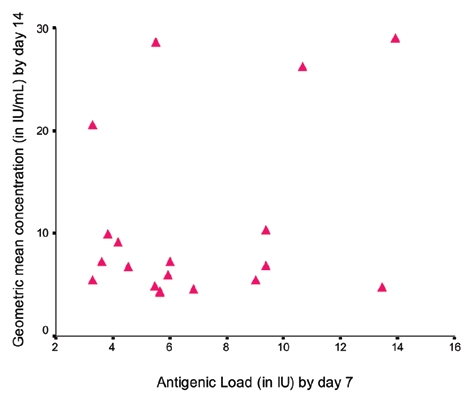
Correlation between antigenic load by day 7 versus GMC by day 14 (r = 0.289, p > 0.230).
Figure 2.
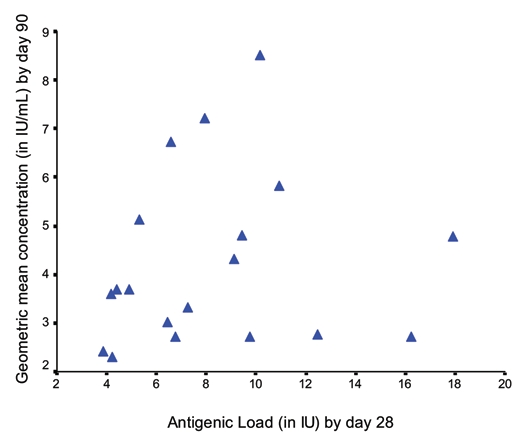
Correlation between antigenic load by day 28 versus GMC by day 90 (r = 0.140, p > 0.568).
Discussion
IDRV involves administration of small quantity i.e., 0.1 mL of vaccine into the dermis layer of skin. Rabies being a practically 100% fatal disease, IDRV is a life saving treatment in PEP. As rabies is endemic in the developing world, IDRV is recommended as a cost effective tool for use in countries of Asia and Africa. Some consider IDRV as an “inferior/weaker cousin” of intramuscular rabies vaccination, throwing doubt on its efficacy. Compounding this problem further, WHO having defined potency for rabies vaccines by IM route, has not done so, for rabies vaccines given by ID route. Consequently, some countries like Thailand, Srilanka and Philippines have mandated a higher potency for IDRV.
In a study using a single lot of PCECV vaccine with potencies ranging from 0.032 IU (1:16 dilution) to 0.506 IU (undiluted) per ID dose of 0.1 mL when administered to subjects showed a clear, almost linear relationship discernible between the amount of antigen administered and immune response in terms of RVNA titres.4 A study involving Vietnamese children which used three batches of PVRV with potencies ranging from 3.5 IU to 12.0 IU per vial (of 0.5 mL) failed to show a definite dose-response relationship.5 Another study using dilutions of HDCV when administered intradermally to healthy volunteers demonstrated a dose-response relationship.6
In this metaanalysis, done on three different types of cell culture vaccines used for intradermal rabies vaccination, having a range of potency from 0.55 IU to 2.32 IU per ID dose of 0.1 mL when administered to 1011 subjects using five different regimens showed no significant linear dose-response relationship between antigenicity and immunogenicity. This may be, to some extent, due to computation of results from different studies in different populations with different vaccines and using different regimens and performing serology with different tests, but it forms a part of any such metaanalysis.
As this is a first study of its kind the authors recommend, that in future the metaanalysis may be done by considering more number of studies as and when papers on IDRV are published, giving a greater power for analysis. For the present, the findings of this study hopefully should convince the authorities, medical profession and the industry to have confidence in the potencies of rabies vaccines currently advocated for use by intradermal route.
Lastly, the manufacturers shall continue to follow the current WHO recommendations of a minimum potency of 2.5 IU per IM dose i.e., 0.5 mL for PVRV and 1 mL for HDCV and PCECV, which amounts to 0.5 IU per ID dose of 0.1 mL for PVRV and 0.25 IU per ID dose of 0.1 mL for HDCV and PCECV for ensuring an efficacious IDRV.
Materials and Methods
The authors used Pubmed and selected ten studies published in peer reviewed national/international journals7–16 and that were conducted to evaluate the immunogenicity of rabies vaccines by ID route. Besides, the clinical trial reports of three Indian studies (not yet published) were also included.17–19 The metadata of these thirteen studies from four countries i.e., India, Thailand, Germany and Lithuania conducted over a period of 23 years (1986–2009) was used for metaanalysis.
The total number of subjects in these thirteen studies having nineteen vaccine cohorts was 1011. The vaccines studied were PCECV (Rabipur, India and Germany), PVRV (Verorab, France and Indirab, India) and HDCV (Merieux inactivated rabies vaccine, France). The different ID regimens used were-updated TRC [2-2-2-0-2] 5 cohorts; TRC [2-2-2-0-1-1] 7 cohorts; 8 site [8-0- 4-0-1-1] 2 cohorts; 4 site [4-0-2-0-1 and 4-4-4-0-1-1] 4 cohorts and KIMS [2-2-2-2-2] 1 cohort. The RVNA assessment was done by using rapid fluorescent focus inhibition test (RFFIT) in 15 cohorts and mouse neutralization test (MNT) in 4 cohorts (Table 1). Both the methods are approved by WHO.20 The data available from nineteen cohorts included potency of the vaccine, ID schedule, number of subjects and geometric mean concentrations of RVNA for days 14 and 90. The potency of rabies vaccine per 0.1 mL of ID dose was calculated depending on the volume and potency of the same vaccine used by IM route. The “antigenic load” is defined as the amount of antigen injected by days 7 and 28 according to the potency of vaccine and ID regimen used. The antigenic load was computed for all the nineteen vaccine cohorts from thirteen studies. The GMCs of RVNA assays were noted for the days 14 and 90 as a measure to assess the immune response to the antigenic load/stimulus by days 7 and 28 respectively (Table 2).
Table 1.
Details of regimens, schedules, cohorts and vaccines
| Regimen | Schedule | Total cohorts | Vaccines (cohorts) |
| Updated TRC | (2-2-2-0-2) | 5 | Rabipur (2) |
| Verorab (1) | |||
| Indirab (2) | |||
| TRC | (2-2-2-0-1-1) | 7 | Rabipur (4) |
| Verorab (3) | |||
| 8 site | (8-0-4-0-1-1) | 2 | Rabipur (2) |
| 4 site | (4-0-2-0-1-1 & 4-4-4-0-1-1) | 4 | Rabipur (2) |
| Verorab (1) | |||
| MIRV (1) | |||
| KIMS | (2-2-2-2-2) | 1 | Rabipur (1) |
Note: RVNA Method: RFFIT (15 cohorts); MNT (4 cohorts).
Table 2.
Details of vaccine cohorts, ID regimens, antigenic loads and immune response
| Cohort | Vaccine | Potency/ID dose | ID Schedule | Number of Subjects | Antigenic load (Day 7) | GMC (Day 14) | Antigenic load (Day 28) | GMC (Day 90) | Type of RVNA analysis |
| 1 | Rabipur7 | 0.55 | 4-0-2-0-1-1 | 86 | 3.30 | 20.50 | 3.85 | 2.39 | RFFIT |
| 2 | Indirab19 | 0.55 | 2-2-2-0-2 | 55 | 3.30 | 5.40 | 4.40 | 3.66 | RFFIT |
| 3 | Rabipur8 | 0.60 | 2-2-2-0-1-1 | 65 | 3.60 | 7.13 | 4.20 | 2.27 | MNT |
| 4 | MIRV9 | 0.32 | 4-4-4-0-1-1 | 19 | 3.84 | 9.88 | 4.16 | 3.57 | RFFIT |
| 5 | Rabipur10 | 0.70 | 2-2-2-0-1-1 | 81 | 4.20 | 9.07 | 4.90 | 3.67 | RFFIT |
| 6 | Rabipur11 | 0.76 | 2-2-2-0-1-1 | 25 | 4.56 | 6.70 | 5.32 | 5.10 | MNT |
| 7 | Rabipur19 | 0.91 | 2-2-2-0-2 | 54 | 5.46 | 4.75 | 7.28 | 3.30 | RFFIT |
| 8 | Rabipur12 | 0.92 | 2-2-2-0-1-1 | 59 | 5.52 | 28.50 | 6.44 | 3.00 | RFFIT |
| 9 | Rabipur13 | 0.94 | 2-2-2-2-2 | 45 | 5.64 | 4.17 | 9.43 | 4.79 | RFFIT |
| 10 | Rabipur14 | 0.94 | 2-2-2-0-1-1 | 49 | 5.64 | 4.30 | 6.58 | 6.70 | RFFIT |
| 11 | Rabipur17 | 0.99 | 2-2-2-0-2 | 81 | 5.94 | 5.83 | 7.92 | 7.20 | RFFIT |
| 12 | Rabipur15 | 0.75 | 4-0-4-0-1-1 | 15 | 6.00 | 7.20 | 6.75 | 2.70 | RFFIT |
| 13 | Indirab18 | 1.14 | 2-2-2-0-2 | 68 | 6.84 | 4.46 | 9.12 | 4.30 | RFFIT |
| 14 | Rabipur15 | 0.75 | 8-0-4-0-1-1 | 15 | 9.00 | 5.40 | 9.75 | 2.70 | RFFIT |
| 15 | Rabipur16 | 0.78 | 8-0-4-0-1-1 | 39 | 9.36 | 10.20 | 10.14 | 8.50 | MNT |
| 16 | Rabipur16 | 1.56 | 2-2-2-0-1-1 | 43 | 9.36 | 6.80 | 10.92 | 5.80 | MNT |
| 17 | Verorab7 | 1.78 | 4-0-2-0-1-1 | 87 | 10.68 | 26.10 | 12.46 | 2.75 | RFFIT |
| 18 | Verorab18 | 2.24 | 2-2-2-0-2 | 66 | 13.44 | 4.67 | 17.92 | 4.75 | RFFIT |
| 19 | Verorab12 | 2.32 | 2-2-2-0-1-1 | 59 | 13.92 | 28.90 | 16.24 | 2.70 | RFFIT |
Note: GMC, Geometric mean concentration; RFFIT, Rapid fluorescent focus inhibition test; MNT, Mouse neutralization test.
The SPSS v11.0 package was used for statistical analysis of the metadata.
Acknowledgements
The authors thank Dr. N.S.N. Rao, Professor of Statistics (retd.) for providing professional guidance in data analysis.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/11934
References
- 1.World Health Organization, author. Rabies vaccines, WHO position paper, weekly epidemiological record 49/50. 2007;82:426–426. [Google Scholar]
- 2.Proposed new WHO rabies control guidelines (Highlights from the WHO consultation on human and dog rabies prevention and control, France, Oct. 2009) Asian Biomed. 2009;3:751–754. [Google Scholar]
- 3.Sudarshan MK, Mahendra BJ, Madhusudana SN, Ashwath Narayana DH, Sanjay TV, Gangaboraiah, et al. Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines: Results of a metaanalysis. Hum Vaccin. 2005;1:187–190. doi: 10.4161/hv.1.5.2110. [DOI] [PubMed] [Google Scholar]
- 4.Beran J, Honegr K, Banzhoff A, Malerczyk C. Potency requirements of rabies vaccines administered intradermally using the Thai Red Cross regimen: investigation of the immunogenicity of serially diluted purified chick embryo cell rabies vaccine. Vaccine. 2005;23:3902–3927. doi: 10.1016/j.vaccine.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Lang J, Hoa DQ, Gioi NV, Vien NC, Nguyen CV, Rouyrre N. Immunogenicity and safety of low dose intradermal rabies vaccine given during an Expanded Programme on immunization session in Vietnam: results of a comparative randomized trial. Trans R Soc Trop Med Hyg. 1999;93:208–213. doi: 10.1016/s0035-9203(99)90309-7. [DOI] [PubMed] [Google Scholar]
- 6.Fishbein DR, Pacer RE, Holmes DF, Ley AB, Yager P, Tong TC. Rabies post-exposure prophylaxis with human diploid cell rabies vaccine: a dose-response study. J Infect Dis. 1987;156:50–55. doi: 10.1093/infdis/156.1.50. [DOI] [PubMed] [Google Scholar]
- 7.Ambrozaitis A, Laiskonis A, Balciuniene L, Banzhoff A, Malerczyk C. Rabies post-exposure prophylaxis vaccination with purified chick embryo cell vaccine (PCECV) and purified vero cell rabies vaccine (PVRV) in a four-site intradermal schedule (4-0-2-0-1-1): An immunogenic, cost-effective and practical regimen. Vaccine. 2006;24:4116–4121. doi: 10.1016/j.vaccine.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Charanasri U, Kingnate D, Meesomboon V, Samuthananon P, Chaeychomsri W. Intradermal simulated rabies post-exposure prophylaxis using purified chick embryo rabies vaccine. J Med Assoc Thai. 1992;75:639–642. [PubMed] [Google Scholar]
- 9.Ubol S, Phanuphak P. An effective economical intradermal regimen of human diploid cell rabies vaccination for post-exposure treatment. Clin Exp Immunol. 1986;63:491–497. [PMC free article] [PubMed] [Google Scholar]
- 10.Suntharasamai P, Chaiprasithikul P, Wasi C, Supanaranond W, Auewarakul P, Chanthavanich P. A simplified and economical intradermal regimen of purified chick embryo cell rabies vaccine for post-exposure prophylaxis. Vaccine. 1994;12:508–512. doi: 10.1016/0264-410x(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 11.Madhusudana SN, Prem Anand N. Response to purified chick embryo cell rabies vaccine administered intra-dermally for post-exposure prophylaxis. The Nat Med J India. 1997;10:115–116. [PubMed] [Google Scholar]
- 12.Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified vero cell rabies vaccine. Bull World Health Organ. 2000;78:693–698. [PMC free article] [PubMed] [Google Scholar]
- 13.Sudarshan MK, Madhusudana SN, Mahendra BJ, Ashwath Narayana DH, Ananda Giri MS, Popova O. Evaluation of a new five-injection, two-site, intradermal schedule for purified chick embryo cell rabies vaccine: A randomized, open-label, active-controlled trial in healthy adult volunteers in India. Current Therapeutic Res. 2005;66:323–334. doi: 10.1016/j.curtheres.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhusudana SN, Sanjay TV, Mahendra BJ, Sudarshan MK, Ashwath Narayana DH, Ananda Giri MS. Comparison of safety and immunogenicity of purified chick embryo cell rabies vaccine (PCEV) and purified vero cell rabies vaccine (PVRV) using the Thai Red Cross intradermal regimen at a dose of 0.1 ml. Hum Vaccin. 2006;2:200–204. doi: 10.4161/hv.2.5.3197. [DOI] [PubMed] [Google Scholar]
- 15.Wilde H, Khawplod P, Hemachudha T, Sitprija V. Postexposure treatment of rabies infection: can it be done without immunoglobulin? CID. 2000;34:477–480. doi: 10.1086/324628. [DOI] [PubMed] [Google Scholar]
- 16.Madhusudana SN, Prem Anand N, Shamsundar R. Evaluation of two intradermal vaccination regimens using purified chick embryo cell vaccine for postexposure prophylaxis of rabies. Nat Med J India. 2001;14:145–147. [PubMed] [Google Scholar]
- 17.A report on “A clinical evaluation of safety and immunogenicity of purified chick embryo cell rabies vaccine (PCECV), administered intradermally using updated Thai Red Cross regimen in animal bite cases”. India: Novartis Vaccines; 2007. [Google Scholar]
- 18.A report on “A comparative, multi center Phase III, randomized (1:1) double blind study to evaluate the safety and immunogenicity of PVRV (Indirab vs. reference vaccine) administered intradermally using simulated updated Thai Red cross regimen in healthy volunteers”. Hyderabad, India: Bharath Biotech International Ltd.; 2009. [Google Scholar]
- 19.A report on “A comparative phase III, randomized (1:1) single blind study to evaluate the safety and immunogenicity of rabies vaccines (Indirab vs. reference vaccine) reconstituted with 1 mL diluent each, administered intradermally using simulated post exposure updated Thai Red Cross regimen in healthy volunteers”. Hyderabad, India: Bharath Biotech International Ltd.; 2009. [Google Scholar]
- 20.Meslin F-X, Kaplan MM, Koprowski H. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]


